Gamma Irradiation Triggers Immune Escape in Glioma-Propagating Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture and Irradiation of Human GPCs
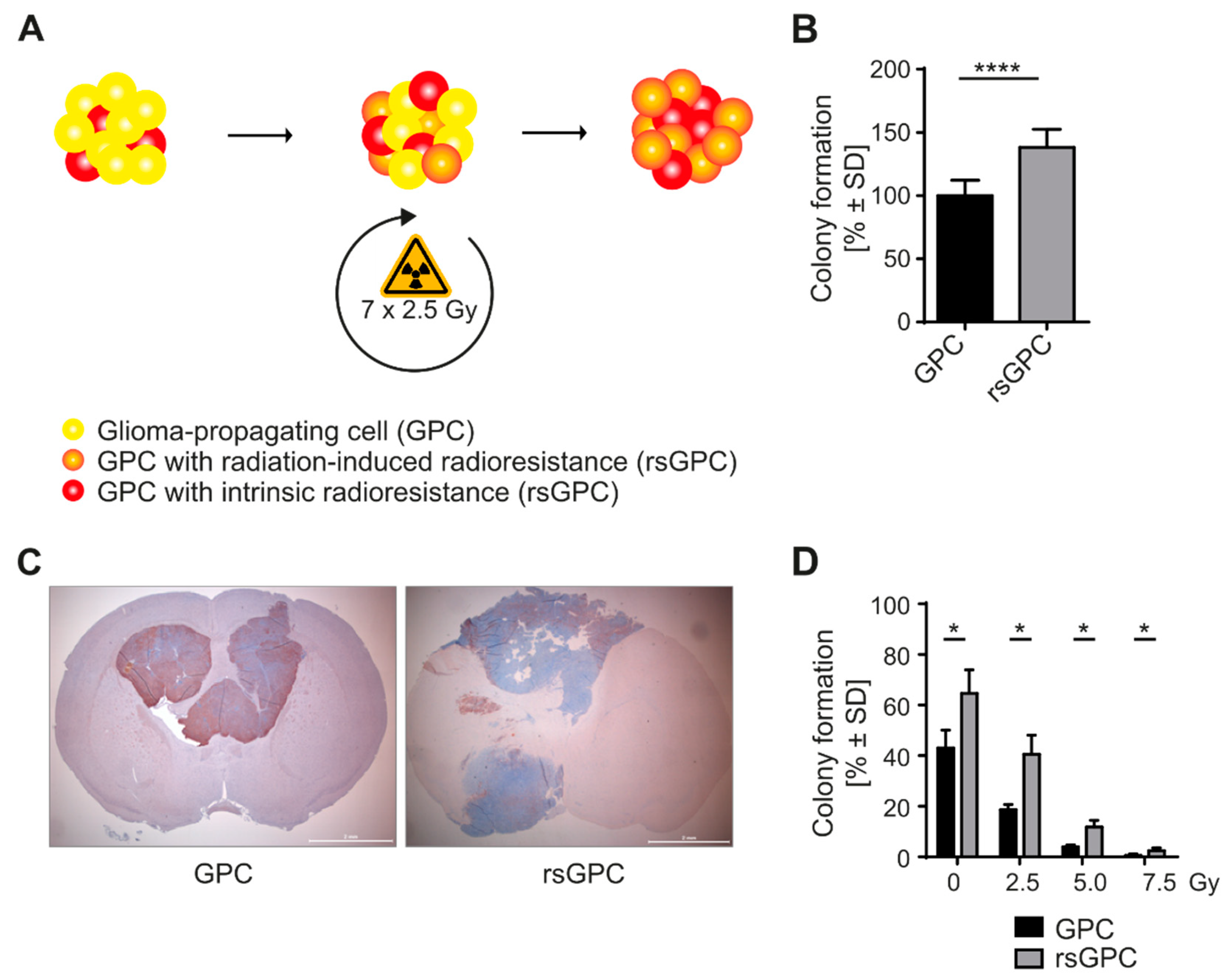
2.2. Colony Formation Assay
2.3. PBMC and CD8+ T-Cell Isolation
2.4. Mixed Lymphocyte Tumor Cultures
2.5. Flow Cytometry
2.6. Isolation of Detergent-Resistant Membrane Fractions
2.7. SDS-PAGE and Western Blot
2.8. Mass Spectrometric Analysis
2.9. Data and Statistical Analysis
3. Results
3.1. Radio-Selection and Phenotypic Analysis of GPCs
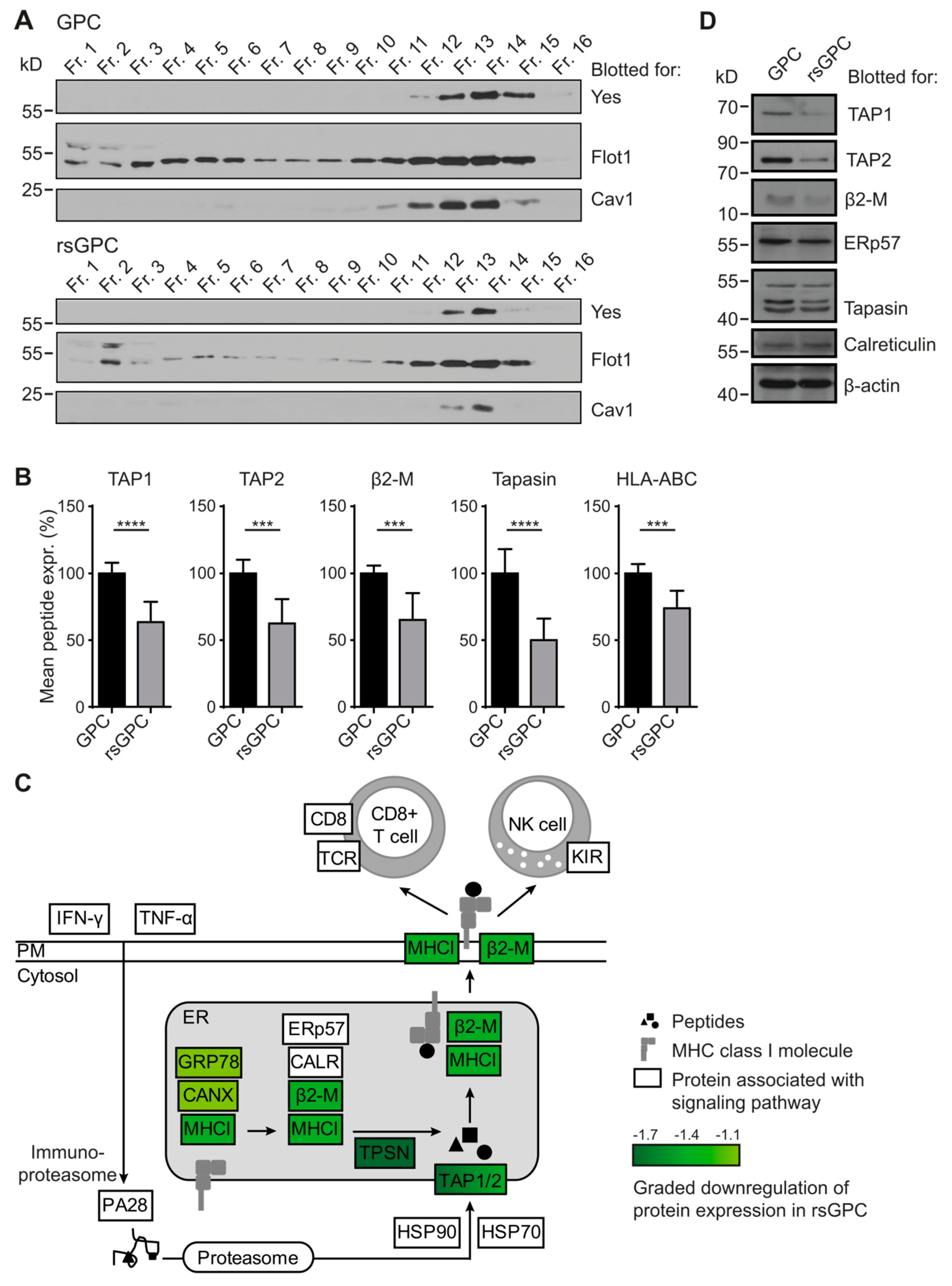
3.2. Comparative Analysis of DRM Composition
3.3. Downregulation of MHC Class I Antigen-Processing and -Presentation Components in rsGPC
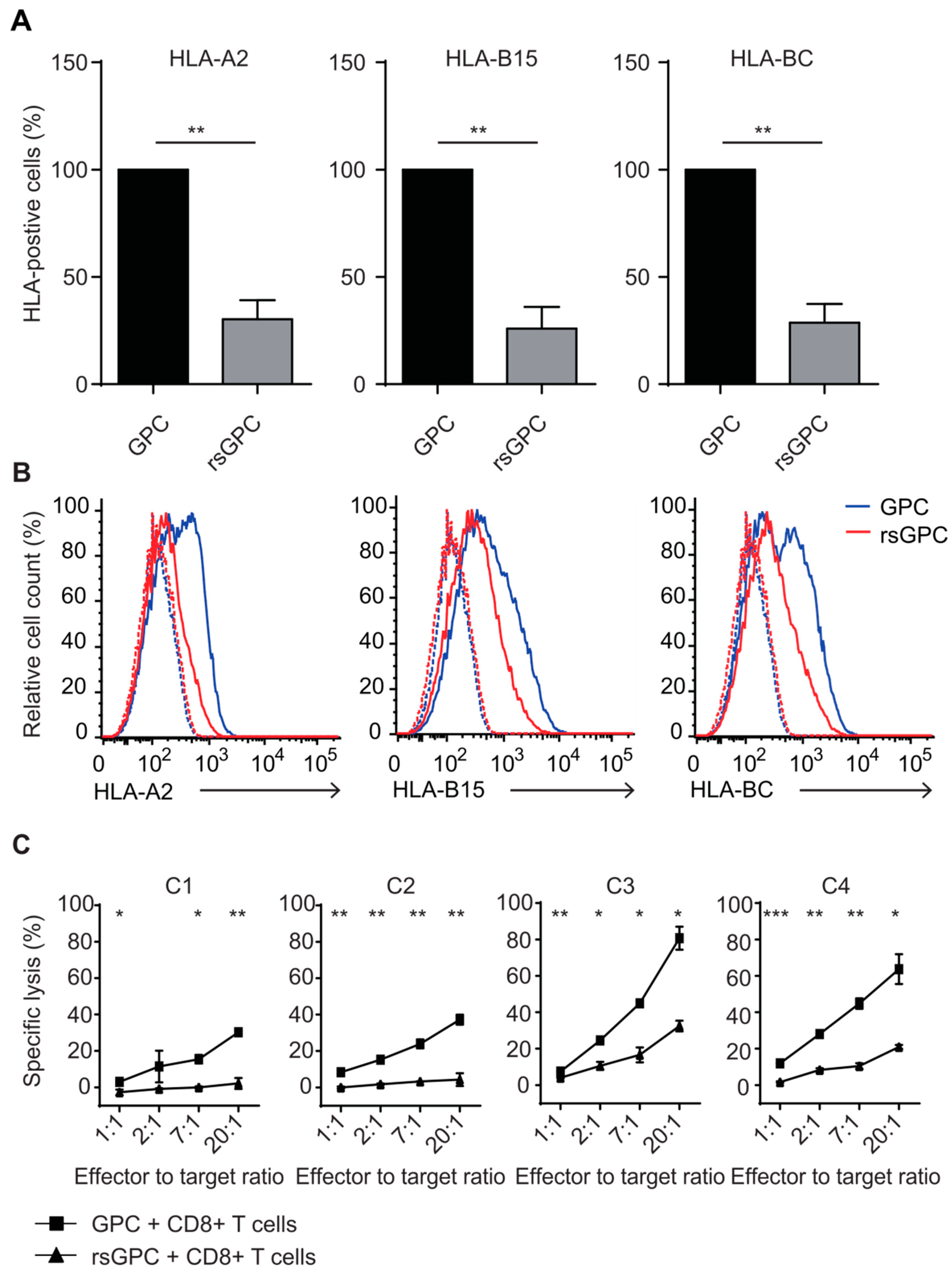
3.4. Evaluation of MHC Class I Surface Expression and Recognition Potential by Cytotoxic CD8+ T Cells after Fractionated Radiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Beier, D.; Schulz, J.B.; Beier, C.P. Chemoresistance of glioblastoma cancer stem cells-much more complex than expected. Mol. Cancer 2011, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jeong, E.K.; Ju, M.K.; Jeon, H.M.; Kim, M.Y.; Kim, C.H.; Park, H.G.; Han, S.I.; Kang, H.S. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol. Cancer 2017, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Nguyen, D.H.; Dong, Q.; Shitaku, P.; Chung, K.; Liu, O.Y.; Tso, J.L.; Liu, J.Y.; Konkankit, V.; Cloughesy, T.F.; et al. Molecular properties of CD133+ glioblastoma stem cells derived from treatment-refractory recurrent brain tumors. J. Neurooncol. 2009, 94, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; Poppleton, H.; Kocak, M.; Hogg, T.L.; Fuller, C.; Hamner, B.; Oh, E.Y.; Gaber, M.; Finklestein, D.; Allen, M.; et al. A Perivascular Niche for Brain Tumor Stem Cells. Cancer Cell 2007, 11, 69–82. [Google Scholar] [CrossRef]
- Crane, C.A.; Ahn, B.J.; Han, S.J.; Parsa, A.T. Soluble factors secreted by glioblastoma cell lines facilitate recruitment, survival, and expansion of regulatory T cells: Implications for immunotherapy. Neuro-Oncol. 2012, 14, 584–595. [Google Scholar] [CrossRef]
- Di Tomaso, T.; Mazzoleni, S.; Wang, E.; Sovena, G.; Clavenna, D.; Franzin, A.; Mortini, P.; Ferrone, S.; Doglioni, C.; Marincola, F.M.; et al. Immunobiological Characterization of Cancer Stem Cells Isolated from Glioblastoma Patients. Clin. Cancer Res. 2010, 16, 800–813. [Google Scholar] [CrossRef]
- Gomez, G.G.; Kruse, C.A. Mechanisms of malignant glioma immune resistance and sources of immunosuppression. Gene Ther. Mol. Biol. 2006, 10, 133–146. [Google Scholar]
- Wei, J.; Barr, J.; Kong, L.-Y.; Wang, Y.; Wu, A.; Sharma, A.K.; Gumin, J.; Henry, V.; Colman, H.; Sawaya, R.; et al. Glioma-Associated Cancer-Initiating Cells Induce Immunosuppression. Clin. Cancer Res. 2010, 16, 461–473. [Google Scholar] [CrossRef]
- Reits, E.A.; Hodge, J.W.; Herberts, C.A.; Groothuis, T.A.; Chakraborty, M.; Wansley, E.K.; Camphausen, K.; Luiten, R.M.; De Ru, A.H.; Neijssen, J.; et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006, 203, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Santin, A.D.; Hermonat, P.L.; Hiserodt, J.C.; Chiriva-Internati, M.; Woodliff, J.; Theus, J.W.; Barclay, D.; Pecorelli, S.; Parham, G.P. Effects of irradiation on the expression of major histocompatibility complex class I antigen and adhesion costimulation molecules ICAM-1 in human cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 1997, 39, 737–742. [Google Scholar] [CrossRef]
- Kim, J.E.; Patel, M.; Mangraviti, A.; Kim, E.S.; Theodros, D.; Velarde, E.; Liu, A.; Sankey, E.W.; Tam, A.; Xu, H.; et al. Combination Therapy with Anti-PD-1, Anti-TIM-3, and Focal Radiation Results in Regression of Murine Gliomas. Clin. Cancer Res. 2016, 23, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Joo, K.M.; Jin, J.; Kim, E.; Kim, K.H.; Kim, Y.; Kang, B.G.; Kang, Y.-J.; Lathia, J.D.; Cheong, K.H.; Song, P.H.; et al. MET Signaling Regulates Glioblastoma Stem Cells. Cancer Res. 2012, 72, 3828–3838. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.-K.; Suh, Y.; Cui, Y.-H.; Hwang, E.; Lim, E.-J.; Yoo, K.-C.; Lee, G.-H.; Yi, J.-M.; Kang, S.-G.; Lee, S.-J. Fractionated radiation-induced nitric oxide promotes expansion of glioma stem-like cells. Cancer Sci. 2013, 104, 1172–1177. [Google Scholar] [CrossRef]
- Wang, J.; Wakeman, T.P.; Lathia, J.D.; Hjelmeland, A.B.; Wang, X.F.; White, R.R.; Rich, J.N.; Sullenger, B.A. Notch promotes radioresistance of glioma stem cells. Stem Cells 2010, 28, 17–28. [Google Scholar] [CrossRef]
- Fadul, C.E.; Fisher, J.L.; Gui, J.; Hampton, T.H.; Côté, A.L.; Ernstoff, M.S. Immune modulation effects of concomitant temozolomide and radiation therapy on peripheral blood mononuclear cells in patients with glioblastoma multiforme. Neuro-oncology 2011, 13, 393–400. [Google Scholar] [CrossRef]
- Friedrich, T.; Scholz, M.; Durante, M. A predictive biophysical model of the combined action of radiotherapy and immunotherapy in cancer. Int. J. Radiat. Oncol. Biol. Phys. 2022, in press. [Google Scholar] [CrossRef]
- Bionda, C.; Hadchity, E.; Alphonse, G.; Chapet, O.; Rousson, R.; Rodriguez-Lafrasse, C.; Ardail, D. Radioresistance of human carcinoma cells is correlated to a defect in raft membrane clustering. Free Radic. Biol. Med. 2007, 43, 681–694. [Google Scholar] [CrossRef]
- Santana-Delgado, P.; Peña, L.A.; Haimovitz-Friedman, A.; Martin, S.; Green, D.; McLoughlin, M.; Cordon-Cardo, C.; Schuchman, E.H.; Fuks, Z.; Kolesnick, R. Acid Sphingomyelinase–Deficient Human Lymphoblasts and Mice Are Defective in Radiation-Induced Apoptosis. Cell 1996, 86, 189–199. [Google Scholar] [CrossRef]
- Hadjipanayis, C.G.; Van Meir, E.G. Tumor initiating cells in malignant gliomas: Biology and implications for therapy. J. Mol. Med. 2009, 87, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Park, I.-H.; Ryu, H.-H.; Li, S.-Y.; Li, C.-H.; Lim, S.-H.; Wen, M.; Jang, W.-Y.; Jung, S. Sublethal dose of irradiation enhances invasion of malignant glioma cells through p53-MMP 2 pathway in U87MG mouse brain tumor model. Radiat. Oncol. 2015, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Barrantes-Freer, A.; Kim, E.; Bielanska, J.; Giese, A.; Mortensen, L.S.; Schulz-Schaeffer, W.J.; Stadelmann, C.; Brück, W.; Pardo, L.A. Human Glioma–Initiating Cells Show a Distinct Immature Phenotype Resembling but Not Identical to NG2 Glia. J. Neuropathol. Exp. Neurol. 2013, 72, 307–324. [Google Scholar] [CrossRef]
- Parham, P.; Bodmer, W.F. Monoclonal antibody to a human histocompatibility alloantigen, HLA-A2. Nature 1978, 276, 397–399. [Google Scholar] [CrossRef]
- Lemonier, F.A.; Rebai, N.; Le Bouteiller, P.P.; Malissen, B.; Caillol, D.H.; Kourilsky, F.M. Epitopic analysis of detergent-solubilized HLA molecules by solid-phase radioimmunoassay. J. Immunol. Methods 1982, 54, 9–22. [Google Scholar] [CrossRef]
- Tenzer, S.; Moro, A.; Kuharev, J.; Francis, A.C.; Vidalino, L.; Provenzani, A.; Macchi, P. Proteome-Wide Characterization of the RNA-Binding Protein RALY-Interactome Using the in Vivo-Biotinylation-Pulldown-Quant (iBioPQ) Approach. J. Proteome Res. 2013, 12, 2869–2884. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Rosfa, S.; Wlodarski, A.; Kuharev, J.; Rekik, A.; Knauer, S.K.; Bantz, C.; Nawroth, T.; Bier, C.; et al. Nanoparticle Size Is a Critical Physicochemical Determinant of the Human Blood Plasma Corona: A Comprehensive Quantitative Proteomic Analysis. ACS Nano 2011, 5, 7155–7167. [Google Scholar] [CrossRef]
- Geromanos, S.J.; Vissers, J.P.C.; Silva, J.C.; Dorschel, C.A.; Li, G.-Z.; Gorenstein, M.V.; Bateman, R.H.; Langridge, J.I. The detection, correlation, and comparison of peptide precursor and product ions from data independent LC-MS with data dependant LC-MS/MS. Proteomics 2009, 9, 1683–1695. [Google Scholar] [CrossRef]
- Distler, U.; Kuharev, J.; Navarro, P.; Levin, Y.; Schild, H.; Tenzer, S. Drift time-specific collision energies enable deep-coverage data-independent acquisition proteomics. Nat. Methods 2013, 11, 167–170. [Google Scholar] [CrossRef]
- Dennis, G., Jr.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome. Biol. 2003, 4, P3. [Google Scholar] [CrossRef] [PubMed]
- Altmann, C.; Keller, S.; Schmidt, M.H.H. The Role of SVZ Stem Cells in Glioblastoma. Cancers 2019, 11, 448. [Google Scholar] [CrossRef] [PubMed]
- Pajonk, F.; Vlashi, E.; McBride, W.H. Radiation Resistance of Cancer Stem Cells: The 4 R’s of Radiobiology Revisited. Stem Cells 2010, 28, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, L.K.; Arvizu, M.; Aguilar-Cordova, E.; Chiocca, E.A. The Spectrum of Vaccine Therapies for Patients With Glioblastoma Multiforme. Curr. Treat. Options Oncol. 2012, 13, 437–450. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stankovic, N.D.; Hoppmann, N.; Teodorczyk, M.; Kim, E.L.; Bros, M.; Giese, A.; Zipp, F.; Schmidt, M.H. No role of IFITM3 in brain tumor formation in vivo. Oncotarget 2016, 7, 86388–86405. [Google Scholar] [CrossRef]
- Perry, J.R.; Laperriere, N.; O’Callaghan, C.J.; Brandes, A.A.; Menten, J.; Phillips, C.; Fay, M.; Nishikawa, R.; Cairncross, J.G.; Roa, W.; et al. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. N. Engl. J. Med. 2017, 376, 1027–1037. [Google Scholar] [CrossRef]
- Balducci, M.; Chiesa, S.; Diletto, B.; D’Agostino, G.R.; Mangiola, A.; Manfrida, S.; Mantini, G.; Albanese, A.; Fiorentino, A.; Frascino, V.; et al. Low-dose fractionated radiotherapy and concomitant chemotherapy in glioblastoma multiforme with poor prognosis: A feasibility study. Neuro-oncology 2011, 14, 79–86. [Google Scholar] [CrossRef]
- Phillips, T.M.; McBride, W.H.; Pajonk, F. The Response of CD24−/low/CD44 + Breast Cancer–Initiating Cells to Radiation. JNCI J. Natl. Cancer Inst. 2006, 98, 1777–1785. [Google Scholar] [CrossRef]
- Woodward, W.A.; Chen, M.S.; Behbod, F.; Alfaro, M.P.; Buchholz, T.A.; Rosen, J.M. WNT/β-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc. Natl. Acad. Sci. USA 2007, 104, 618–623. [Google Scholar] [CrossRef]
- Lomonaco, S.L.; Finniss, S.; Xiang, C.; Decarvalho, A.C.; Umansky, F.; Kalkanis, S.N.; Mikkelsen, T.; Brodie, C. The induction of autophagy by γ-radiation contributes to the radioresistance of glioma stem cells. Int. J. Cancer 2009, 125, 717–722. [Google Scholar] [CrossRef]
- Wild-Bode, C.; Weller, M.; Rimner, A.; Dichgans, J.; Wick, W. Sublethal irradiation promotes migration and invasiveness of glioma cells: Implications for radiotherapy of human glioblastoma. Cancer Res. 2001, 61, 2744–2750. [Google Scholar]
- Bionda, C.; Athias, A.; Poncet, D.; Alphonse, G.; Guezguez, A.; Gambert, P.; Rodriguez-Lafrasse, C.; Ardail, D. Differential regulation of cell death in head and neck cell carcinoma through alteration of cholesterol levels in lipid rafts microdomains. Biochem. Pharmacol. 2008, 75, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Kirschke, C.P.; Huang, L. ZnT7, a Novel Mammalian Zinc Transporter, Accumulates Zinc in the Golgi Apparatus. J. Biol. Chem. 2003, 278, 4096–4102. [Google Scholar] [CrossRef]
- Pardo, L.A.; Stühmer, W. The roles of K+ channels in cancer. Nat. Cancer 2013, 14, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Sakai, H.; Kuragari, M.; Suzuki, T.; Tauchi, K.; Minamimura, T.; Tsukada, K.; Asano, S.; Takeguchi, N. Expression of ATP1AL1, a Non-Gastric Proton Pump, in Human Colorectum. Jpn. J. Physiol. 2002, 52, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Streif, D.; Iglseder, E.; Hauser-Kronberger, C.; Fink, K.G.; Jakab, M.; Ritter, M. Expression of the Non-gastric H+/K+ ATPase ATP12A in Normal and Pathological Human Prostate Tissue. Cell. Physiol. Biochem. 2011, 28, 1287–1294. [Google Scholar] [CrossRef]
- Facoetti, A.; Nano, R.; Zelini, P.; Morbini, P.; Benericetti, E.; Ceroni, M.; Campoli, M.; Ferrone, S. Human Leukocyte Antigen and Antigen Processing Machinery Component Defects in Astrocytic Tumors. Clin. Cancer Res. 2005, 11, 8304–8311. [Google Scholar] [CrossRef]
- Pedersen, M.H.; Hood, B.L.; Beck, H.C.; Conrads, T.P.; Ditzel, H.J.; Leth-Larsen, R. Downregulation of antigen presentation-associated pathway proteins is linked to poor outcome in triple-negative breast cancer patient tumors. Oncoimmunology 2017, 6, e1305531. [Google Scholar] [CrossRef]
- Seliger, B.; Harders, C.; Lohmann, S.; Momburg, F.; Urlinger, S.; Tampé, R.; Huber, C. Down-regulation of the MHC class I antigen-processing machinery after oncogenic transformation of murine fibroblasts. Eur. J. Immunol. 1998, 28, 122–133. [Google Scholar] [CrossRef]
- Bicknell, D.C.; Kaklamanis, L.; Hampson, R.; Bodmer, W.F.; Karran, P. Selection for β2-microglobulin mutation in mismatch repair-defective colorectal carcinomas. Curr. Biol. 1996, 6, 1695–1697. [Google Scholar] [CrossRef]
- Challa-Malladi, M.; Lieu, Y.K.; Califano, O.; Holmes, A.B.; Bhagat, G.; Murty, V.V.; Dominguez-Sola, D.; Pasqualucci, L.; Dalla-Favera, R. Combined Genetic Inactivation of β2-Microglobulin and CD58 Reveals Frequent Escape from Immune Recognition in Diffuse Large B Cell Lymphoma. Cancer Cell 2011, 20, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Klug, F.; Prakash, H.; Huber, P.E.; Seibel, T.; Bender, N.; Halama, N.; Pfirschke, C.; Voss, R.H.; Timke, C.; Umansky, L.; et al. Low-Dose Irradiation Programs Macrophage Differentiation to an iNOS+/M1 Phenotype that Orchestrates Effective T Cell Immunotherapy. Cancer Cell 2013, 24, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Derer, A.; Frey, B.; Fietkau, R.; Gaipl, U.S. Immune-modulating properties of ionizing radiation: Rationale for the treatment of cancer by combination radiotherapy and immune checkpoint inhibitors. Cancer Immunol. Immunother. 2015, 65, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Genard, G.; Lucas, S.; Michiels, C. Reprogramming of Tumor-Associated Macrophages with Anticancer Therapies: Radiotherapy versus Chemo- and Immunotherapies. Front. Immunol. 2017, 8, 828. [Google Scholar] [CrossRef]
- Peters, H.L.; Yan, Y.; Solheim, J.C. APLP2 regulates the expression of MHC class I molecules on irradiated Ewing’s sarcoma cells. Oncoimmunology 2013, 2, e26293. [Google Scholar] [CrossRef]
- Heimberger, A.B.; Sun, W.; Hussain, S.F.; Dey, M.; Crutcher, L.; Aldape, K.; Gilbert, M.; Hassenbusch, S.J.; Sawaya, R.; Schmittling, B.; et al. Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: Case study. Neuro Oncol. 2008, 10, 98–103. [Google Scholar] [CrossRef]
- Grossman, S.A.; Ye, X.; Lesser, G.; Sloan, A.; Carraway, H.; Desideri, S.; Piantadosi, S.; Consortium, N.C. Immunosuppression in Patients with High-Grade Gliomas Treated with Radiation and Temozolomide. Clin. Cancer Res. 2011, 17, 5473–5480. [Google Scholar] [CrossRef]
- Dai, D.; Tian, Q.; Shui, Y.; Li, J.; Wei, Q. The impact of radiation induced lymphopenia in the prognosis of head and neck cancer: A systematic review and meta-analysis. Radiother. Oncol. 2022, 168, 28–36. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Cropet, C.; Van Glabbeke, M.; Sebban, C.; Le Cesne, A.; Judson, I.; Tredan, O.; Verweij, J.; Biron, P.; Labidi-Galy, S.I.; et al. Lymphopenia as a Prognostic Factor for Overall Survival in Advanced Carcinomas, Sarcomas, and Lymphomas. Cancer Res. 2009, 69, 5383–5391. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoppmann, N.; Heinig, N.; Distler, U.; Kim, E.; Lennerz, V.; Krauß, Y.; Schumann, U.; Giese, A.; Tenzer, S.; Bitar, L.; et al. Gamma Irradiation Triggers Immune Escape in Glioma-Propagating Cells. Cancers 2022, 14, 2728. https://doi.org/10.3390/cancers14112728
Hoppmann N, Heinig N, Distler U, Kim E, Lennerz V, Krauß Y, Schumann U, Giese A, Tenzer S, Bitar L, et al. Gamma Irradiation Triggers Immune Escape in Glioma-Propagating Cells. Cancers. 2022; 14(11):2728. https://doi.org/10.3390/cancers14112728
Chicago/Turabian StyleHoppmann, Nicola, Nora Heinig, Ute Distler, Ella Kim, Volker Lennerz, Yvonne Krauß, Ulrike Schumann, Alf Giese, Stefan Tenzer, Lynn Bitar, and et al. 2022. "Gamma Irradiation Triggers Immune Escape in Glioma-Propagating Cells" Cancers 14, no. 11: 2728. https://doi.org/10.3390/cancers14112728
APA StyleHoppmann, N., Heinig, N., Distler, U., Kim, E., Lennerz, V., Krauß, Y., Schumann, U., Giese, A., Tenzer, S., Bitar, L., & Schmidt, M. H. H. (2022). Gamma Irradiation Triggers Immune Escape in Glioma-Propagating Cells. Cancers, 14(11), 2728. https://doi.org/10.3390/cancers14112728






