Comparison of Proclarix, PSA Density and MRI-ERSPC Risk Calculator to Select Patients for Prostate Biopsy after mpMRI
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Design, Setting and Participants
2.2. Intervention
2.3. Endpoint Measurements
2.4. Statistical Analyses
3. Results
3.1. Characteristics of the Study Population and Distribution of Overall PCa, csPCa, and iPCa by PI-RADS Category
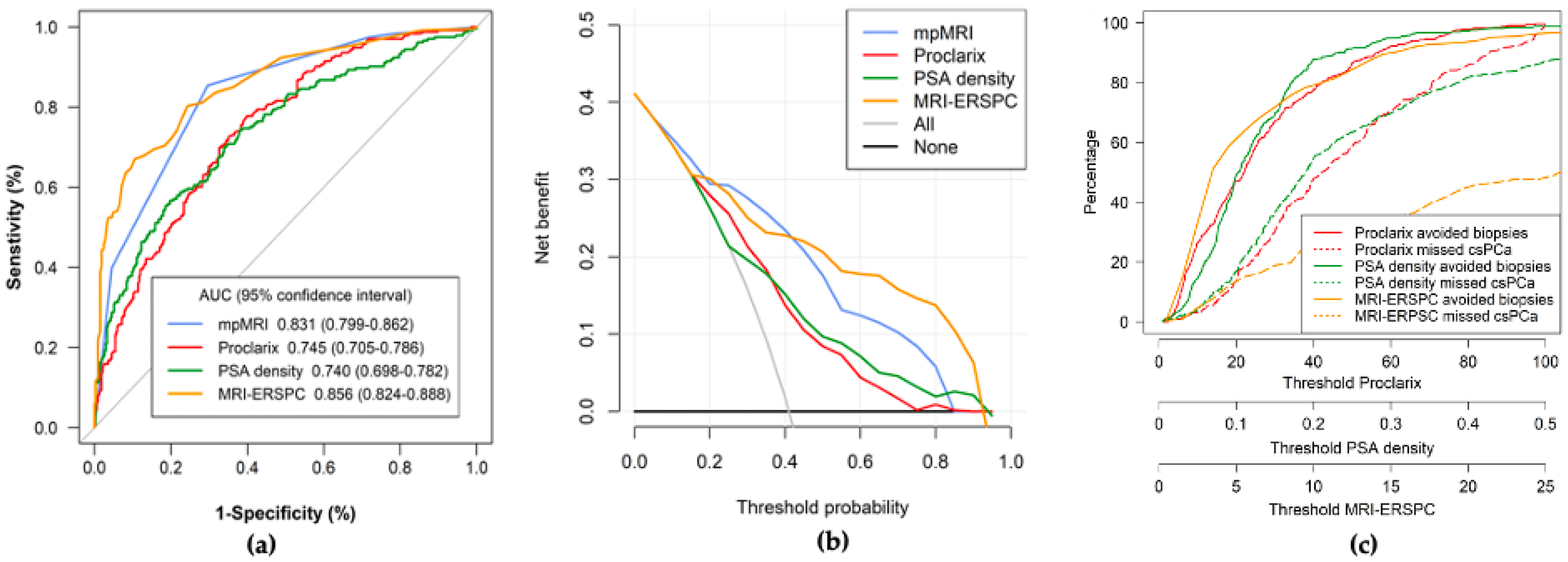
3.2. Overall Efficacy, Net Benefit, and Clinical Utility of mpMRI, PSAD, MRI-ERSPC RC, and Proclarix, and Overall Performances after the Selection of Appropriate Thresholds
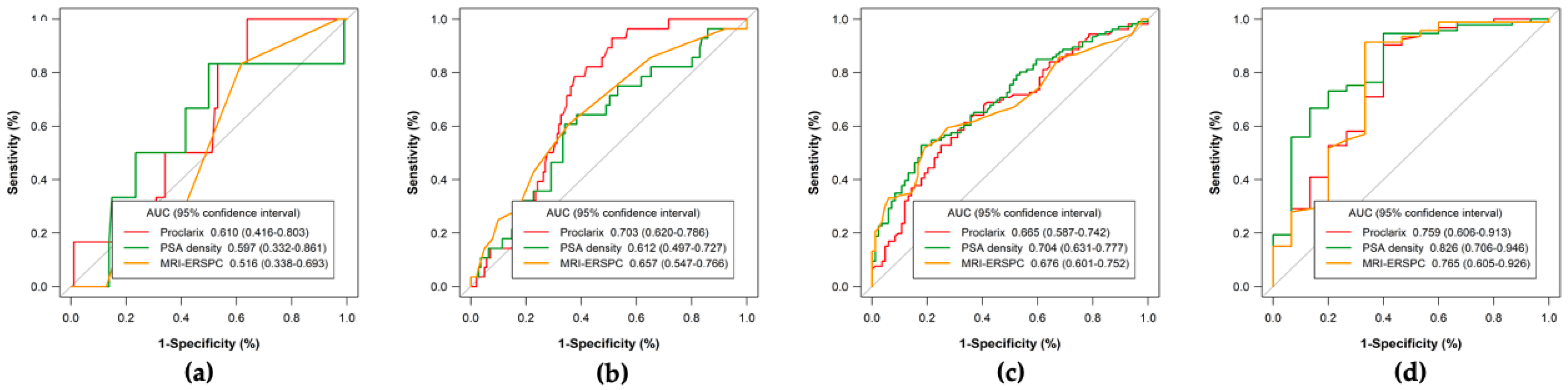
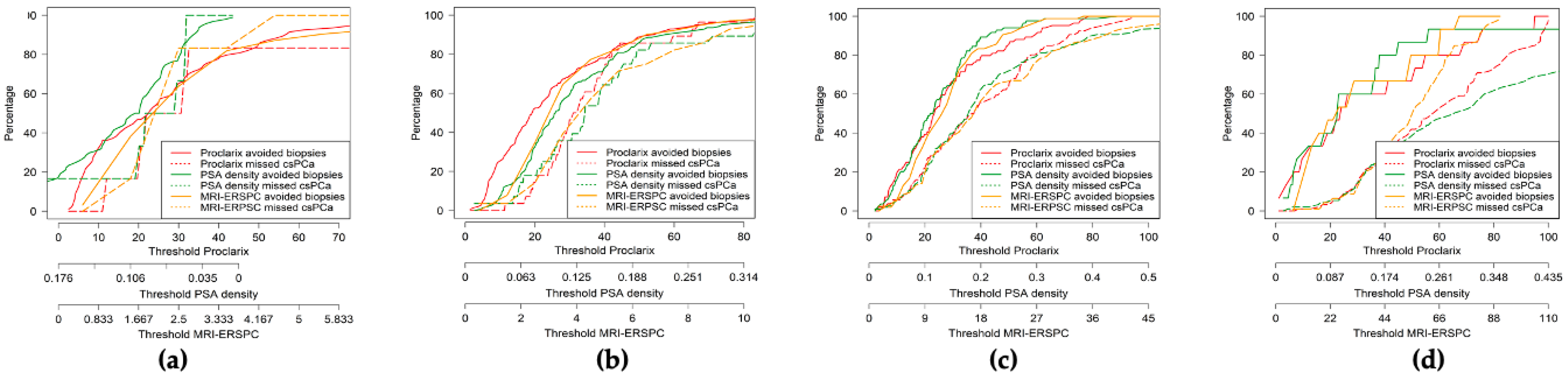
3.3. Efficacy, Net Benefit, Clinical Utility, and Performance of PSAD, MRI-ERSPC RC, and Proclarix by PI-RADS Category
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hugosson, J.; Roobol, M.J.; Månsson, M.; Tammela, T.L.J.; Zappa, M.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Carlsson, S.V.; Talala, K.M.; et al. A 16-Yr Follow-up of the European Randomized Study of Screening for Prostate Cancer. Eur. Urol. 2019, 76, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; van den Broeck, T.; Cumberbatch, M.G.; de Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Grossman, D.C.; Curry, S.J.; Owens, D.K.; Bibbins-Domingo, K.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Ebell, M.; Epling, J.W.; Kemper, A.R.; et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 319, 1901–1913. [Google Scholar] [CrossRef] [PubMed]
- Sathianathen, N.J.; Omer, A.; Harriss, E.; Davies, L.; Kasivisvanathan, V.; Punwani, S.; Moore, C.M.; Kastner, C.; Barrett, T.; van den Bergh, R.C.; et al. Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in the Detection of Clinically Significant Prostate Cancer in the Prostate Imaging Reporting and Data System Era: A Systematic Review and Meta-Analysis. Eur. Urol. 2020, 78, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, P.C.; van den Broeck, T.; Sylvester, R.; Marconi, L.; Bellmunt, J.; van den Bergh, R.C.N.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; Fossati, N.; et al. What Is the Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in Excluding Prostate Cancer at Biopsy? A Systematic Review and Meta-Analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur. Urol. 2017, 72, 250–266. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, E.; Stabile, A.; Pellegrino, F.; Basile, G.; Cignoli, D.; Cirulli, G.O.; Sorce, G.; Barletta, F.; Scuderi, S.; Bravi, C.A.; et al. Positive Predictive Value of Prostate Imaging Reporting and Data System Version 2 for the Detection of Clinically Significant Prostate Cancer: A Systematic Review and Meta-Analysis. Eur Urol. Oncol. 2021, 4, 697–713. [Google Scholar] [CrossRef]
- Schoots, I.G. MRI in Early Prostate Cancer Detection: How to Manage Indeterminate or Equivocal PI-RADS 3 Lesions? Transl Androl. Urol. 2018, 7, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Maggi, M.; Panebianco, V.; Mosca, A.; Salciccia, S.; Gentilucci, A.; di Pierro, G.; Busetto, G.M.; Barchetti, G.; Campa, R.; Sperduti, I.; et al. Prostate Imaging Reporting and Data System 3 Category Cases at Multiparametric Magnetic Resonance for Prostate Cancer: A Systematic Review and Meta-Analysis. Eur. Urol. Focus 2020, 6, 463–478. [Google Scholar] [CrossRef]
- Dianat, S.S.; Rancier Ruiz, R.M.; Bonekamp, D.; Carter, H.B.; Macura, K.J. Prostate Volumetric Assessment by Magnetic Resonance Imaging and Transrectal Ultrasound: Impact of Variation in Calculated Prostate-Specific Antigen Density on Patient Eligibility for Active Surveillance Program. J. Comput. Assist. Tomogr. 2013, 37, 589–595. [Google Scholar] [CrossRef]
- Morote, J.; Celma, A.; Diaz, F.; Regis, L.; Roche, S.; Mast, R.; Semidey, M.E.; de Torres, I.M.; Planas, J.; Trilla, E. Prostatic-Specific Antigen Density Behavior According to Multiparametric Magnetic Resonance Imaging Result. Urol. Oncol. 2020, 38, 410–417. [Google Scholar] [CrossRef]
- Osses, D.F.; Roobol, M.J.; Schoots, I.G. Prediction Medicine: Biomarkers, Risk Calculators and Magnetic Resonance Imaging as Risk Stratification Tools in Prostate Cancer Diagnosis. Int. J. Mol. Sci. 2019, 20, 1637. [Google Scholar] [CrossRef]
- Steuber, T.; Tennstedt, P.; Macagno, A.; Athanasiou, A.; Wittig, A.; Huber, R.; Golding, B.; Schiess, R.; Gillessen, S. Thrombospondin 1 and Cathepsin D Improve Prostate Cancer Diagnosis by Avoiding Potentially Unnecessary Prostate Biopsies. BJU Int. 2019, 123, 826–833. [Google Scholar] [CrossRef]
- Steuber, T.; Heidegger, I.; Kafka, M.; Roeder, M.A.; Chun, F.; Preisser, F.; Palisaar, R.-J.; Hanske, J.; Budaeus, L.; Schiess, R.; et al. PROPOSe: A Real-Life Prospective Study of Proclarix, a Novel Blood-Based Test to Support Challenging Biopsy Decision-Making in Prostate Cancer. Eur. Urol. Oncol. 2021. [Google Scholar] [CrossRef]
- Klocker, H.; Golding, B.; Weber, S.; Steiner, E.; Tennstedt, P.; Keller, T.; Schiess, R.; Gillessen, S.; Horninger, W.; Steuber, T. Development and Validation of a Novel Multivariate Risk Score to Guide Biopsy Decision for the Diagnosis of Clinically Significant Prostate Cancer. BJUI Compass 2020, 1, 15–20. [Google Scholar] [CrossRef]
- Alberts, A.R.; Roobol, M.J.; Verbeek, J.F.M.; Schoots, I.G.; Chiu, P.K.; Osses, D.F.; Tijsterman, J.D.; Beerlage, H.P.; Mannaerts, C.K.; Schimmöller, L.; et al. Prediction of High-Grade Prostate Cancer Following Multiparametric Magnetic Resonance Imaging: Improving the Rotterdam European Randomized Study of Screening for Prostate Cancer Risk Calculators. Eur. Urol. 2019, 75, 310–318. [Google Scholar] [CrossRef]
- Endt, K.; Goepfert, J.; Omlin, A.; Athanasiou, A.; Tennstedt, P.; Guenther, A.; Rainisio, M.; Engeler, D.S.; Steuber, T.; Gillessen, S.; et al. Development and Clinical Testing of Individual Immunoassays for the Quantification of Serum Glycoproteins to Diagnose Prostate Cancer. PLoS ONE 2017, 12, e0181557. [Google Scholar] [CrossRef]
- Macagno, A.; Athanasiou, A.; Wittig, A.; Huber, R.; Weber, S.; Keller, T.; Rhiel, M.; Golding, B.; Schiess, R. Analytical Performance of Thrombospondin-1 and Cathepsin D Immunoassays Part of a Novel CE-IVD Marked Test as an Aid in the Diagnosis of Prostate Cancer. PLoS ONE 2020, 15, e0233442. [Google Scholar] [CrossRef]
- Roobol, M.J.; Steyerberg, E.W.; Kranse, R.; Wolters, T.; van den Bergh, R.C.N.; Bangma, C.H.; Schröder, F.H. A Risk-Based Strategy Improves Prostate-Specific Antigen-Driven Detection of Prostate Cancer. Eur. Urol. 2010, 57, 79–85. [Google Scholar] [CrossRef]
- Weinreb, J.C.; Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J.; Margolis, D.; Schnall, M.D.; Shtern, F.; Tempany, C.M.; et al. PI-RADS Prostate Imaging—Reporting and Data System: 2015, Version 2. Eur. Urol. 2016, 69, 16–40. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef]
- Epstein, J.I.; Zelefsky, M.J.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W.; et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef]
- Creelman, C.D.; Wayne, D. ROC Curves for Discrimination of Linear Extent. J. Exp. Psychol. 1968, 77, 514–516. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Vickers, A.J.; Elkin, E.B. Decision Curve Analysis: A Novel Method for Evaluating Prediction Models. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef]
- Borque-Fernando, Á.; Esteban-Escaño, L.M.; Rubio-Briones, J.; Lou-Mercadé, A.C.; García-Ruiz, R.; Tejero-Sánchez, A.; Muñoz-Rivero, M.V.; Cabañuz-Plo, T.; Alfaro-Torres, J.; Marquina-Ibáñez, I.M.; et al. A Preliminary Study of the Ability of the 4Kscore Test, the Prostate Cancer Prevention Trial-Risk Calculator and the European Research Screening Prostate-Risk Calculator for Predicting High-Grade Prostate Cancer. Actas Urológicas Españolas 2016, 40, 155–163. [Google Scholar] [CrossRef][Green Version]
- Morote, J.; Campistol, M.; Triquell, M.; Celma, A.; Regis, L.; de Torres, I.; Semidey, M.E.; Mast, R.; Santamaria, A.; Planas, J.; et al. Improving the Early Detection of Clinically Significant Prostate Cancer in Men in the Challenging Prostate Imaging-Reporting and Data System 3 Category. Eur. Urol. Open Sci. 2022, 37, 38–44. [Google Scholar] [CrossRef]
- Boesen, L.; Nørgaard, N.; Løgager, V.; Balslev, I.; Bisbjerg, R.; Thestrup, K.C.; Jakobsen, H.; Thomsen, H.S. Prebiopsy Biparametric Magnetic Resonance Imaging Combined with Prostate-Specific Antigen Density in Detecting and Ruling out Gleason 7–10 Prostate Cancer in Biopsy-Naïve Men. Eur. Urol. Oncol. 2019, 2, 311–319. [Google Scholar] [CrossRef]
- Abreu-Gomez, J.; Wu, M.; McInnes, M.D.F.; Thornhill, R.E.; Flood, T.A.; Schieda, N. Shape Analysis of Peripheral Zone Observations on Prostate DWI: Correlation to Histopathology Outcomes after Radical Prostatectomy. Am. J. Roentgenol. 2020, 214, 1239–1247. [Google Scholar] [CrossRef]
- Boschheidgen, M.; Schimmöller, L.; Arsov, C.; Ziayee, F.; Morawitz, J.; Valentin, B.; Radke, K.L.; Giessing, M.; Esposito, I.; Albers, P.; et al. MRI Grading for the Prediction of Prostate Cancer Aggressiveness. Eur. Radiol. 2021, 32, 2351–2359. [Google Scholar] [CrossRef]
| Characteristic | Measurement |
|---|---|
| Number of cases | 567 |
| Median age, years (IQR) | 69 (63–74) |
| Median total PSA, ng/mL (IQR) | 7.0 (4.9–11.2) |
| Abnormal DRE, n (%) | 109 (19.2) |
| Median free PSA, ng/mL (IQR) | 1.1 (0.7–1.7) |
| Median prostate volume, mL (IQR) | 55 (40–76) |
| Median percent free PSA, % (IQR) | 15.1 (10.7–20.6) |
| Median PSA density, ng/mL/cc (IQR) | 0.13 (0.09–0.21) |
| Repeat biopsy, n (%) | 133 (23.5) |
| Family history of PCa, n (%) | 48 (8.6%) |
| PI-RADS, n (%) | |
| 1–2 | 100 (17.6) |
| 3 | 169 (29.8) |
| 4 | 190 (33.5) |
| 5 | 108 (19.0) |
| Overall PCa detection, n (%) | 298 (52.6) |
| csPCa detection, n (%) | 232 (40.9) |
| iPCa detection, n (%) | 66 (11.7) |
| Parameter | mpMRI | Proclarix | PSAD | MRI-ERSPC |
|---|---|---|---|---|
| Cut-off | 1–2 PI-RADS | 10% | 0.07 ng/mL/cc | 3% |
| Sensitivity (%) | 226/232 (97.4) | 226/232 (97.4) | 209/232 (90.1) | 219/232 (94.4) |
| Specificity (%) | 94/335 (28.1) | 89/335 (26.6) | 96/335 (28.7) | 104/335 (31.0) |
| Negative predictive value (%) | 94/100 (94.0) | 89/95 (93.7) | 96/119 (80.7) | 109/117 (88.9) |
| Positive predictive value (%) | 226/467 (48.4) | 226/472 (47.9) | 209/448 (46.7) | 219/450 (48.7) |
| Accuracy (%) | 320/567 (56.4) | 315/567 (55.6) | 305/567 (53.8) | 323/567 (57.3) |
| Avoidable biopsies | 100/567 (17.6) | 95/567 (16.8) | 119/567 (21.0) | 117/567 (20.6) |
| Misdiagnosis of csPCa (%) | 6/232 (2.6) | 6/232 (2.6) | 23/232 (9.9) | 13/232 (5.6) |
| GG2 | 4 | 3 | 10 | 8 |
| GG3 | 1 | 2 | 6 | 1 |
| GG4 | 1 | 1 | 4 | 2 |
| GG5 | 0 | 0 | 3 | 0 |
| PI-RADS | Sensitivity | Specificity | NPV | PPV | Accuracy | Avoidable Biopsies | Misdiagnosis of csPCa | GG2 | GG3 | GG4 | GG5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Proclarix (cut-off 10%) | |||||||||||
| 1–2 | 6/6(100) | 30/94 (31.9) | 30/30 (100) | 6/70 (8.6) | 36/100 (36) | 30/100 (30) | 0/6 (0) | 0 | 0 | 0 | 0 |
| 3 | 25/25 (100) | 36/144 (25.0) | 36/36 (100) | 25/133 (19.8) | 61/169 (36.1) | 36/169 (21.3) | 0/25 (0) | 0 | 0 | 0 | 0 |
| 4 | 100/105 (95.2) | 18/85 (21.2) | 18/23 (78.3) | 100/167 (59.9) | 118/190 (62.1) | 23/190 (12.1) | 5/105 (4.8) | 2 | 1 | 1 | 1 |
| 5 | 95/96 (99.0) | 5/12 (41.7) | 5/6 (83.3) | 95/102 (93.1) | 100/108 (92.6) | 6/108 (5.6) | 1/96 (1.0) | 1 | 0 | 0 | 0 |
| PSAD (cut-off 0.07 ng/mL/cc) | |||||||||||
| 1–2 | 3/6 (50) | 26/94 (27.7) | 26/29 (89.7) | 3/71 (4.2) | 29/100 (29.0) | 29/100 (29.0) | 3/6 (50.0) | 1 | 1 | 1 | 0 |
| 3 | 21/25 (84.0) | 41/144 (28.5) | 41/45 (91.1) | 21/124 (16.0) | 62/169 (36.7) | 45/169 (26.2) | 4/25 (16.0) | 4 | 0 | 0 | 0 |
| 4 | 93/105 (88.6) | 13/85 (27.1) | 23/35 (65.7) | 93/155 (60.0) | 116/190 (61.1) | 35/190 (18.4) | 12/105 (11.4) | 4 | 3 | 3 | 2 |
| 5 | 92/96 (95.8) | 6/12 (50.0) | 6/10 (60.0) | 92/98 (93.9) | 98/108 (90.7) | 10/108 (9.3) | 4/96 (4.2) | 0 | 2 | 1 | 1 |
| MRI-ERSPC model (cut-off 3%) | |||||||||||
| 1–2 | 1/6 (16.7) | 58/94 (61.7) | 58/63 (92.1) | 1/37 (2.7) | 59/100 (59) | 63/100 (63) | 5/6 (83.3) | 3 | 1 | 1 | 0 |
| 3 | 21/25 (84) | 46 (31.9) | 46/50 (92) | 21/119 (17.6) | 67/169 (39.6) | 50/169 (29.6) | 4/25 (16) | 4 | 0 | 0 | 0 |
| 4 | 103/105 (98.1) | 2/86 (2.3) | 2/4 (50) | 103/186 (15.3) | 104/190 (54.8) | 4/190 (2.1) | 2/105 (1.9) | 1 | 1 | 0 | 2 |
| 5 | 96/99 (100) | NA | NA | 96/108 (88.9) | 96/108 (87.9) | 0/108 (0) | 0/96 (0) | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campistol, M.; Morote, J.; Triquell, M.; Regis, L.; Celma, A.; de Torres, I.; Semidey, M.E.; Mast, R.; Santamaría, A.; Planas, J.; et al. Comparison of Proclarix, PSA Density and MRI-ERSPC Risk Calculator to Select Patients for Prostate Biopsy after mpMRI. Cancers 2022, 14, 2702. https://doi.org/10.3390/cancers14112702
Campistol M, Morote J, Triquell M, Regis L, Celma A, de Torres I, Semidey ME, Mast R, Santamaría A, Planas J, et al. Comparison of Proclarix, PSA Density and MRI-ERSPC Risk Calculator to Select Patients for Prostate Biopsy after mpMRI. Cancers. 2022; 14(11):2702. https://doi.org/10.3390/cancers14112702
Chicago/Turabian StyleCampistol, Miriam, Juan Morote, Marina Triquell, Lucas Regis, Ana Celma, Inés de Torres, María E. Semidey, Richard Mast, Anna Santamaría, Jacques Planas, and et al. 2022. "Comparison of Proclarix, PSA Density and MRI-ERSPC Risk Calculator to Select Patients for Prostate Biopsy after mpMRI" Cancers 14, no. 11: 2702. https://doi.org/10.3390/cancers14112702
APA StyleCampistol, M., Morote, J., Triquell, M., Regis, L., Celma, A., de Torres, I., Semidey, M. E., Mast, R., Santamaría, A., Planas, J., & Trilla, E. (2022). Comparison of Proclarix, PSA Density and MRI-ERSPC Risk Calculator to Select Patients for Prostate Biopsy after mpMRI. Cancers, 14(11), 2702. https://doi.org/10.3390/cancers14112702






