Fusogenic Hybrid Extracellular Vesicles with PD-1 Membrane Proteins for the Cytosolic Delivery of Cargos
Abstract
Simple Summary
Abstract
1. Introduction
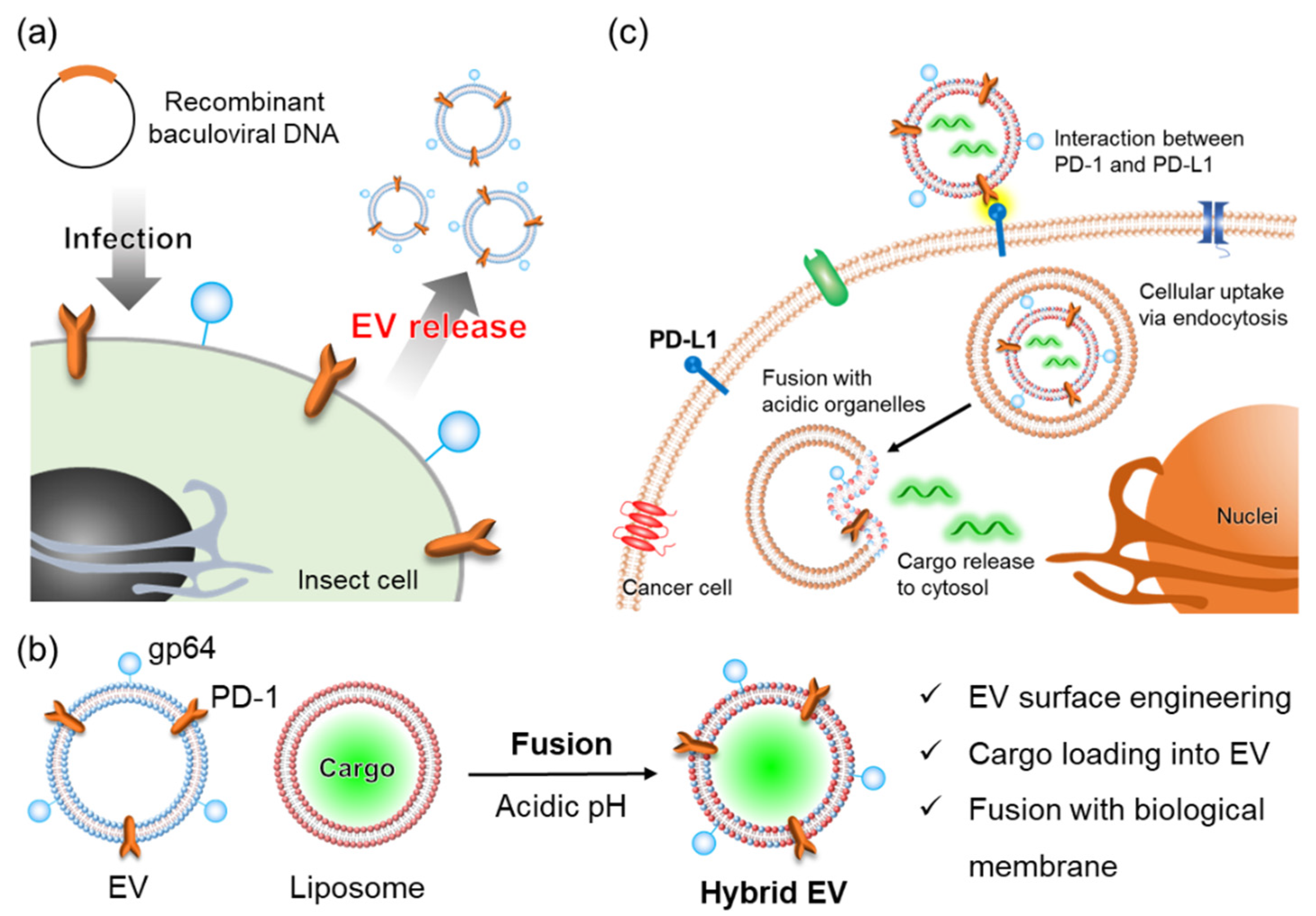
2. Materials and Methods
2.1. Materials
2.2. Construction of Recombinant Baculoviruses
2.3. Isolation of Extracellular Vesicles
2.4. Liposome Preparation
2.5. Nanoparticle Tracking Analysis
2.6. Transmission Electron Microscopy
2.7. Western Blotting
2.8. Imaging Flow Cytometry
2.9. Cellular Uptake of Hybrid Extracellular Vesicles
2.10. Evaluation of the Fusion of Hybrid Extracellular Vesicles with Acidic Organelles
2.11. Cytosolic Delivery of Hybrid Extracellular Vesicle Cargo
2.12. Statistical Analysis
3. Results
3.1. Preparation and Characterization of Hybrid Extracellular Vesicles
3.2. Intracellular Uptake and Organelle Localization of Hybrid Extracellular Vesicles
3.3. Membrane Fusion of Hybrid Extracellular Vesicles with Cell Organelles
3.4. Cargo Delivery to the Cytosol by Fusion between Hybrid Extracellular Vesicles and Acidic Organelles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morshedi Rad, D.; Alsadat Rad, M.; Razavi Bazaz, S.; Kashaninejad, N.; Jin, D.; Ebrahimi Warkiani, M. A Comprehensive Review on Intracellular Delivery. Adv. Mater. 2021, 33, e2005363. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.A.; Witzigmann, D.; Thomson, S.B.; Chen, S.; Leavitt, B.R.; Cullis, P.R.; van der Meel, R. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 2021, 16, 630–643. [Google Scholar] [CrossRef]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Sil, S.; Dagur, R.S.; Liao, K.; Peeples, E.S.; Hu, G.; Periyasamy, P.; Buch, S. Strategies for the use of Extracellular Vesicles for the Delivery of Therapeutics. J. Neuroimmune Pharmacol. 2020, 15, 422–442. [Google Scholar] [CrossRef]
- Fu, S.; Wang, Y.; Xia, X.; Zheng, J.C. Exosome engineering: Current progress in cargo loading and targeted delivery. NanoImpact. 2020, 20, 100261. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; Stremersch, S.; Braeckmans, K.; de Smedt, S.C.; Hendrix, A.; Wood, M.J.A.; Schiffelers, R.M.; Raemdonck, K.; Vader, P. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Control Release 2013, 172, 229–238. [Google Scholar] [CrossRef]
- Louzoun-Zada, S.; Jaber, Q.Z.; Fridman, M. Guiding Drugs to Target-Harboring Organelles: Stretching Drug-Delivery to a Higher Level of Resolution. Angew. Chem. Int. Ed. Engl. 2019, 58, 15584–15594. [Google Scholar] [CrossRef]
- Nakase, I.; Futaki, S. Combined treatment with a pH-sensitive fusogenic peptide and cationic lipids achieves enhanced cytosolic delivery of exosomes. Sci. Rep. 2015, 5, 10112. [Google Scholar] [CrossRef]
- Nakase, I.; Noguchi, K.; Aoki, A.; Takatani-Nakase, T.; Fujii, I.; Futaki, S. Arginine-rich cell-penetrating peptide-modified extracellular vesicles for active macropinocytosis induction and efficient intracellular delivery. Sci. Rep. 2017, 7, 1991. [Google Scholar] [CrossRef]
- Sawada, S.; Sato, Y.T.; Kawasaki, R.; Yasuoka, J.I.; Mizuta, R.; Sasaki, Y.; Akiyoshi, K. Nanogel hybrid assembly for exosome intracellular delivery: Effects on endocytosis and fusion by exosome surface polymer engineering. Biomater. Sci. 2020, 8, 619–630. [Google Scholar] [CrossRef]
- Evers, M.J.W.; van de Wakker, S.I.; de Groot, E.M.; de Jong, O.G.; Gitz-Francois, J.J.J.; Seinen, C.S.; Sluijter, J.P.G.; Schiffelers, R.M.; Vader, P. Functional siRNA Delivery by Extracellular Vesicle-Liposome Hybrid Nanoparticles. Adv. Healthc. Mater. 2022, 11, e2101202. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.F.; Zuo, F.F.; Yin, B.C.; Ye, B.C. Delivery of siRNA based on engineered exosomes for glioblastoma therapy by targeting STAT3. Biomater. Sci. 2022, 10, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Xing, H.; Xun, Z.; Yang, T.; Ding, P.; Cai, C.; Wang, D.; Zhao, X. Exosome-based small RNA delivery: Progress and prospects. Asian J. Pharm. Sci. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Guo, Y.; Li, D.; Zhang, S.; Yang, Y.; Liu, J.J.; Wang, X.; Liu, C.; Milkie, D.E.; Moore, R.P.; Tulu, U.S.; et al. Visualizing Intracellular Organelle and Cytoskeletal Interactions at Nanoscale Resolution on Millisecond Timescales. Cell 2018, 175, 1430–1442. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, P.S.; Baylies, M.K.; Fleissner, A.; Helming, L.; Inoue, N.; Podbilewicz, B.; Wang, H.; Wong, M. Genetic basis of cell-cell fusion mechanisms. Trends Genet. 2013, 29, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Jenni, S.; Stanifer, M.L.; Roth, E.; Whelan, S.P.; van Oijen, A.M.; Harrison, S.C. Mechanism of membrane fusion induced by vesicular stomatitis virus G protein. Proc. Natl. Acad. Sci. USA 2017, 114, E28–E36. [Google Scholar] [CrossRef]
- White, J.M.; Delos, S.E.; Brecher, M.; Schornberg, K. Structures and mechanisms of viral membrane fusion proteins: Multiple variations on a common theme. Crit. Rev. Biochem. Mol. Biol. 2008, 43, 189–219. [Google Scholar] [CrossRef]
- Jahn, R.; Lang, T.; Südhof, T.C. Membrane Fusion. Cell 2003, 112, 519–533. [Google Scholar] [CrossRef]
- Mora, N.L.; Boyle, A.L.; Kolck, B.J.V.; Rossen, A.; Pokorna, S.; Koukalova, A.; Sachl, R.; Hof, M.; Kros, A. Controlled Peptide-Mediated Vesicle Fusion Assessed by Simultaneous Dual-Colour Time-Lapsed Fluorescence Microscopy. Sci. Rep. 2020, 10, 3087. [Google Scholar] [CrossRef]
- Lentz, B.R.; Lee, J.K. Poly(ethylene glycol) (PEG)-mediated fusion between pure lipid bilayers: A mechanism in common with viral fusion and secretory vesicle release? Mol. Membr. Biol. 1999, 16, 279–296. [Google Scholar] [CrossRef]
- Piffoux, M.; Silva, A.K.A.; Wilhelm, C.; Gazeau, F.; Tareste, D. Modification of Extracellular Vesicles by Fusion with Liposomes for the Design of Personalized Biogenic Drug Delivery Systems. ACS Nano. 2018, 12, 6830–6842. [Google Scholar] [CrossRef]
- Dimitrov, D.S. Virus entry: Molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2004, 2, 109–122. [Google Scholar] [CrossRef]
- Yee, C.M.; Zak, A.J.; Hill, B.D.; Wen, F. The Coming Age of Insect Cells for Manufacturing and Development of Protein Therapeutics. Ind. Eng. Chem. Res. 2018, 57, 10061–10070. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, R.; Yoshida, S.; Sawada, S.; Sasaki, Y.; Akiyoshi, K. Preparation of engineered extracellular vesicles with full-length functional PD-1 membrane proteins by baculovirus expression system. Biochem. Biophys. Res. Commun. 2020, 526, 967–972. [Google Scholar] [CrossRef]
- Blissard, G.W.; Wenz, J.R. Baculovirus Gp64 Envelope Glycoprotein Is Sufficient to Mediate Ph-Dependent Membrane-Fusion. J. Virol. 1992, 66, 6829–6835. [Google Scholar] [CrossRef] [PubMed]
- Kadlec, J.; Loureiro, S.; Abrescia, N.G.; Stuart, D.I.; Jones, I.M. The postfusion structure of baculovirus gp64 supports a unified view of viral fusion machines. Nat. Struct. Mol. Biol. 2008, 15, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, H.; Mizutani, M.; Imamura, K.; Morino, K.; Kobayashi, J.; Okumura, K.; Tsumoto, K.; Yoshimura, T. Development of a novel preparation method of recombinant proteoliposomes using baculovirus gene expression systems. J. Biochem. 2008, 144, 763–770. [Google Scholar] [CrossRef]
- Kamiya, K.; Tsumoto, K.; Arakawa, S.; Shimizu, S.; Morita, I.; Yoshimura, T.; Akiyoshi, K. Preparation of connexin43-integrated giant Liposomes by a baculovirus expression-liposome fusion method. Biotechnol. Bioeng. 2010, 107, 836–843. [Google Scholar] [CrossRef]
- Kamiya, K.; Tsumoto, K.; Yoshimura, T.; Akiyoshi, K. Cadherin-integrated liposomes with potential application in a drug delivery system. Biomaterials 2011, 32, 9899–9907. [Google Scholar] [CrossRef]
- Ishikawa, R.; Yoshida, S.; Sawada, S.; Sasaki, Y.; Akiyoshi, K. Development and single-particle analysis of hybrid extracellular vesicles fused with liposomes using viral fusogenic proteins. FEBS Open Bio. 2022; in press. [Google Scholar] [CrossRef]
- Das, C.K.; Jena, B.C.; Banerjee, I.; Das, S.; Parekh, A.; Bhutia, S.K.; Mandal, M. Exosome as a Novel Shuttle for Delivery of Therapeutics across Biological Barriers. Mol. Pharm. 2019, 16, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.T.; Umezaki, K.; Sawada, S.; Mukai, S.A.; Sasaki, Y.; Harada, N.; Shiku, H.; Akiyoshi, K. Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep. 2016, 6, 21933. [Google Scholar] [CrossRef] [PubMed]
- Ramasubramanian, L.; Kumar, P.; Wang, A. Engineering Extracellular Vesicles as Nanotherapeutics for Regenerative Medicine. Biomolecules 2019, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.B.; Dammer, E.B.; Ren, R.J.; Wang, G. The endosomal-lysosomal system: From acidification and cargo sorting to neurodegeneration. Transl. Neurodegener. 2015, 4, 18. [Google Scholar] [CrossRef]
- Zhou, J.; Blissard, G.W. Mapping the conformational epitope of a neutralizing antibody (AcV1) directed against the AcMNPV GP64 protein. Virology 2006, 352, 427–437. [Google Scholar] [CrossRef][Green Version]
- Akinc, A.; Thomas, M.; Klibanov, A.M.; Langer, R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J. Gene Med. 2005, 7, 657–663. [Google Scholar] [CrossRef]
- Badazhkova, V.D.; Raik, S.V.; Polyakov, D.S.; Poshina, D.N.; Skorik, Y.A. Effect of Double Substitution in Cationic Chitosan Derivatives on DNA Transfection Efficiency. Polymers 2020, 12, 1057. [Google Scholar] [CrossRef]
- Bus, T.; Traeger, A.; Schubert, U.S. The great escape: How cationic polyplexes overcome the endosomal barrier. J. Mater. Chem. B 2018, 6, 6904–6918. [Google Scholar] [CrossRef]
- Xie, J.; Bi, Y.; Zhang, H.; Dong, S.; Teng, L.; Lee, R.J.; Yang, Z. Cell-Penetrating Peptides in Diagnosis and Treatment of Human Diseases: From Preclinical Research to Clinical Application. Front. Pharmacol. 2020, 11, 697. [Google Scholar] [CrossRef]
- Sahni, A.; Qian, Z.; Pei, D. Cell-Penetrating Peptides Escape the Endosome by Inducing Vesicle Budding and Collapse. ACS Chem. Biol. 2020, 15, 2485–2492. [Google Scholar] [CrossRef]
- Clark, S.R.; Lee, K.Y.; Lee, H.; Khetan, J.; Kim, H.C.; Choi, Y.H.; Shin, K.; Won, Y.Y. Determining the effects of PEI adsorption on the permeability of 1,2-dipalmitoylphosphatidylcholine/bis(monoacylglycero)phosphate membranes under osmotic stress. Acta Biomater. 2018, 65, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Colman, P.M.; Lawrence, M.C. The structural biology of type I viral membrane fusion. Nat. Rev. Mol. Cell Biol. 2003, 4, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Petitjean, S.J.L.; Koehler, M.; Zhang, Q.; Dumitru, A.C.; Chen, W.; Derclaye, S.; Vincent, S.P.; Soumillion, P.; Alsteens, D. Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor. Nat. Commun. 2020, 11, 4541. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, C.; Xu, X.F.; Xu, W.; Liu, S.W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Gui, M.; Xiang, Y. Structural intermediates in the low pH-induced transition of influenza hemagglutinin. PLoS Pathog. 2020, 16, e1009062. [Google Scholar] [CrossRef]
- Beilstein, F.; Abou Hamdan, A.; Raux, H.; Belot, L.; Ouldali, M.; Albertini, A.A.; Gaudin, Y. Identification of a pH-Sensitive Switch in VSV-G and a Crystal Structure of the G Pre-fusion State Highlight the VSV-G Structural Transition Pathway. Cell Rep. 2020, 32, 108042. [Google Scholar] [CrossRef]
- Mashel, T.V.; Tarakanchikova, Y.V.; Muslimov, A.R.; Zyuzin, M.V.; Timin, A.S.; Lepik, K.V.; Fehse, B. Overcoming the delivery problem for therapeutic genome editing: Current status and perspective of non-viral methods. Biomaterials 2020, 258, 120282. [Google Scholar] [CrossRef]
- Airenne, K.J.; Hu, Y.C.; Kost, T.A.; Smith, R.H.; Kotin, R.M.; Ono, C.; Matsuura, Y.; Wang, S.; Yla-Herttuala, S. Baculovirus: An insect-derived vector for diverse gene transfer applications. Mol. Ther. 2013, 21, 739–749. [Google Scholar] [CrossRef]
- Mansouri, M.; Bellon-Echeverria, I.; Rizk, A.; Ehsaei, Z.; Cianciolo Cosentino, C.; Silva, C.S.; Xie, Y.; Boyce, F.M.; Davis, M.W.; Neuhauss, S.C.; et al. Highly efficient baculovirus-mediated multigene delivery in primary cells. Nat. Commun. 2016, 7, 11529. [Google Scholar] [CrossRef]
- Glenn, G.M.; Smith, G.; Fries, L.; Raghunandan, R.; Lu, H.; Zhou, B.; Thomas, D.N.; Hickman, S.P.; Kpamegan, E.; Boddapati, S.; et al. Safety and immunogenicity of a Sf9 insect cell-derived respiratory syncytial virus fusion protein nanoparticle vaccine. Vaccine 2013, 31, 524–532. [Google Scholar] [CrossRef]
- Premanand, B.; Zhong Wee, P.; Prabakaran, M. Baculovirus Surface Display of Immunogenic Proteins for Vaccine Development. Viruses 2018, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Gwon, Y.D.; Kim, S.; Cho, Y.; Heo, Y.; Cho, H.; Park, K.; Lee, H.J.; Choi, J.; Poo, H.; Kim, Y.B. Immunogenicity of Virus Like Particle Forming Baculoviral DNA Vaccine against Pandemic Influenza H1N1. PLoS ONE 2016, 11, e0154824. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.; Khalil, F.; Antonia, S. PD-L1 expression is increased in a subset of basal type breast cancer cells. PLoS ONE 2014, 9, e88557. [Google Scholar] [CrossRef]
- Zheng, Y.; Fang, Y.C.; Li, J. PD-L1 expression levels on tumor cells affect their immunosuppressive activity. Oncol. Lett. 2019, 18, 5399–5407. [Google Scholar] [CrossRef]
- Lim, W.J.; Lee, M.; Oh, Y.; Fang, X.Q.; Lee, S.; Lim, C.H.; Park, J.; Lim, J.H. Statins Decrease Programmed Death-Ligand 1 (PD-L1) by Inhibiting AKT and beta-Catenin Signaling. Cells 2021, 10, 2488. [Google Scholar] [CrossRef]
- Butte, M.J.; Pena-Cruz, V.; Kim, M.J.; Freeman, G.J.; Sharpe, A.H. Interaction of human PD-L1 and B7-1. Mol. Immunol. 2008, 45, 3567–3572. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishikawa, R.; Yoshida, S.; Sawada, S.-i.; Sasaki, Y.; Akiyoshi, K. Fusogenic Hybrid Extracellular Vesicles with PD-1 Membrane Proteins for the Cytosolic Delivery of Cargos. Cancers 2022, 14, 2635. https://doi.org/10.3390/cancers14112635
Ishikawa R, Yoshida S, Sawada S-i, Sasaki Y, Akiyoshi K. Fusogenic Hybrid Extracellular Vesicles with PD-1 Membrane Proteins for the Cytosolic Delivery of Cargos. Cancers. 2022; 14(11):2635. https://doi.org/10.3390/cancers14112635
Chicago/Turabian StyleIshikawa, Raga, Shosuke Yoshida, Shin-ichi Sawada, Yoshihiro Sasaki, and Kazunari Akiyoshi. 2022. "Fusogenic Hybrid Extracellular Vesicles with PD-1 Membrane Proteins for the Cytosolic Delivery of Cargos" Cancers 14, no. 11: 2635. https://doi.org/10.3390/cancers14112635
APA StyleIshikawa, R., Yoshida, S., Sawada, S.-i., Sasaki, Y., & Akiyoshi, K. (2022). Fusogenic Hybrid Extracellular Vesicles with PD-1 Membrane Proteins for the Cytosolic Delivery of Cargos. Cancers, 14(11), 2635. https://doi.org/10.3390/cancers14112635





