An International Retrospective Observational Study of Liver Functional Deterioration after Repeat Liver Resection for Patients with Hepatocellular Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Participating Centers and Registered Patients
2.2. Selection of Patients and Data Collection
2.3. Analysis of the Indicators of Liver Function before and 3 Months after RLR
2.4. Comparison between the Patients Who Underwent ORLR after Previous Open LR (OO group) and LRLR after Previous Laparoscopic LR (LL Group): Background Factors, Indicators for Liver Function before RLR, Their Changes after RLR, and Overall Survival after RLR
2.5. Statistical Analyses
3. Results
3.1. Analyses of the Indicators for Liver Function before and 3 Months after RLR
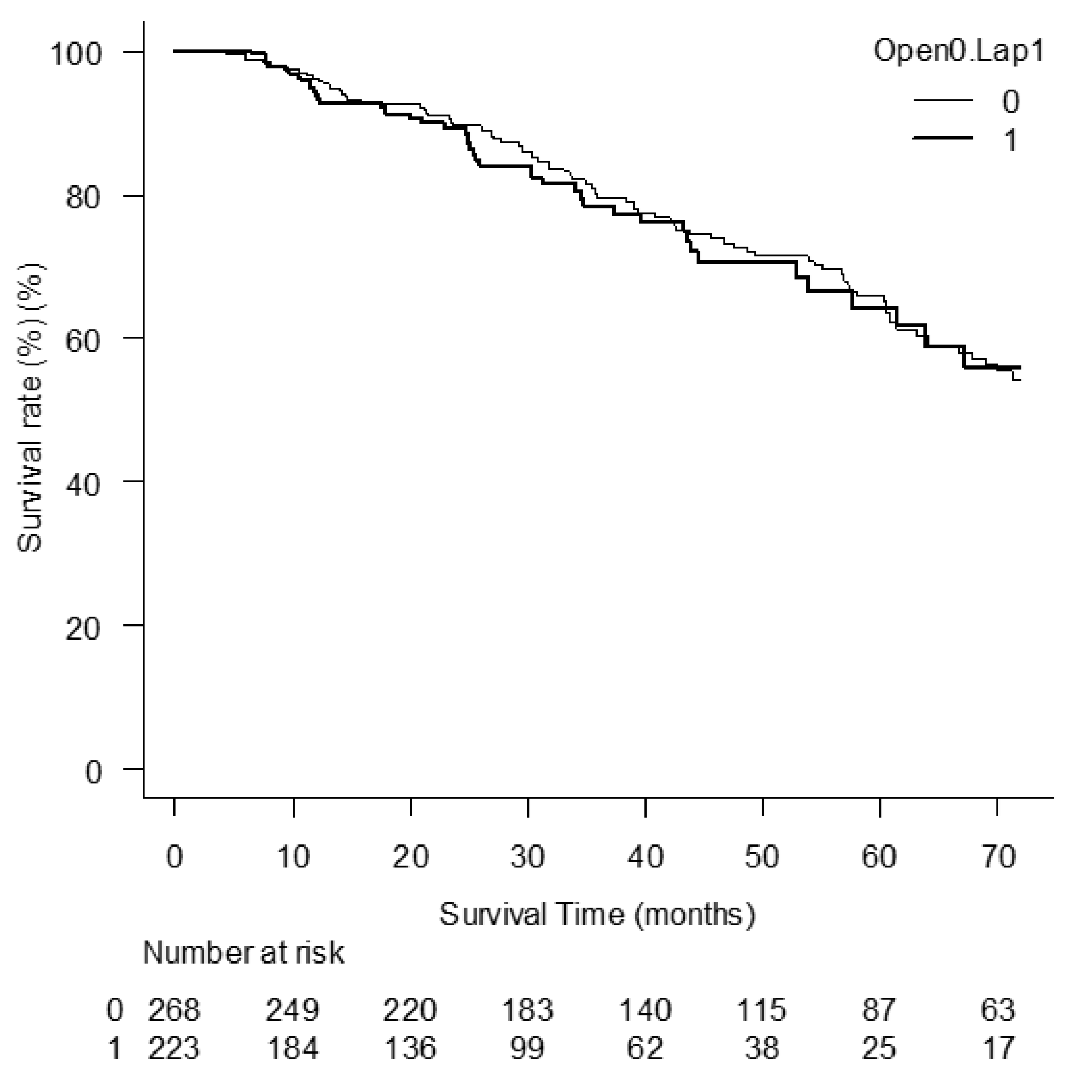
3.2. Comparison between OO Group and LL Group: Background Factors, Indicators for Liver Function before RLR, and Those, Their Changes, and Overall Survivals after RLR
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capussotti, L.; Ferrero, A.; Viganò, L.; Polastri, R.; Tabone, M. Liver resection for HCC with cirrhosis: Surgical perspectives out of EASL/AASLD guidelines. Eur. J. Surg. Oncol. 2009, 35, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Lee, S.G.; Belghiti, J. Liver transplantation for HCC: Its role: Eastern and Western perspectives. J. Hepatobiliary Pancreat. Sci. 2010, 17, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.Y.; Leung, T.W.; Yu, S.C.; Ho, S.K. Percutaneous local ablative therapy for hepatocellular carcinoma: A review and look into the future. Ann. Surg. 2003, 237, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Cheng, A.L.; Baron, A.; Han, G.; Lopez, C.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [Green Version]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Cheng, A.L.; Kudo, M.; Merle, P.; Li, D.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Gennari, L.; Montalto, F.; Ammatuna, M.; Morabito, A. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996, 334, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Ryder, S.D. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut 2003, 52, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahbari, N.N.; Mehrabi, A.; Mollberg, N.M.; Müller, S.A.; Koch, M.; Büchler, M.W.; Weitz, J. Hepatocellular carcinoma: Current management and perspectives for the future. Ann. Surg. 2011, 253, 453–469. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Marrero, J.A.; Rudolph, L.; Reddy, K.R. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 2008, 134, 1752–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llovet, J.M.; Fuster, J.; Bruix, J. The Barcelona approach: Diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004, 10, S115–S120. [Google Scholar] [CrossRef] [PubMed]
- Morise, Z.; Aldrighetti, L.; Belli, G.; Ratti, F.; Belli, A.; Cherqui, D.; Alzoubi, M.; Lo, M.; Kubo, S.; Monden, K.; et al. ILLS-Tokyo Collaborator group. Laparoscopic repeat liver resection for hepatocellular carcinoma: A multicentre propensity score-based study. Br. J. Surg. 2020, 107, 889–895. [Google Scholar] [CrossRef] [PubMed]
- 3Miyama, A.; Morise, Z.; Aldrighetti, L.; Belli, G.; Ratti, F.; Cheung, T.-T.; Lo, C.-M.; Tanaka, S.; Kubo, S.; Okamura, Y.; et al. Multicenter Propensity Score-Based Study of Laparoscopic Repeat Liver Resection for Hepatocellular Carcinoma: A Subgroup Analysis of Cases with Tumors Far from Major Vessels. Cancers 2021, 13, 3187. [Google Scholar]
- Morise, Z.; Ciria, R.; Cherqui, D.; Chen, K.H.; Belli, G.; Wakabayashi, G. Can we expand the indications for laparoscopic liver resection? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J. Hepatobiliary Pancreat. Sci. 2015, 22, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; Toyoda, H.; Fox, R.; Palmer, D.; Lai, P.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Sharma, R.; Allara, E.; Yen, C.; Arizumi, T.; Kubota, K.; Carr, B.I.; Kim, Y.-W.; Kudo, M.; Guerra, V.; et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J. Hepatol. 2017, 66, 338–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, G.; Taura, K.; Ikai, I.; Fujikawa, T.; Nishitai, R.; Kaihara, S.; Uemoto, S.; Okuda, Y.; Tanabe, K.; Nishio, T.; et al. ALPlat criterion for the resection of hepatocellular carcinoma based on a predictive model of posthepatectomy liver failure. Surgery 2020, 167, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, A.; Tsukamoto, T.; Shimizu, S.; Kodai, S.; Yamamoto, S.; Yamazoe, S.; Ohira, G.; Nakajima, T. Laparoscopic liver resection for treating recurrent hepatocellular carcinoma. J. Hepatobiliary Pancreat. Sci. 2013, 20, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Tomishige, H.; Morise, Z.; Kawabe, N.; Nagata, H.; Ohshima, H.; Kawase, J.; Arawaka, S.; Isetani, M.; Yoshida, R. Caudal approach to pure laparoscopic posterior sectionectomy under the laparoscopy-specific view. World J. Gastrointest. Surg. 2013, 5, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Han, H.S.; Kaneko, H.; Buell, J.F. Laparoscopic hepatectomy is theoretically better than open hepatectomy: Preparing for the 2nd International Consensus Conference on Laparoscopic Liver Resection. J. Hepatobiliary Pancreat. Sci. 2014, 21, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Soubrane, O.; Schwarz, L.; Cauchy, F.; Perotto, L.O.; Brustia, R.; Bernard, D.; Scatton, O. A conceptual technique for laparoscopic right hepatectomybased on facts and oncologic principles: The caudal approach. Ann. Surg. 2015, 261, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
| ORLR, n = 450 | LRLR, n = 425 | p Value | |
|---|---|---|---|
| Age (years old) | 66.07 ± 10.77 | 68.03 ± 10.60 | 0.007 * |
| Sex (male:female) | 355:95 | 322:103 | 0.270 |
| BMI | 22.98 ± 3.43 | 23.98 ± 3.96 | <0.001 * |
| Performance status (0:1:2) | 411:37:1 | 365:55:5 | 0.016 * |
| Size of tumor (mm) | 23.59 ± 17.52 | 20.49 ± 10.74 | 0.002 * |
| Number of tumors (1:2:3:>4) | 315:87:23:25 | 335:70:13:7 | 0.003 * |
| Tumor location (AL:PS) | 159:107 | 145:77 | 0.223 |
| Extent of resection (Segment or less: Section: 2 or more sections) | 329:72:49 | 382:33:10 | <0.001 * |
| Albumin (g/dL) | 4.09 ± 0.41 | 4.01 ± 0.48 | 0.006 * |
| Total Bilirubin (mg/dL) | 0.73 ± 0.32 | 0.76 ± 0.35 | 0.095 |
| Platelet (X104/microL) | 14.77 ± 6.11 | 13.93 ± 5.10 | 0.026 * |
| Presence of fibrosis (NL:CH:LF:LC) | 73:56:114:202 # | 49:39:120:21 3 ## | 0.056 |
| Child–Pugh score (5:6:7:>8) | 393:46:9:2 | 322:84:14:5 | <0.001 * |
| Pre-Operative Data | Post-Operative Data | p Value | |
|---|---|---|---|
| Albumin (g/dL) | 4.04 ± 0.45 | 3.97 ± 0.53 | 0.006 * |
| Total Bilirubin (mg/dL) | 0.76 ± 0.33 | 0.81 ± 0.40 | 0.010 * |
| Platelet (×104/microL) | 14.07 ± 5.02 | 14.12 ± 5.20 | 0.862 |
| ALBI score | −2.73 ± 0.40 | −2.65 ± 0.48 | 0.001 * |
| AlPlat score | 504.49 ± 70.46 | 498.24 ± 77.05 | 0.125 |
| Before LR | OO | LL | p Value |
|---|---|---|---|
| Age (years old) | 67.37 ± 10.36 | 68.62 ± 9.96 | 0.176 |
| Sex (male:female) | 214:54 | 167:57 | 0.194 |
| BMI | 22.94 ± 3.44 | 23.96 ± 3.98 | 0.002 * |
| Performance status (1:2:3) | 250:17:1 | 194:29:1 | 0.043 * |
| Number of tumors (1:2:3:>4) | 188:58:14:8 | 176:38:6:4 | 0.209 |
| Size of tumor (mm) | 20.93 ± 15.21 | 19.00 ± 9.52 | 0.089 |
| Tumor location (AL:PS) | 159:107 | 145:77 | 0.223 |
| Albumin (g/dL) | 4.09 ± 0.39 | 3.94 ± 0.49 | <0.001 * |
| Total Bilirubin (mg/dL) | 0.73 ± 0.31 | 0.75 ± 0.35 | 0.698 |
| Platelet (×104/microL) | 14.58 ± 4.89 | 13.57 ± 5.41 | 0.031 * |
| ALBI score | −2.78 ± 0.34 | −2.65 ± 0.46 | <0.001 * |
| AlPlat score | 514.32 ± 61.09 | 490.43 ± 79.95 | <0.001 * |
| Presence of fibrosis (NL:CH:LF:LC) | 48:41:70:106 | 21:23:63:114 | 0.006 * |
| Child-Pugh score (5:6:7:>8) | 239:25:4:0 | 160:53:7:4 | <0.001 * |
| 3 months after LR | |||
| Albumin (g/dL) | 4.03 ± 0.47 | 3.89 ± 0.55 | 0.003 * |
| Total Bilirubin (mg/dL) | 0.77 ± 0.36 | 0.80 ± 0.39 | 0.461 |
| Platelet (X104/microL) | 14.77 ± 5.09 | 13.68 ± 5.56 | 0.025 * |
| ALBI score | −2.71 ± 0.42 | −2.59 ± 0.52 | 0.003 * |
| AlPlat score | 510.10 ± 69.08 | 486.70 ± 83.60 | 0.001 * |
| OO | LL | p Value | |
|---|---|---|---|
| Change of Alb (g/dL) | 0.068 ± 0.40 | 0.054 ± 0.42 | 0.710 |
| Change of Total Bilirubin (mg/dL) | −0.036 ± 0.34 | −0.049 ± 0.33 | 0.653 |
| Change of Platelet (×104/microL) | −0.19 ± 4.26 | −0.11 ± 3.34 | 0.830 |
| Change of ALBI score | −0.064 ± 0.35 | −0.063 ± 0.38 | 0.969 |
| Change of ALPlat score | 4.23 ± 53.46 | 3.73 ± 53.59 | 0.919 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morise, Z.; Aldrighetti, L.; Belli, G.; Ratti, F.; Cheung, T.T.; Lo, C.M.; Tanaka, S.; Kubo, S.; Okamura, Y.; Uesaka, K.; et al. An International Retrospective Observational Study of Liver Functional Deterioration after Repeat Liver Resection for Patients with Hepatocellular Carcinoma. Cancers 2022, 14, 2598. https://doi.org/10.3390/cancers14112598
Morise Z, Aldrighetti L, Belli G, Ratti F, Cheung TT, Lo CM, Tanaka S, Kubo S, Okamura Y, Uesaka K, et al. An International Retrospective Observational Study of Liver Functional Deterioration after Repeat Liver Resection for Patients with Hepatocellular Carcinoma. Cancers. 2022; 14(11):2598. https://doi.org/10.3390/cancers14112598
Chicago/Turabian StyleMorise, Zenichi, Luca Aldrighetti, Giulio Belli, Francesca Ratti, Tan To Cheung, Chung Mau Lo, Shogo Tanaka, Shoji Kubo, Yukiyasu Okamura, Katsuhiko Uesaka, and et al. 2022. "An International Retrospective Observational Study of Liver Functional Deterioration after Repeat Liver Resection for Patients with Hepatocellular Carcinoma" Cancers 14, no. 11: 2598. https://doi.org/10.3390/cancers14112598
APA StyleMorise, Z., Aldrighetti, L., Belli, G., Ratti, F., Cheung, T. T., Lo, C. M., Tanaka, S., Kubo, S., Okamura, Y., Uesaka, K., Monden, K., Sadamori, H., Hashida, K., Kawamoto, K., Gotohda, N., Chen, K., Kanazawa, A., Takeda, Y., Ohmura, Y., ... Wakabayashi, G. (2022). An International Retrospective Observational Study of Liver Functional Deterioration after Repeat Liver Resection for Patients with Hepatocellular Carcinoma. Cancers, 14(11), 2598. https://doi.org/10.3390/cancers14112598












