Tumor-Infiltrating Lymphocytes (TILs) in Early Breast Cancer Patients: High CD3+, CD8+, and Immunoscore Are Associated with a Pathological Complete Response
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Immunohistochemistry (IHC) and Digital Image Analysis
2.3. Pathological Assessment
2.4. Immunoscore® for Clinical Research
2.5. Statistical Methods
2.6. Ethics Approval
3. Results
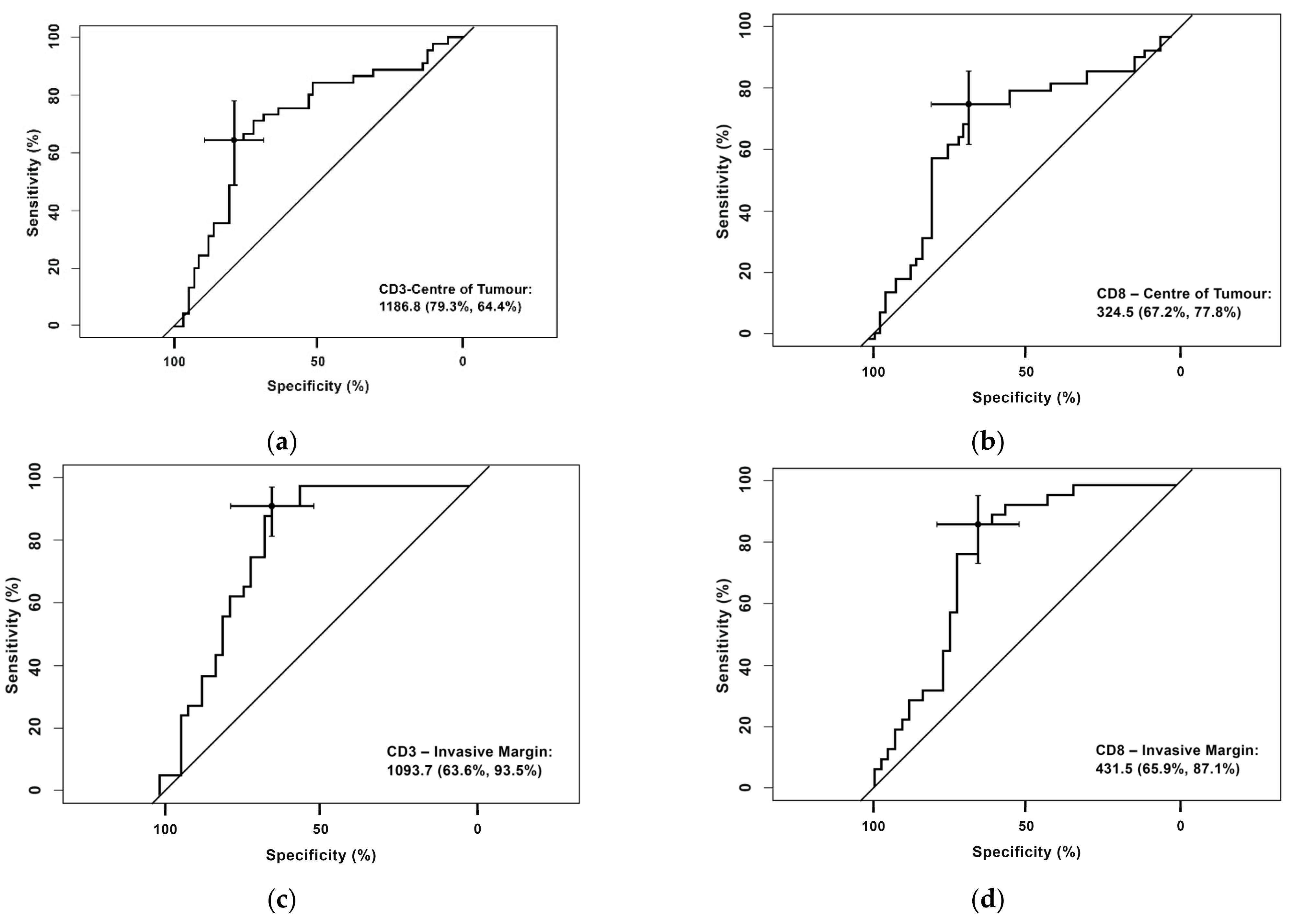
3.1. Receiver Operating Characteristic (ROC) Curve for CD3+ and CD8+ Cells in the Center of the Tumor and the Invasive Margin
- CD3+ T-cell densities in the center of the tumor were 1186.80 cells/mm2 (sensitivity 79.3% and specificity 64.4%)
- CD8+ T-cell densities in the center of the tumor were 324,50 cells/mm2 (sensitivity 77.8%% and specificity 67.2%)
- CD3+ T-cell densities in the invasive margin were 1093.70 cells/mm2 (sensitivity 93.5% % and specificity 63.9%)
- CD8+ T-cell densities in the invasive margin were 431.5 cells/mm2 (sensitivity 87.1% and specificity 65.9%).
3.2. Categorization of Patients According to Numbers of CD3+ and CD8+ Cells in the Center of the Tumor and Invasive Margin
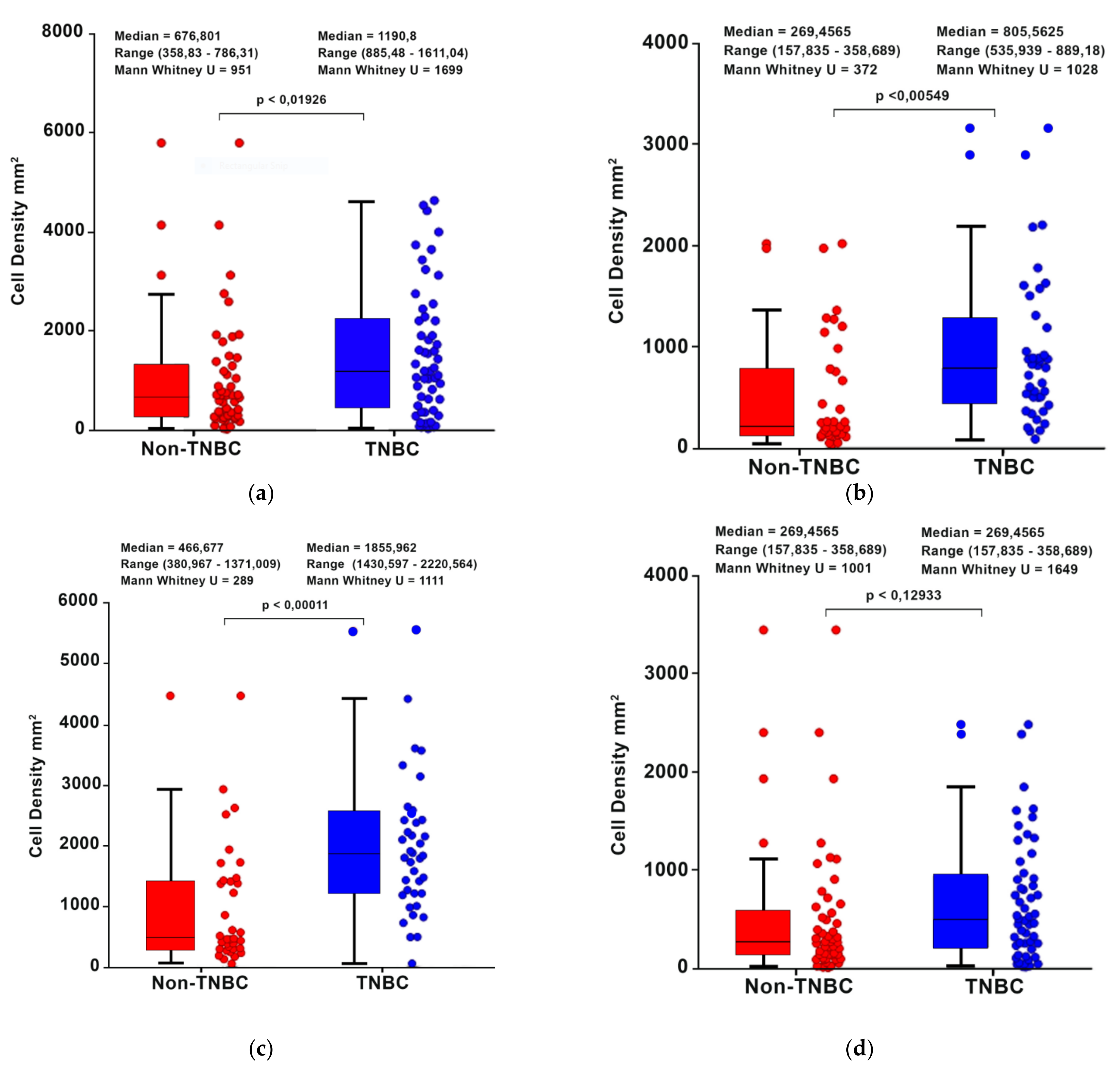
3.3. Comparison of T-Cell Density between TNBC vs. Non-TNBC Patients
3.4. Pathological Complete Response According to CD3+ and CD8+ Cell Counts in the Center of the Tumor and the Invasive Margin
3.5. Pathological Complete Response by Immunoscore® Clinical Research
3.6. Logistic Regression Analysis for Patient Characteristics Associated with a Pathological Complete Response
3.7. Positive Predictive Values and Negative Predictive Values for CD3+ IM, CD8+ IM, CD3+ CT, CD8+ CT, and ISCR for pCR
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- MacCarty, W.C.; Mahle, A.E. Relation of differentiation and lymphocytic infiltration to postoperative longevity in gastric carcinoma. J. Lab. Clin. Med. 1921, 6, 473–480. [Google Scholar]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (Impassion031): A randomised, double-blind, phase 3 trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Henschel, V.; Molinero, L.; Impassion130 Investigators; et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (Impassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 44–59. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; KEYNOTE-522 Investigators; et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 397–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cserni, G.; Chmielik, E.; Cserni, B.; Tot, T. The new TNM-based staging of breast cancer. Virchows Arch. 2018, 472, 697–703. [Google Scholar] [CrossRef]
- Galon, J.; Pagès, F.; Marincola, F.M.; Angell, H.K.; Thurin, M.; Lugli, A.; Zlobec, I.; Berger, A.; Bifulco, C.; Botti, G.; et al. Cancer classification using the Immunoscore: A worldwide task force. J. Transl. Med. 2012, 10, 205. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Fridman, W.H.; Galon, J. The prognostic impact of anti-cancer immune response: A novel classification of cancer patients. Semin. Immunopathol. 2011, 33, 335–340. [Google Scholar] [CrossRef] [Green Version]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Anitei, M.G.; Zeitoun, G.; Mlecnik, B.; Marliot, F.; Haicheur, N.; Todosi, A.M.; Kirilovsky, A.; Lagorce, C.; Bindea, G.; Ferariu, D.; et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin. Cancer Res. 2014, 20, 1891–1899. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, T.; Argilés, G.; Oki, E.; Martinelli, E.; Taniguchi, H.; Arnold, D.; Mishima, S.; Li, Y.; Smruti, B.K.; Ahn, J.B.; et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the diagnosis treatment and follow-up of patients with localised colon cancer. Ann. Oncol. 2021, 32, 1496–1510. [Google Scholar] [CrossRef]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef]
- Hermitte, F. Biomarkers immune monitoring technology primer: Immunoscore® Colon. J. Immunother. Cancer 2016, 4, 57. [Google Scholar] [CrossRef] [Green Version]
- Van Den Eynde, M.; Mlecnik, B.; Bindea, G.; Fredriksen, T.; Church, S.E.; Lafontain, L.; Haicheur, N.; Marliot, F.; Angelova, M.; Vasaturo, A.; et al. The link between the multiverse of immune microenvironments in metastases and the survival of colorectal cancer patients. Cancer Cell 2018, 34, 1012–1026. [Google Scholar] [CrossRef] [Green Version]
- Shankaran, V.; Ikeda, H.; Bruce, A.T.; White, J.M.; Swanson, P.E.; Old, L.J.; Schreiber, R.D. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001, 410, 1107–1111. [Google Scholar] [CrossRef]
- Pagès, F.; Berger, A.; Camus, M.; Sanchez-Cabo, F.; Costes, A.; Molidor, R.; Mlecnik, B.; Kirilovsky, A.; Nilsson, M.; Damotte, D.; et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N. Engl. J. Med. 2005, 353, 2654–2666. [Google Scholar] [CrossRef]
- Angell, H.; Galon, J. From the immune contexture to the Immunoscore: The role of prognostic and predictive immune markers in cancer. Curr. Opin. Immunol. 2013, 25, 261–267. [Google Scholar] [CrossRef]
- Koletsa, T.; Kotoula, V.; Koliou, G.A.; Manousou, K.; Chrisafi, S.; Zagouri, F.; Sotiropoulou, M.; Pentheroudakis, G.; Papoudou-Bai, A.; Christodoulou, C.; et al. Prognostic impact of stromal and intratumoral CD3, CD8 and FOXP3 in adjuvantly treated breast cancer: Do they add information over stromal tumor-infiltrating lymphocyte density? Cancer Immunol. Immunother. 2020, 69, 1549–1564. [Google Scholar] [CrossRef]
- Li, X.; Gruosso, T.; Zuo, D.; Omeroglu, A.; Meterissian, S.; Guiot, M.C.; Salazar, A.; Park, M.; Levine, H. Infiltration of CD8(+) T cells into tumor cell clusters in triple-negative breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 3678–3687. [Google Scholar] [CrossRef] [Green Version]
- Mi, H.; Gong, C.; Sulam, J.; Fertig, E.J.; Szalay, A.S.; Jaffee, E.M.; Stearns, V.; Emens, L.A.; Cimino-Mathews, A.M.; Popel, A.S. Digital pathology analysis quantifies spatial heterogeneity of CD3, CD4, CD8, CD20, and FoxP3 immune markers in triple-negative breast cancer. Front. Physiol. 2020, 11, 583333. [Google Scholar] [CrossRef] [PubMed]
- Miyan, M.; Schmidt-Mende, J.; Kiessling, R.; Poschke, I.; De Boniface, J. Differential tumor infiltration by T-cells characterizes intrinsic molecular subtypes in breast cancer. J. Transl. Med. 2016, 14, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egelston, C.A.; Avalos, C.; Tu, T.Y.; Rosario, A.; Wang, R.; Solomon, S.; Srinivasan, G.; Nelson, M.S.; Huang, Y.; Lim, M.H.; et al. Resident memory CD8+ T cells within cancer islands mediate survival in breast cancer patients. JCI Insight 2019, 4, e130000. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Li, C.; Cai, X.; Xie, Z.; Zhou, L.; Cheng, B.; Zhong, R.; Xiong, S.; Li, J.; Chen, Z.; et al. The association between CD8+ tumor-infiltrating lymphocytes and the clinical outcome of cancer immunotherapy: A systematic review and meta-analysis. eClinicalMedicine 2021, 41, 101134. [Google Scholar] [CrossRef] [PubMed]
- Fortis, S.P.; Sofopoulos, M.; Sotiriadou, N.N.; Haritos, C.; Vaxevanis, C.K.; Anastasopoulou, E.A.; Janssen, N.; Arnogiannaki, N.; Ardavanis, A.; Pawelec, G.; et al. Differential intratumoral distributions of CD8 and CD163 immune cells as prognostic biomarkers in breast cancer. J. Immunother. Cancer 2017, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Kos, Z.; Roblin, E.; Kim, R.S.; Michiels, S.; Gallas, B.D.; Chen, W.; Van De Vijver, K.K.; Goel, S.; Adams, S.; Demaria, S.; et al. Pitfalls in assessing stromal tumor infiltrating lymphocytes (sTILs) in breast cancer. NPJ Breast Cancer 2020, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van Den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. International TILs Working Group 2014. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef] [Green Version]
- Denkert, C.; Von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Stanton, S.E.; Adams, S.; Disis, M.L. Variation in the incidence and magnitude of tumour-infiltrating lymphocytes in breast cancer subtypes: A systematic review. JAMA Oncol. 2016, 2, 1354–1360. [Google Scholar] [CrossRef]
- Colbeck, E.J.; Ager, A.; Gallimore, A.; Jones, G.W. Tertiary lymphoid structures in cancer: Drivers of antitumour immunity, immunosuppression, or bystander sentinels in disease? Front. Immunol. 2017, 8, 1830. [Google Scholar] [CrossRef] [Green Version]
- Buisseret, L.; Garaud, S.; De Wind, A.; Van Den Eynden, G.; Boisson, A.; Solinas, C.; Gu-Trantien, C.; Naveaux, C.; Lodewyckx, J.N.; Duvillier, H.; et al. Tumour-infiltrating lymphocyte composition, organization and PD-1/ PD-L1 expression are linked in breast cancer. Oncoimmunology 2016, 6, e1257452. [Google Scholar] [CrossRef]
- Solinas, C.; Garaud, S.; De Silva, P.; Boisson, A.; Van Den Eynden, G.; De Wind, A.; Risso, P.; Rodrigues Vitória, J.; Richard, F.; Migliori, E.; et al. Immune checkpoint molecules on tumour-infiltrating lymphocytes and their association with tertiary lymphoid structures in human breast cancer. Front. Immunol. 2017, 8, 1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buisseret, L.; Desmedt, C.; Garaud, S.; Fornili, M.; Wang, X.; Van Den Eyden, G.; De Wind, A.; Duquenne, S.; Boisson, A.; Naveaux, C.; et al. Reliability of tumour-infiltrating lymphocyte and tertiary lymphoid structure assessment in human breast cancer. Mod. Pathol. 2017, 30, 1204–1212. [Google Scholar] [CrossRef]
- Kroeger, D.; Milne, K.; Nelson, B.H. Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic T-cell responses, and superior prognosis in ovarian cancer. Clin. Cancer Res. 2016, 22, 3005–3015. [Google Scholar] [CrossRef] [Green Version]
- Mlecnik, B.; Bifulco, C.; Bindea, G.; Marliot, F.; Lugli, A.; Lee, J.J.; Zlobec, I.; Rau, T.T.; Berger, M.D.; Nagtegaal, I.D.; et al. Multicenter international society for immunotherapy of cancer study of the consensus immunoscore for the prediction of survival and response to chemotherapy in stage III colon cancer. J. Clin. Oncol. 2020, 38, 3638–3651. [Google Scholar] [CrossRef]
- Pagès, F.; André, T.; Taieb, J.; Vernerey, D.; Henriques, J.; Borg, C.; Marliot, F.; Ben Jannet, R.; Louvet, C.; Mineur, L.; et al. Prognostic and predictive value of the Immunoscore in stage III colon cancer patients treated with oxaliplatin in the prospective IDEA France PRODIGE-GERCOR cohort study. Ann. Oncol. 2020, 31, 921–929. [Google Scholar] [CrossRef]
- El Sissy, C.; Kirilovsky, A.; Van Den Eynde, M.; Muşină, A.M.; Anitei, M.G.; Romero, A.; Marliot, F.; Junca, A.; Doyen, J.; Mlecnik, B.; et al. A diagnostic biopsy-adapted Immunoscore predicts response to neoadjuvant treatment and selects patients with rectal cancer eligible for a watch-and-wait strategy. Clin. Cancer Res. 2020, 26, 5198–5207. [Google Scholar] [CrossRef]


| Total Number of Patients n = 103 | ||
|---|---|---|
| Age Median Age (Range/Min–Max) 52 Years (26–84) | ||
| Total | % | |
| Histology | ||
| Ductal | 99 | 96% |
| Lobular | 2 | 2% |
| Other | 2 | 2% |
| Menopausal Status | ||
| Pre-menopausal | 41 | 40% |
| Post-menopausal | 62 | 60% |
| Tumor Size | ||
| T1 | 23 | 22% |
| T2 | 65 | 63% |
| T3 + T4 | 15 | 15% |
| Nodal Status | ||
| Negative | 45 | 44% |
| Positive | 54 | 52% |
| Unknown | 4 | 4% |
| Stage | ||
| IA | 8 | 7% |
| IB | 49 | 48% |
| IC | 10 | 10% |
| IIA | 1 | 1% |
| IIB | 26 | 25% |
| IIC | 7 | 7% |
| IIIC | 2 | 2% |
| Ki-67 Pre-treatment | ||
| Ki-67 Median (Range) | 40% (5–90%) | |
| ≥40% | 51 | 50% |
| 15–39% | 37 | 35% |
| <15% | 13 | 13% |
| Unknown | 2 | 2% |
| Molecular subtype | ||
| Luminal A | 9 | 9% |
| Luminal B | 23 | 22% |
| HER-2 Positive | 18 | 18% |
| CD3+ and CD8+ Count | Median Cells/mm2 | Range (Min Max) Cells/mm2 |
|---|---|---|
| CD3+ center of tumor | 884 | 25–5771 |
| CD3+ invasive margin * | 1409 | 53–6197 |
| CD8+ center of tumor | 358 | 10–3448 |
| CD8+ invasive margin * | 535 | 38–3117 |
| Cell Density CD3+ Center of Tumor | Total | Percentages |
| CD3+ CT ≥ 1186.81 cells/mm2 | 40 | 39% |
| CD3+ CT < 1186.80 cells/mm2 | 63 | 61% |
| Cell density CD3+ Invasive Margin * | ||
| CD3+ IM ≥ 1093.71 cells/mm2 | 46 | 61% |
| CD3+ IM < 1093.70 cells/mm2 | 29 | 39% |
| Cell density CD8+ Center of Tumor | ||
| CD8+ ≥ 324.51 cells/mm2 | 55 | 53% |
| CD8+ < 324.50 cells/mm2 | 48 | 47% |
| Cell density CD8+ Invasive Margin * | ||
| CD8+ IM ≥ 431.51 cells/mm2 | 41 | 55% |
| CD8+ IM < 431.50 cells/mm2 | 34 | 45% |
| Immunoscore CR * | ||
| 0 | 27 | 36% |
| 1 | 4 | 5% |
| 2 | 8 | 11% |
| 3 | 13 | 17% |
| 4 | 23 | 31% |
| Variable | pCR | Non-pCR | Fisher’s Exact |
|---|---|---|---|
| Tumor Size | |||
| T1 | 10 (43%) | 13 (57%) | 0.020 |
| T2 | 34 (52%) | 31 (48%) | |
| T3 & T4 | 1 (0.1%) | 14 (99.9%) | |
| Nodal Status | |||
| Positive | 19 (35%) | 35 (65%) | 0.024 |
| Negative | 24 (53%) | 21 (47%) | |
| Stage | |||
| Stage I | 6 (67%) | 3 (33%) | 0.020 |
| Stage IIA | 25 (51%) | 24 (49%) | |
| Stage IIB | 11 (42%) | 15 (58%) | |
| Stage III | 3 (16%) | 16 (84%) | |
| ER | |||
| Positive | 8 (18%) | 37 (82%) | ≤0.0001 |
| Negative | 37 (64%) | 21 (36%) | |
| PR | |||
| Positive | 5 (13%) | 33 (86% | |
| Negative | 40 (61%) | 25 (38%) | ≤0.0001 |
| HER-2 | |||
| Positive | 9 (50%) | 9 (50%) | 0.6070 |
| Negative | 36 (42%) | 49 (58%) | |
| Biological type | |||
| Luminal | 3 (9%) | 29 (91%) | ≤0.0001 |
| HER-2 Positive | 9 (50%) | 9 (50%) | |
| TNBC | 33 (62%) | 20 (38%) | |
| Ki-67 | |||
| ≥40% | 29 (57%) | 22 (43%) | 0.0001 |
| 15–39% | 15 (41%) | 22 (59%) | |
| <15% | 0 (0%) | 13 (100%) | |
| CD3+ Cell density at Center of Tumor | |||
| CD3+ CT ≥ 1186.81 mm2 | 27 (68%) | 13 (33%) | |
| CD3+ CT < 1186.80 mm2 | 18 (29%) | 45 (71%) | 0.0002 |
| CD3+ Cell density—Invasive Margin * | |||
| CD3+ IM ≥ 1093.71 mm2 | 29 (63%) | 17 (37%) | |
| CD3+ IM < 1093.70 mm2 | 2 (7%) | 27 (93%) | ≤0.0001 |
| CD8+ Cell Density at Center of Tumor | |||
| CD8+ CT ≥ 324,51 mm2 | 35 (64%) | 20 (36%) | |
| CD8+ CT < 324,50 mm2 | 10 (21%) | 38 (79%) | ≤0.0001 |
| CD8+ Cell density—Invasive Margin * | |||
| CD8+ IM ≥ 431.51 mm2 | 26 (63%) | 15 (37%) | |
| CD8+ IM < 431.50 mm2 | 5 (15%) | 29 (85%) | ≤0.0001 |
| Immunoscore CR | |||
| High | 25 (69%) | 11 (31%) | ≤0.0001 |
| Intermediate | 4 (50%) | 4 (50%) | |
| Low | 2 (6%) | 29 (94%) | |
| Variable | Wald p-Value | Odds Ratio | 95% CI |
|---|---|---|---|
| Tumor Size | |||
| T1 0.0360 | 1.98 | 0.04 to 1.32 | |
| T2 0.1360 | 0.51 | −1.56 to 0.21 | |
| T3 & T4 | 0.0160 | 5.49 | 0.31 to 3.09 |
| Nodal Status | 0.2460 | 1.59 | 1.06 to 2.40 |
| Stage | |||
| Stage IIA | 0.2905 | 0.70 | −1.01 to 0.30 |
| Stage IIB | 0.9951 | 0.99 | −0.76 to 0.76 |
| Stage III | 0.0087 | 3.92 | 0.35 to 2.38 |
| ER Status | <0.0001 | 1.98 | 0.50 to 0.86 |
| PR Status | <0.0001 | 1.89 | 0.46 to 0.81 |
| HER-2-Status | <0.0001 | 0.60 | −0.51 to−0.51 |
| Biological type | |||
| Luminal—HER-2+ | 0.0001 | 3.33 | 0.62 to 1.78 |
| TNBC—HER-2+ | 0.0052 | 0.47 | −1.28 to −0.23 |
| Ki-67 (continuous) | 0.0014 | 0.97 | −0.05 to −0.01 |
| ISCR * (0 vs. 1 vs. 2 vs. 3 vs. 4) | 0.0001 | 0.42 | −1.26 to −0.49 |
| ISCR * (0 & 1 & 2 vs. 3 & 4) | <0.0001 | 12.50 | 1.40 to 3.65 |
| IS (0–1 vs. 2–3–4) (n = 75) | CD3 CT (n = 75) | CD3 IM (n = 75) | CD8 CT (n = 75) | CD8 IM (n = 75) | |
|---|---|---|---|---|---|
| pCR | 41.3 | 41.3 | 41.3 | 41.3 | 41.3 |
| PPV | 60.4% | 62.2% | 63.2% | 66.7% | 63.4% |
| 95% CI | (45.3–74.2) | (44.8–77.5) | (46.0–78.2) | (49.0–81.4) | (46.9–77.9) |
| NPV | 92.6% | 79.0% | 81.1% | 80.0% | 85.3% |
| 95% CI | (75.7–99.1) | (62.7–90.4) | (64.8–92.0) | (64.3–91.0) | (68.9–95.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapoport, B.L.; Nayler, S.; Mlecnik, B.; Smit, T.; Heyman, L.; Bouquet, I.; Martel, M.; Galon, J.; Benn, C.-A.; Anderson, R. Tumor-Infiltrating Lymphocytes (TILs) in Early Breast Cancer Patients: High CD3+, CD8+, and Immunoscore Are Associated with a Pathological Complete Response. Cancers 2022, 14, 2525. https://doi.org/10.3390/cancers14102525
Rapoport BL, Nayler S, Mlecnik B, Smit T, Heyman L, Bouquet I, Martel M, Galon J, Benn C-A, Anderson R. Tumor-Infiltrating Lymphocytes (TILs) in Early Breast Cancer Patients: High CD3+, CD8+, and Immunoscore Are Associated with a Pathological Complete Response. Cancers. 2022; 14(10):2525. https://doi.org/10.3390/cancers14102525
Chicago/Turabian StyleRapoport, Bernardo Leon, Simon Nayler, Bernhard Mlecnik, Teresa Smit, Liezl Heyman, Isabelle Bouquet, Marine Martel, Jérôme Galon, Carol-Ann Benn, and Ronald Anderson. 2022. "Tumor-Infiltrating Lymphocytes (TILs) in Early Breast Cancer Patients: High CD3+, CD8+, and Immunoscore Are Associated with a Pathological Complete Response" Cancers 14, no. 10: 2525. https://doi.org/10.3390/cancers14102525
APA StyleRapoport, B. L., Nayler, S., Mlecnik, B., Smit, T., Heyman, L., Bouquet, I., Martel, M., Galon, J., Benn, C.-A., & Anderson, R. (2022). Tumor-Infiltrating Lymphocytes (TILs) in Early Breast Cancer Patients: High CD3+, CD8+, and Immunoscore Are Associated with a Pathological Complete Response. Cancers, 14(10), 2525. https://doi.org/10.3390/cancers14102525






