Diagnostic and Prognostic Value of pH- and Oxygen-Sensitive Magnetic Resonance Imaging in Glioma: A Retrospective Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
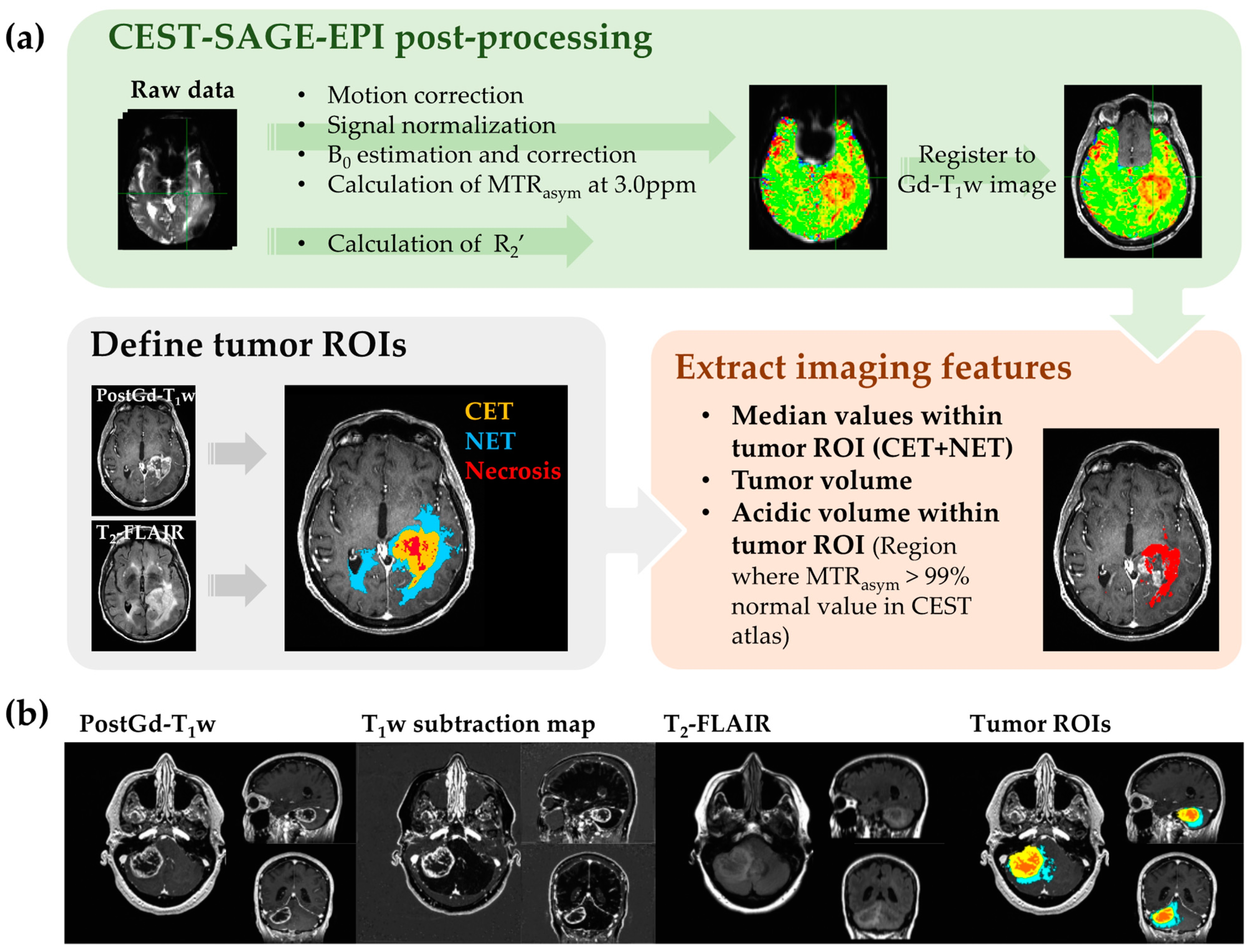
2.2. MRI Acquisition and Post-Processing
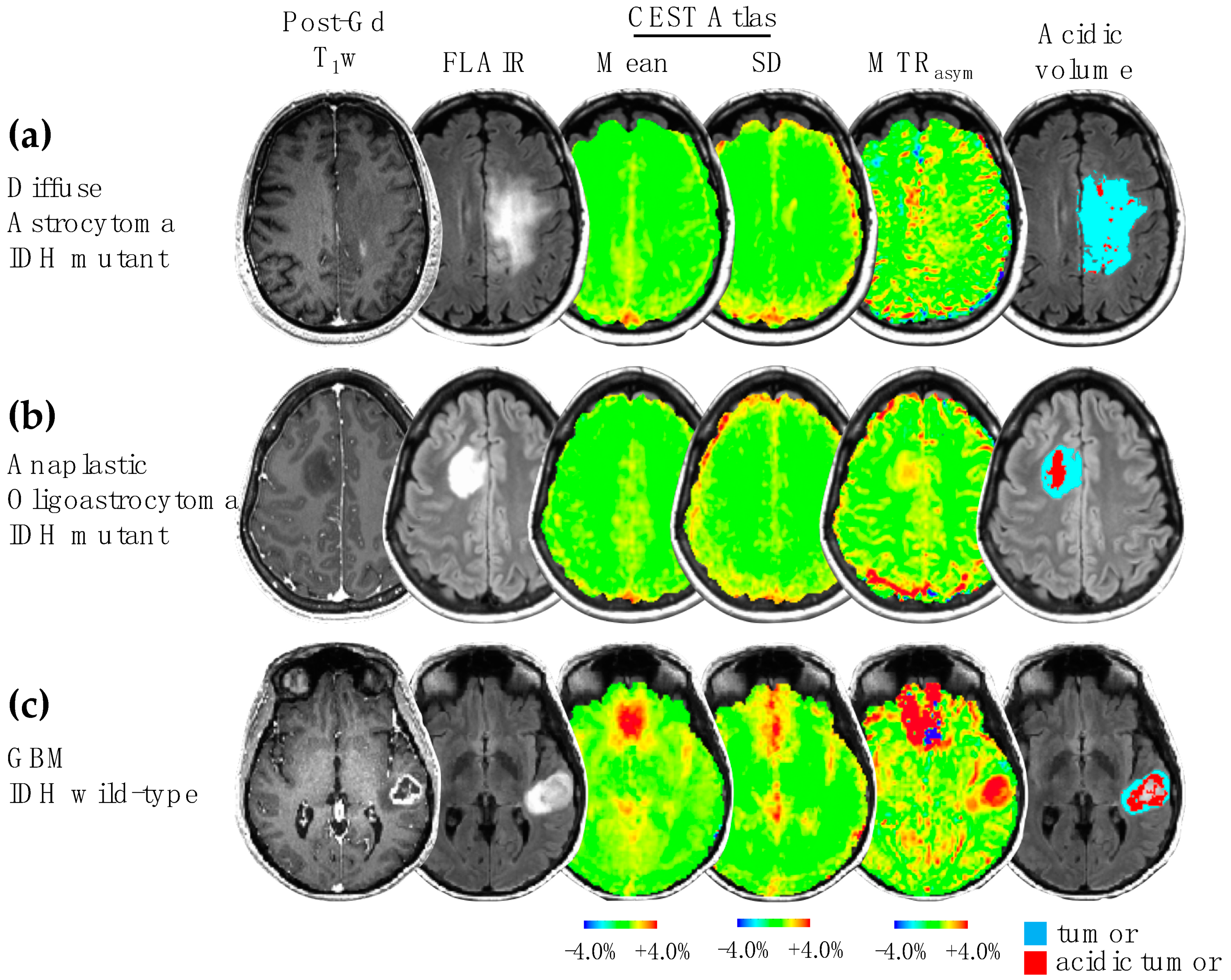
2.3. MRI Features Extraction
2.4. Data Analysis and Statistics
3. Results
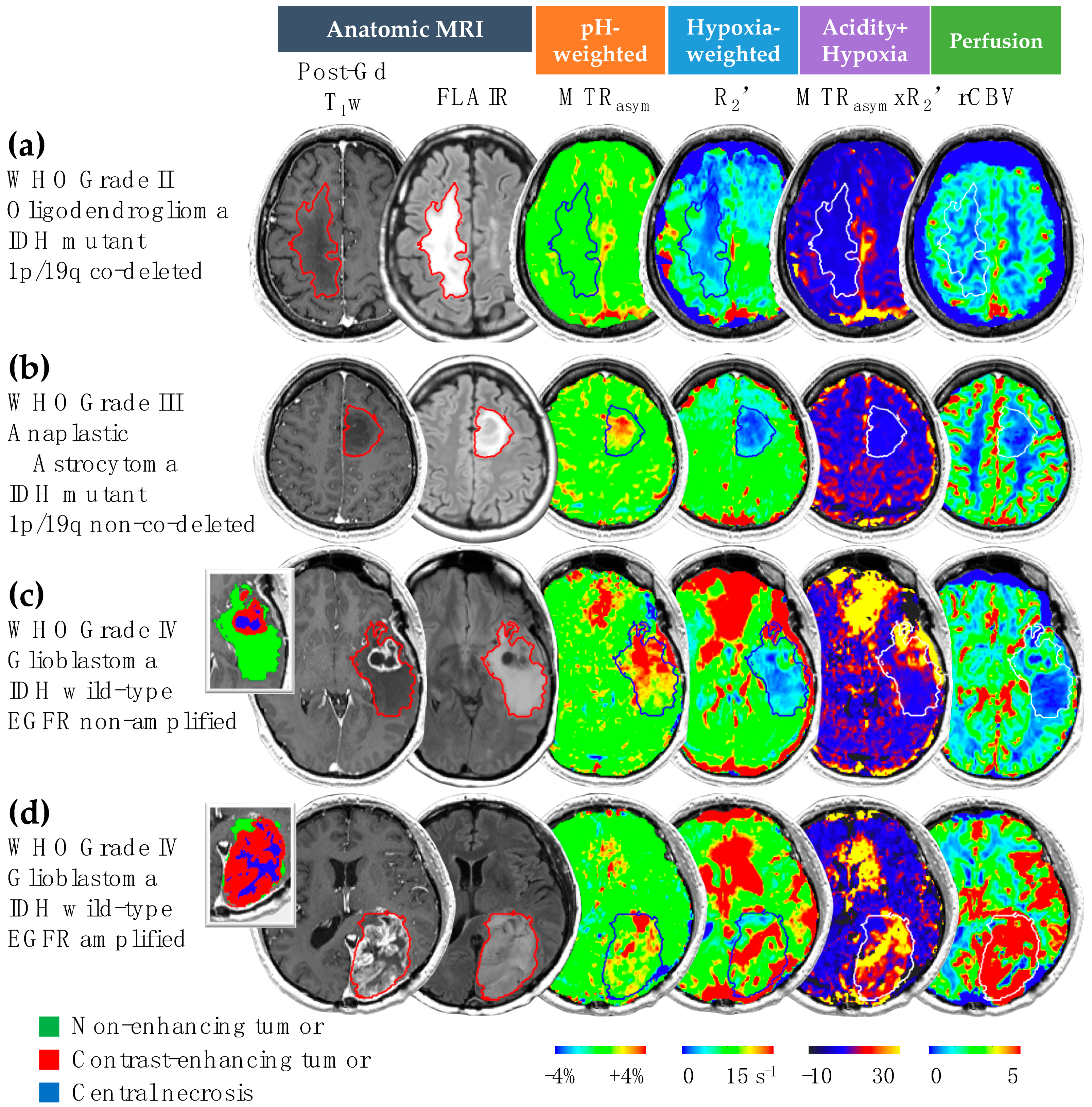
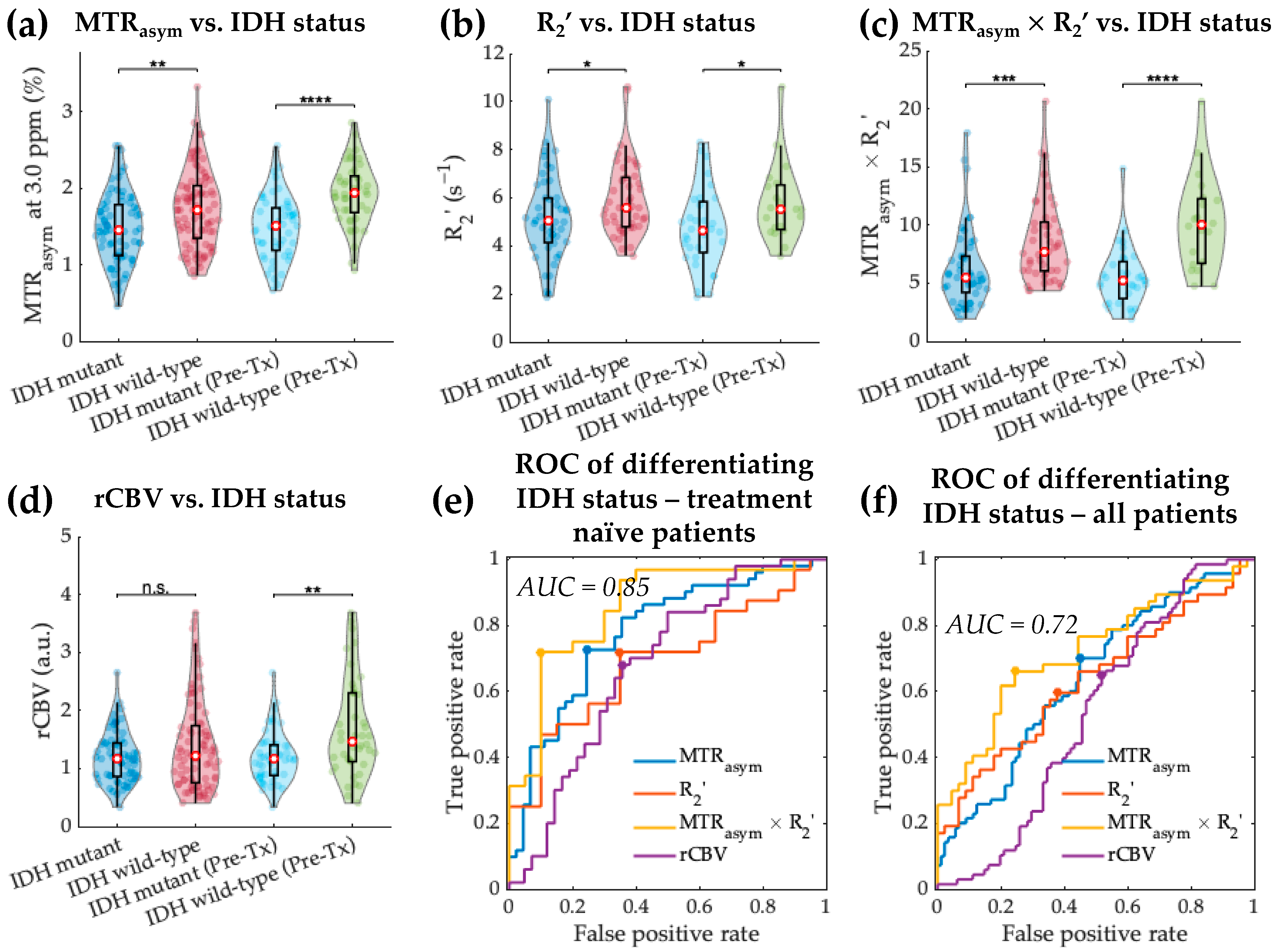
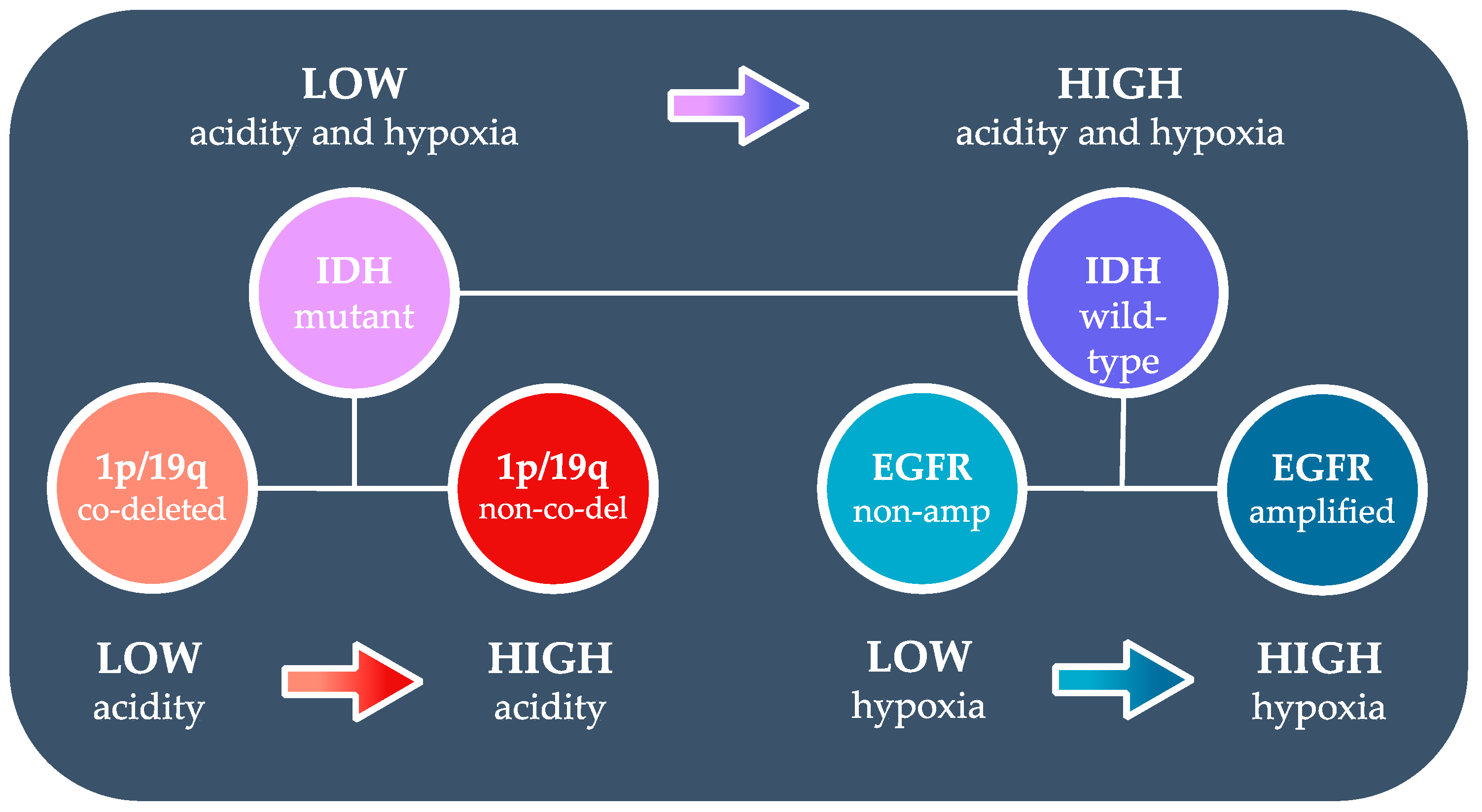
3.1. Acidity and Hypoxia in IDH Mutant and Wild-Type Gliomas
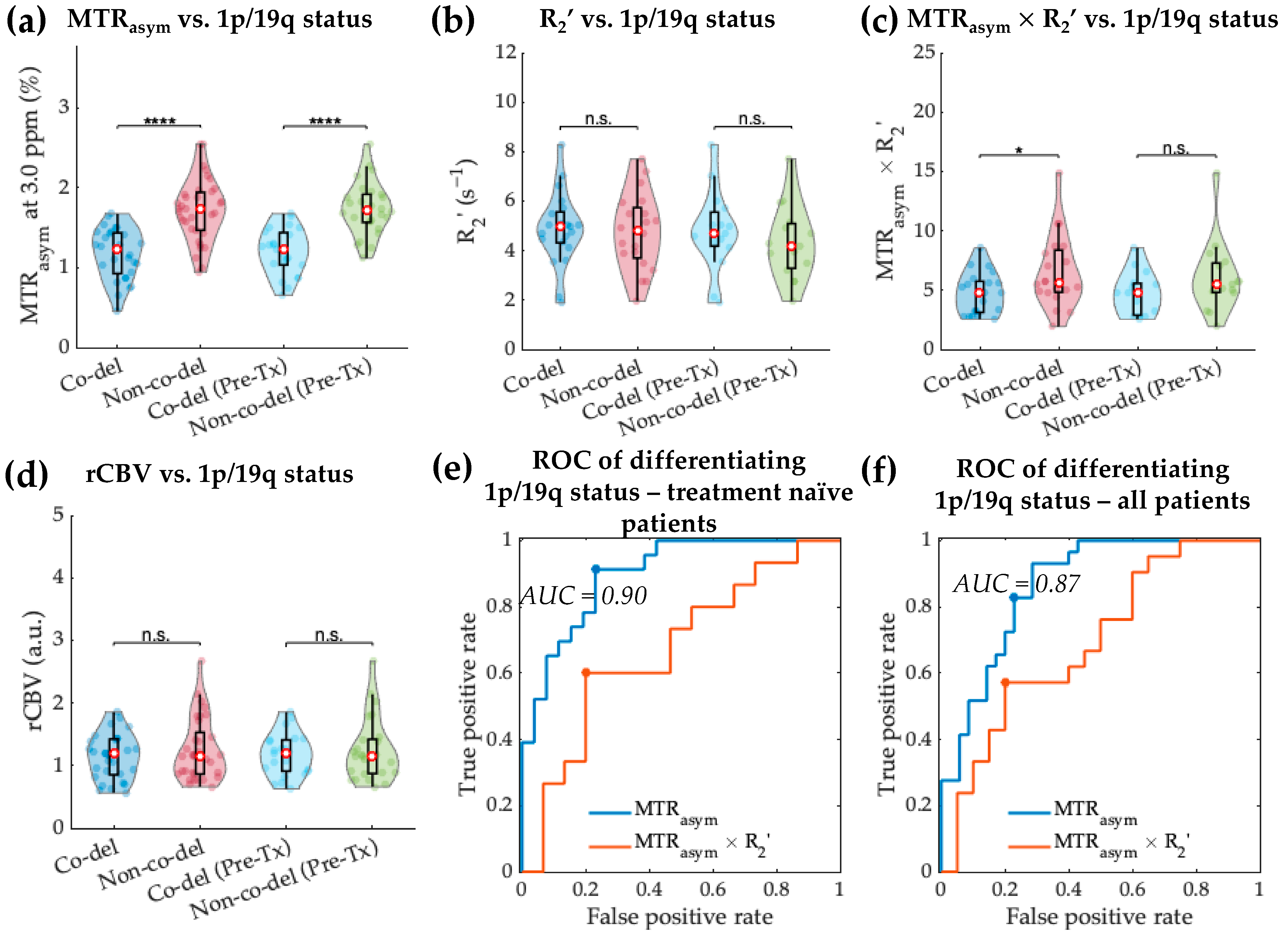
3.2. Acidity and Hypoxia in 1p/19q Co-Deleted and Non-Co-Deleted IDH Mutant Gliomas
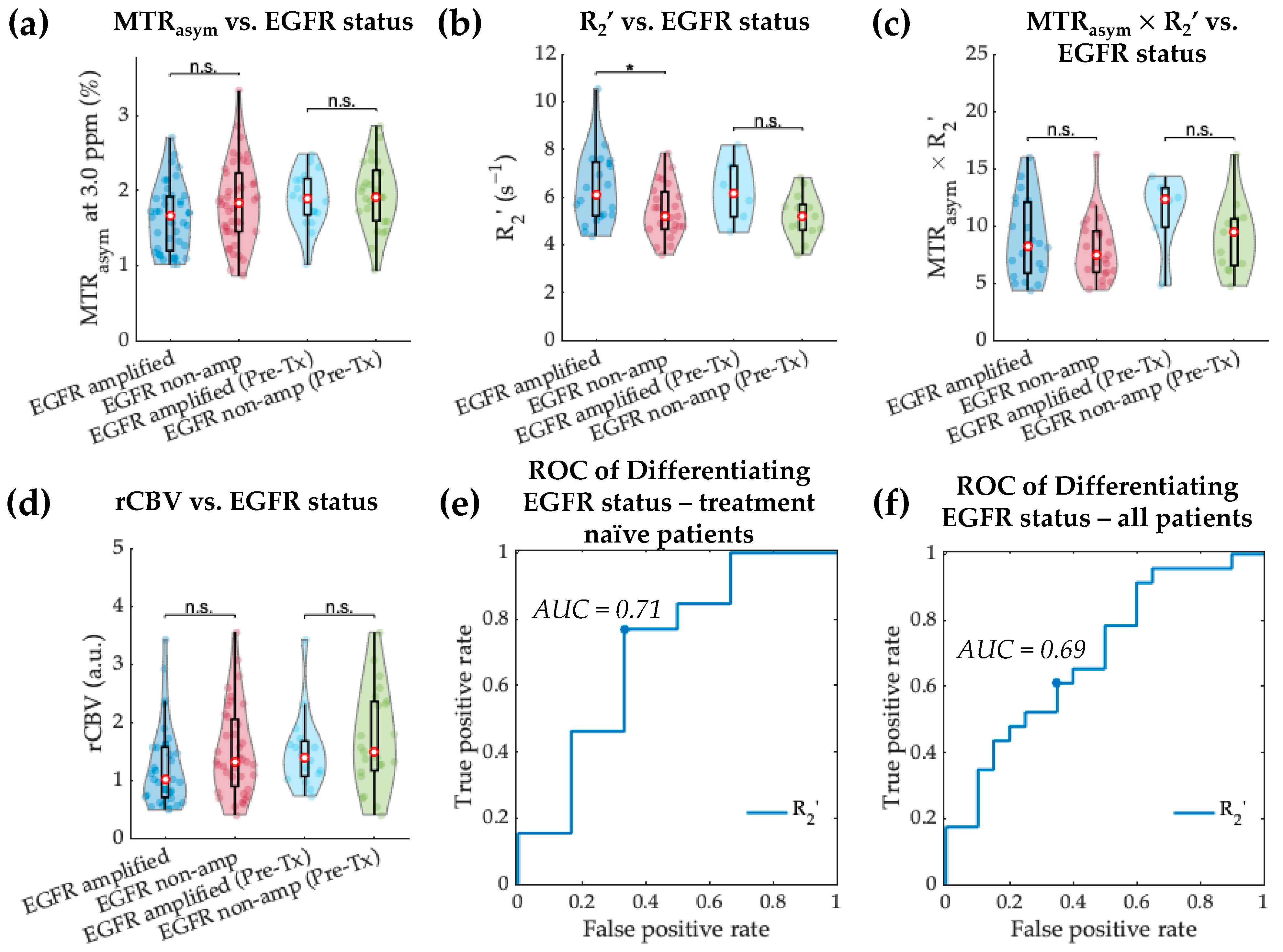
3.3. Acidity and Hypoxia in EGFR-Amplified and Non-Amplified IDH Wild-Type Gliomas
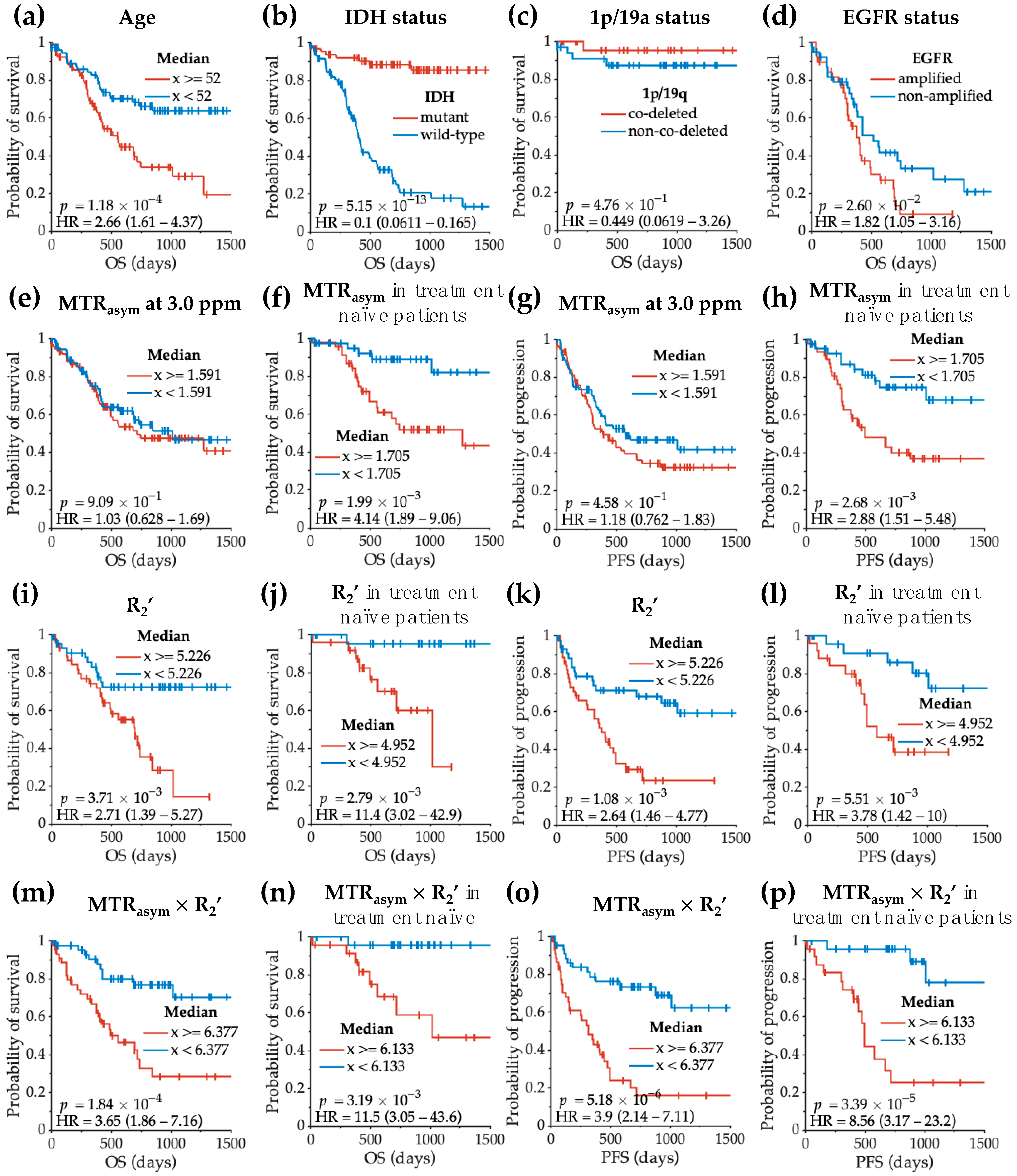
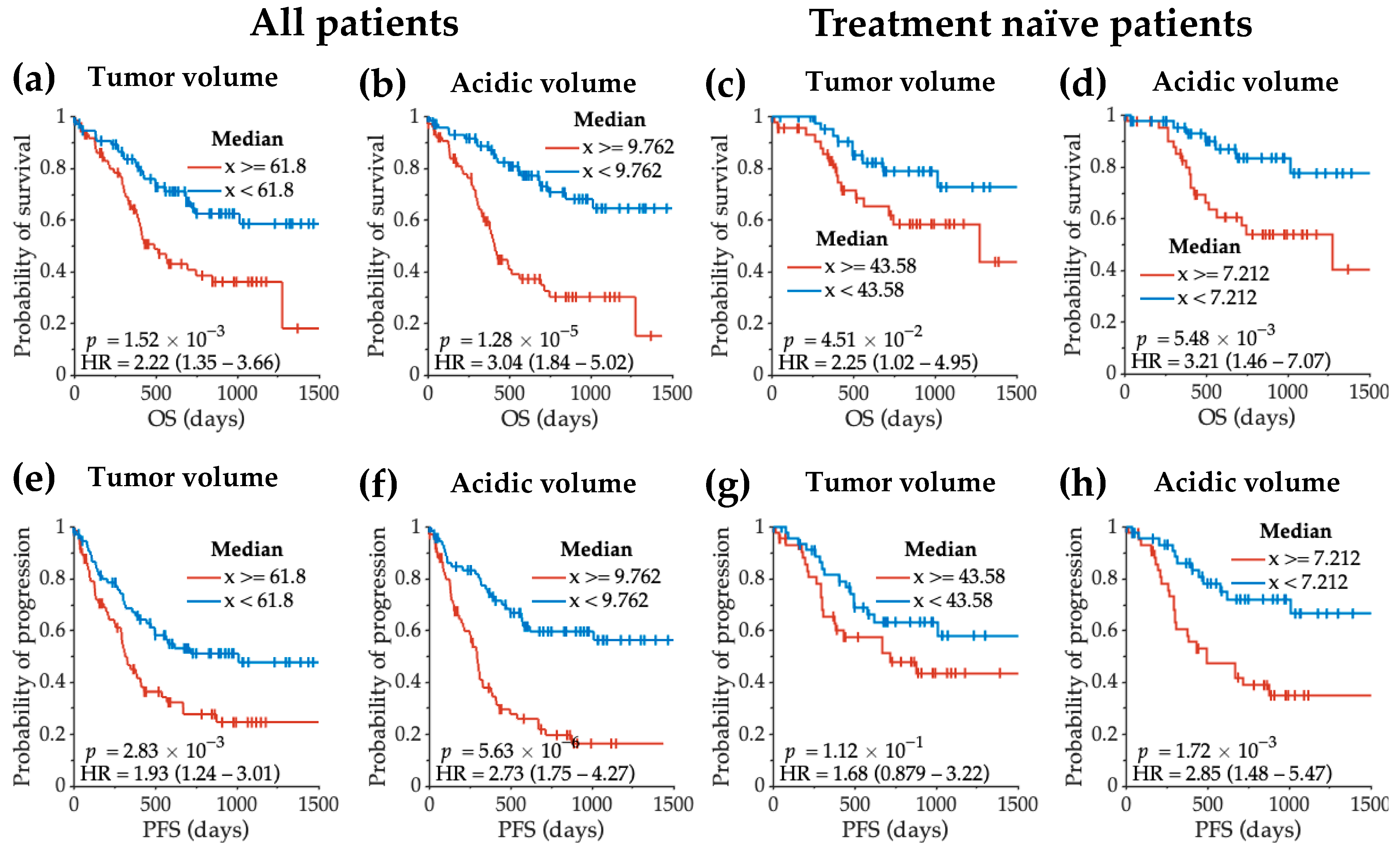
3.4. Acidity and Hypoxia Imaging Features Correlating with Glioma Patient Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venneti, S.; Thompson, C.B. Metabolic Reprogramming in Brain Tumors. Annu. Rev. Pathol. 2017, 12, 515–545. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Khwaja, F.W.; Severson, E.A.; Matheny, S.L.; Brat, D.J.; Van Meir, E.G. Hypoxia and the Hypoxia-Inducible-Factor Pathway in Glioma Growth and Angiogenesis. Neuro Oncol. 2005, 7, 134–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honasoge, A.; Sontheimer, H. Involvement of Tumor Acidification in Brain Cancer Pathophysiology. Front. Physiol. 2013, 4, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbet, C.; Feron, O. Tumour Acidosis: From the Passenger to the Driver’s Seat. Nat. Rev. Cancer 2017, 17, 577–593. [Google Scholar] [CrossRef]
- Colwell, N.; Larion, M.; Giles, A.J.; Seldomridge, A.N.; Sizdahkhani, S.; Gilbert, M.R.; Park, D.M. Hypoxia in the Glioblastoma Microenvironment: Shaping the Phenotype of Cancer Stem-like Cells. Neuro Oncol. 2017, 19, 887–896. [Google Scholar] [CrossRef]
- Monteiro, A.R.; Hill, R.; Pilkington, G.J.; Madureira, P.A. The Role of Hypoxia in Glioblastoma Invasion. Cells 2017, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Harris, R.J.; Yao, J.; Chakhoyan, A.; Raymond, C.; Leu, K.; Liau, L.M.; Nghiemphu, P.L.; Lai, A.; Salamon, N.; Pope, W.B.; et al. Simultaneous PH-Sensitive and Oxygen-Sensitive MRI of Human Gliomas at 3 T Using Multi-Echo Amine Proton Chemical Exchange Saturation Transfer Spin-and-Gradient Echo Echo-Planar Imaging (CEST-SAGE-EPI). Magn. Reson. Med. 2018, 80, 1962–1978. [Google Scholar] [CrossRef]
- Tóth, V.; Förschler, A.; Hirsch, N.M.; den Hollander, J.; Kooijman, H.; Gempt, J.; Ringel, F.; Schlegel, J.; Zimmer, C.; Preibisch, C. MR-Based Hypoxia Measures in Human Glioma. J. Neurooncol. 2013, 115, 197–207. [Google Scholar] [CrossRef]
- Yao, J.; Chakhoyan, A.; Nathanson, D.A.; Yong, W.H.; Salamon, N.; Raymond, C.; Mareninov, S.; Lai, A.; Nghiemphu, P.L.; Prins, R.M.; et al. Metabolic Characterization of Human IDH Mutant and Wild Type Gliomas Using Simultaneous PH- and Oxygen-Sensitive Molecular MRI. Neuro Oncol. 2019, 21, 1184–1196. [Google Scholar] [CrossRef]
- Yao, J.; Tan, C.H.P.; Schlossman, J.; Chakhoyan, A.; Raymond, C.; Pope, W.B.; Salamon, N.; Lai, A.; Ji, M.; Nghiemphu, P.L. PH-Weighted Amine Chemical Exchange Saturation Transfer Echoplanar Imaging (CEST-EPI) as a Potential Early Biomarker for Bevacizumab Failure in Recurrent Glioblastoma. J. Neuro-Oncol. 2019, 142, 587–595. [Google Scholar] [CrossRef]
- Leather, T.; Jenkinson, M.D.; Das, K.; Poptani, H. Magnetic Resonance Spectroscopy for Detection of 2-Hydroxyglutarate as a Biomarker for IDH Mutation in Gliomas. Metabolites 2017, 7, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Choi, S.H.; Ryoo, I.; Yoon, T.J.; Kim, T.M.; Lee, S.-H.; Park, C.-K.; Kim, J.-H.; Sohn, C.-H.; Park, S.-H.; et al. Evaluation of the Microenvironmental Heterogeneity in High-Grade Gliomas with IDH1/2 Gene Mutation Using Histogram Analysis of Diffusion-Weighted Imaging and Dynamic-Susceptibility Contrast Perfusion Imaging. J. Neurooncol. 2015, 121, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Kickingereder, P.; Sahm, F.; Radbruch, A.; Wick, W.; Heiland, S.; von Deimling, A.; Bendszus, M.; Wiestler, B. IDH Mutation Status Is Associated with a Distinct Hypoxia/Angiogenesis Transcriptome Signature Which Is Non-Invasively Predictable with RCBV Imaging in Human Glioma. Sci. Rep. 2015, 5, 16238. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, G.S.; Gocke, T.A.; Chawla, S.; Whitmore, R.G.; Nabavizadeh, A.; Krejza, J.; Lopinto, J.; Plaum, J.; Maloney-Wilensky, E.; Poptani, H.; et al. Magnetic Resonance Perfusion-Weighted Imaging Defines Angiogenic Subtypes of Oligodendroglioma According to 1p19q and EGFR Status. J. Neurooncol. 2009, 92, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Young, R.J.; Shah, A.D.; Schweitzer, A.D.; Graber, J.J.; Shi, W.; Zhang, Z.; Huse, J.; Omuro, A.M.P. Pretreatment Dynamic Susceptibility Contrast MRI Perfusion in Glioblastoma: Prediction of EGFR Gene Amplification. Clin. Neuroradiol. 2015, 25, 143–150. [Google Scholar] [CrossRef]
- Park, Y.W.; Ahn, S.S.; Park, C.J.; Han, K.; Kim, E.H.; Kang, S.-G.; Chang, J.H.; Kim, S.H.; Lee, S.-K. Diffusion and Perfusion MRI May Predict EGFR Amplification and the TERT Promoter Mutation Status of IDH-Wildtype Lower-Grade Gliomas. Eur. Radiol. 2020, 30, 6475–6484. [Google Scholar] [CrossRef]
- Jiang, S.; Zou, T.; Eberhart, C.G.; Villalobos, M.A.V.; Heo, H.-Y.; Zhang, Y.; Wang, Y.; Wang, X.; Yu, H.; Du, Y.; et al. Predicting IDH Mutation Status in Grade II Gliomas Using Amide Proton Transfer-Weighted (APTw) MRI. Magn. Reson. Med. 2017, 78, 1100–1109. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Bendszus, M.; Boxerman, J.; Barboriak, D.; Erickson, B.J.; Smits, M.; Nelson, S.J.; Gerstner, E.; Alexander, B.; Goldmacher, G.; et al. Consensus Recommendations for a Standardized Brain Tumor Imaging Protocol in Clinical Trials. Neuro Oncol. 2015, 17, 1188–1198. [Google Scholar] [CrossRef] [Green Version]
- Harris, R.J.; Cloughesy, T.F.; Liau, L.M.; Nghiemphu, P.L.; Lai, A.; Pope, W.B.; Ellingson, B.M. Simulation, Phantom Validation, and Clinical Evaluation of Fast PH-Weighted Molecular Imaging Using Amine Chemical Exchange Saturation Transfer Echo Planar Imaging (CEST-EPI) in Glioma at 3 T. NMR Biomed. 2016, 29, 1563–1576. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Kim, H.J.; Woodworth, D.C.; Pope, W.B.; Cloughesy, J.N.; Harris, R.J.; Lai, A.; Nghiemphu, P.L.; Cloughesy, T.F. Recurrent Glioblastoma Treated with Bevacizumab: Contrast-Enhanced T1-Weighted Subtraction Maps Improve Tumor Delineation and Aid Prediction of Survival in a Multicenter Clinical Trial. Radiology 2014, 271, 200–210. [Google Scholar] [CrossRef]
- Oughourlian, T.C.; Yao, J.; Hagiwara, A.; Nathanson, D.A.; Raymond, C.; Pope, W.B.; Salamon, N.; Lai, A.; Ji, M.; Nghiemphu, P.L.; et al. Relative Oxygen Extraction Fraction (ROEF) MR Imaging Reveals Higher Hypoxia in Human Epidermal Growth Factor Receptor (EGFR) Amplified Compared with Non-Amplified Gliomas. Neuroradiology 2021, 63, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Hagiwara, A.; Raymond, C.; Shabani, S.; Pope, W.B.; Salamon, N.; Lai, A.; Ji, M.; Nghiemphu, P.L.; Liau, L.M.; et al. Human IDH Mutant 1p/19q Co-Deleted Gliomas Have Low Tumor Acidity as Evidenced by Molecular MRI and PET: A Retrospective Study. Sci. Rep. 2020, 10, 11922. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Wesseling, P. Histologic Classification of Gliomas. Handb. Clin. Neurol. 2016, 134, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.; Kornblum, H.I. Molecular Markers in Glioma. J. Neurooncol. 2017, 134, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.J.; Fuller, G.N.; Abi-Said, D.; Lang, F.F.; Gokaslan, Z.L.; Shi, W.M.; Wildrick, D.M.; Sawaya, R. Limitations of Stereotactic Biopsy in the Initial Management of Gliomas. Neuro Oncol. 2001, 3, 193–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutt-Will, M.; Murek, M.; Schwarz, C.; Hewer, E.; Vulcu, S.; Beck, J.; Raabe, A.; Schucht, P. Frequent Diagnostic Under-Grading in Isocitrate Dehydrogenase Wild-Type Gliomas Due to Small Pathological Tissue Samples. Neurosurgery 2019, 85, 689–694. [Google Scholar] [CrossRef]
- Choi, Y.S.; Bae, S.; Chang, J.H.; Kang, S.-G.; Kim, S.H.; Kim, J.; Rim, T.H.; Choi, S.H.; Jain, R.; Lee, S.-K. Fully Automated Hybrid Approach to Predict the IDH Mutation Status of Gliomas via Deep Learning and Radiomics. Neuro Oncol. 2021, 23, 304–313. [Google Scholar] [CrossRef]
- Bhandari, A.P.; Liong, R.; Koppen, J.; Murthy, S.V.; Lasocki, A. Noninvasive Determination of IDH and 1p19q Status of Lower-Grade Gliomas Using MRI Radiomics: A Systematic Review. Am. J. Neuroradiol. 2021, 42, 94–101. [Google Scholar] [CrossRef]
- Patel, S.H.; Poisson, L.M.; Brat, D.J.; Zhou, Y.; Cooper, L.; Snuderl, M.; Thomas, C.; Franceschi, A.M.; Griffith, B.; Flanders, A.E.; et al. T2-FLAIR Mismatch, an Imaging Biomarker for IDH and 1p/19q Status in Lower-Grade Gliomas: A TCGA/TCIA Project. Clin. Cancer Res. 2017, 23, 6078–6085. [Google Scholar] [CrossRef] [Green Version]
- Stockhammer, F.; Thomale, U.-W.; Plotkin, M.; Hartmann, C.; Von Deimling, A. Association between Fluorine-18-Labeled Fluorodeoxyglucose Uptake and 1p and 19q Loss of Heterozygosity in World Health Organization Grade II Gliomas. J. Neurosurg. 2007, 106, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-F.; Hsu, F.-T.; Hsieh, K.L.-C.; Kao, Y.-C.J.; Cheng, S.-J.; Hsu, J.B.-K.; Tsai, P.-H.; Chen, R.-J.; Huang, C.-C.; Yen, Y.; et al. Machine Learning-Based Radiomics for Molecular Subtyping of Gliomas. Clin. Cancer Res. 2018, 24, 4429–4436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, R.J.; Gupta, A.; Shah, A.D.; Graber, J.J.; Schweitzer, A.D.; Prager, A.; Shi, W.; Zhang, Z.; Huse, J.; Omuro, A.M.P. Potential Role of Preoperative Conventional MRI Including Diffusion Measurements in Assessing Epidermal Growth Factor Receptor Gene Amplification Status in Patients with Glioblastoma. AJNR Am. J. Neuroradiol. 2013, 34, 2271–2277. [Google Scholar] [CrossRef]
- Aghi, M.; Gaviani, P.; Henson, J.W.; Batchelor, T.T.; Louis, D.N.; Barker, F.G. Magnetic Resonance Imaging Characteristics Predict Epidermal Growth Factor Receptor Amplification Status in Glioblastoma. Clin. Cancer Res. 2005, 11, 8600–8605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chesnelong, C.; Chaumeil, M.M.; Blough, M.D.; Al-Najjar, M.; Stechishin, O.D.; Chan, J.A.; Pieper, R.O.; Ronen, S.M.; Weiss, S.; Luchman, H.A.; et al. Lactate Dehydrogenase A Silencing in IDH Mutant Gliomas. Neuro Oncol. 2014, 16, 686–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khurshed, M.; Molenaar, R.J.; Lenting, K.; Leenders, W.P.; van Noorden, C.J.F. In Silico Gene Expression Analysis Reveals Glycolysis and Acetate Anaplerosis in IDH1 Wild-Type Glioma and Lactate and Glutamate Anaplerosis in IDH1-Mutated Glioma. Oncotarget 2017, 8, 49165–49177. [Google Scholar] [CrossRef] [Green Version]
- Blough, M.D.; Al-Najjar, M.; Chesnelong, C.; Binding, C.E.; Rogers, A.D.; Luchman, H.A.; Kelly, J.J.; Fliegel, L.; Morozova, O.; Yip, S.; et al. DNA Hypermethylation and 1p Loss Silence NHE-1 in Oligodendroglioma. Ann. Neurol. 2012, 71, 845–849. [Google Scholar] [CrossRef]
- Franovic, A.; Gunaratnam, L.; Smith, K.; Robert, I.; Patten, D.; Lee, S. Translational Up-Regulation of the EGFR by Tumor Hypoxia Provides a Nonmutational Explanation for Its Overexpression in Human Cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 13092–13097. [Google Scholar] [CrossRef] [Green Version]
- Nicholas, M.K.; Lukas, R.V.; Jafri, N.F.; Faoro, L.; Salgia, R. Epidermal Growth Factor Receptor—Mediated Signal Transduction in the Development and Therapy of Gliomas. Clin. Cancer Res. 2006, 12, 7261–7270. [Google Scholar] [CrossRef] [Green Version]
- Spence, A.M.; Muzi, M.; Swanson, K.R.; O’Sullivan, F.; Rockhill, J.K.; Rajendran, J.G.; Adamsen, T.C.H.; Link, J.M.; Swanson, P.E.; Yagle, K.J.; et al. Regional Hypoxia in Glioblastoma Multiforme Quantified with [18F] Fluoromisonidazole Positron Emission Tomography before Radiotherapy: Correlation with Time to Progression and Survival. Clin. Cancer Res. 2008, 14, 2623–2630. [Google Scholar] [CrossRef] [Green Version]
- Paech, D.; Dreher, C.; Regnery, S.; Meissner, J.-E.; Goerke, S.; Windschuh, J.; Oberhollenzer, J.; Schultheiss, M.; Deike-Hofmann, K.; Bickelhaupt, S.; et al. Relaxation-Compensated Amide Proton Transfer (APT) MRI Signal Intensity Is Associated with Survival and Progression in High-Grade Glioma Patients. Eur. Radiol. 2019, 29, 4957–4967. [Google Scholar] [CrossRef] [PubMed]
- Mărginean, L.; Ștefan, P.A.; Lebovici, A.; Opincariu, I.; Csutak, C.; Lupean, R.A.; Coroian, P.A.; Suciu, B.A. CT in the Differentiation of Gliomas from Brain Metastases: The Radiomics Analysis of the Peritumoral Zone. Brain Sci. 2022, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Csutak, C.; Ștefan, P.-A.; Lenghel, L.M.; Moroșanu, C.O.; Lupean, R.-A.; Șimonca, L.; Mihu, C.M.; Lebovici, A. Differentiating High-Grade Gliomas from Brain Metastases at Magnetic Resonance: The Role of Texture Analysis of the Peritumoral Zone. Brain Sci. 2020, 10, 638. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.; Kharbanda, S.; Pope, W.B.; Tran, A.; Solis, O.E.; Peale, F.; Forrest, W.F.; Pujara, K.; Lai, J.A.A.; Kharbanda, S.; et al. Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J. Clin. Oncol. 2011, 29, 4482–4490. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Ruan, D.; Raymond, C.; Liau, L.M.; Salamon, N.; Pope, W.B.; Nghiemphu, P.L.; Lai, A.; Cloughesy, T.F.; Ellingson, B.M. Improving B0 Correction for pH-Weighted Amine Proton Chemical Exchange Saturation Transfer (CEST) Imaging by Use of k-Means Clustering and Lorentzian Estimation. Tomography 2018, 4, 123–137. [Google Scholar] [CrossRef]
- Schmiedeskamp, H.; Straka, M.; Newbould, R.D.; Zaharchuk, G.; Andre, J.B.; Olivot, J.M.; Moseley, M.E.; Albers, G.W.; Bammer, R. Combined spin- and gradient-echo perfusion-weighted imaging. Magn. Reson. Med. 2012, 68, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Leu, K.; Boxerman, J.L.; Lai, A.; Nghiemphu, P.L.; Pope, W.B.; Cloughesy, T.F.; Ellingson, B.M. Bidirectional Contrast agent leakage correction of dynamic susceptibility contrast (DSC)-MRI improves cerebral blood volume estimation and survival prediction in recurrent glioblastoma treated with bevacizumab. J. Magn. Reson. Imaging 2016, 44, 1229–1237. [Google Scholar] [CrossRef] [Green Version]
| WHO 2016 Grading | ||||
|---|---|---|---|---|
| All Patients | Grade II | Grade III | Grade IV | |
| Number of patients (treatment naïve/on treatment) | 159 (96/63) | 42 (33/9) | 38 (28/10) | 79 (35/44) |
| Age median (range) | 52 (19–90) | 41 (22–90) | 48.5 (21–70) | 59 (19–83) |
| Sex male/female | 101/58 | 24/18 | 24/14 | 53/26 |
| IDH status wild-type/mutant | 89/70 | 3/39 | 13/25 | 73/6 |
| 1p/19q status in IDH mutant non-co-deleted/co-deleted/NA | 35/29/6 | 16/20/3 | 15/9/1 | 4/0/2 |
| EGFR status in IDH wild-type non-amplified/amplified/NA | 42/40/7 | 2/1/0 | 9/3/1 | 31/36/6 |
| Characteristics | OS (Univariate) | OS (Multivariate) | ||||
|---|---|---|---|---|---|---|
| p-Value | HR | HR [95% CI] | p-Value | HR | HR [95% CI] | |
| Age | *** 1.753 × 10−4 | 1.035 | 1.017–1.054 | Covariate | ||
| Treatment status | **** 3.837 × 10−7 | 3.748 | 2.250–6.241 | Covariate | ||
| IDH | **** 4.400 × 10−9 | 0.093 | 0.042–0.206 | Covariate | ||
| MTRasym at 3.0 ppm | 0.2280 | 1.360 | 0.825–2.242 | 0.5474 | 1.1841 | 0.683–2.053 |
| R2’ | *** 2.019 × 10−4 | 1.440 | 1.188–1.746 | * 0.0445 | 1.2703 | 1.006–1.604 |
| MTRasym × R2’ | *** 7.767 × 10−4 | 1.140 | 1.056–1.231 | ** 0.0019 | 1.1655 | 1.058–1.284 |
| rCBV | 0.9646 | 0.991 | 0.661–1.486 | 0.9702 | 0.9924 | 0.664–1.483 |
| CET + NET volume | **** 7.784 × 10−5 | 1.007 | 1.003–1.010 | 0.1526 | 1.0027 | 0.999–1.006 |
| Acidic volume | *** 2.742 × 10−4 | 1.020 | 1.009–1.031 | 0.0931 | 1.0103 | 0.998–1.023 |
| Acidic volume fraction | * 0.0410 | 1.021 | 1.001–1.041 | 0.3563 | 1.0098 | 0.989–1.031 |
| Characteristics | PFS (Univariate) | PFS (Multivariate) | ||||
|---|---|---|---|---|---|---|
| p-Value | HR | HR [95% CI] | p-Value | HR | HR [95% CI] | |
| Age | ** 0.0014 | 1.025 | 1.010–1.041 | Covariate | ||
| Treatment status | **** 5.032 × 10−7 | 3.142 | 2.010–4.910 | Covariate | ||
| IDH | **** 4.788 × 10−11 | 0.138 | 0.077–0.249 | Covariate | ||
| MTRasym at 3.0 ppm | * 0.0287 | 1.653 | 1.054–2.594 | 0.1661 | 1.4278 | 0.862–2.364 |
| R2’ | **** 4.587 × 10−5 | 1.436 | 1.207–1.709 | * 0.0102 | 1.2972 | 1.064–1.582 |
| MTRasym × R2’ | **** 8.290 × 10−6 | 1.177 | 1.096–1.265 | **** 8.617 × 10−5 | 1.1919 | 1.092–1.301 |
| rCBV | 0.2076 | 1.244 | 0.886–1.747 | 0.4957 | 1.1293 | 0.796–1.602 |
| CET + NET volume | *** 1.903 × 10−4 | 1.006 | 1.003–1.009 | 0.5049 | 1.0011 | 0.998–1.004 |
| Acidic volume | *** 1.563 × 10−4 | 1.019 | 1.009–1.030 | 0.1072 | 1.0094 | 0.998–1.021 |
| Acidic volume fraction | * 0.0125 | 1.022 | 1.005–1.040 | 0.2023 | 1.0120 | 0.994–1.031 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.; Hagiwara, A.; Oughourlian, T.C.; Wang, C.; Raymond, C.; Pope, W.B.; Salamon, N.; Lai, A.; Ji, M.; Nghiemphu, P.L.; et al. Diagnostic and Prognostic Value of pH- and Oxygen-Sensitive Magnetic Resonance Imaging in Glioma: A Retrospective Study. Cancers 2022, 14, 2520. https://doi.org/10.3390/cancers14102520
Yao J, Hagiwara A, Oughourlian TC, Wang C, Raymond C, Pope WB, Salamon N, Lai A, Ji M, Nghiemphu PL, et al. Diagnostic and Prognostic Value of pH- and Oxygen-Sensitive Magnetic Resonance Imaging in Glioma: A Retrospective Study. Cancers. 2022; 14(10):2520. https://doi.org/10.3390/cancers14102520
Chicago/Turabian StyleYao, Jingwen, Akifumi Hagiwara, Talia C. Oughourlian, Chencai Wang, Catalina Raymond, Whitney B. Pope, Noriko Salamon, Albert Lai, Matthew Ji, Phioanh L. Nghiemphu, and et al. 2022. "Diagnostic and Prognostic Value of pH- and Oxygen-Sensitive Magnetic Resonance Imaging in Glioma: A Retrospective Study" Cancers 14, no. 10: 2520. https://doi.org/10.3390/cancers14102520
APA StyleYao, J., Hagiwara, A., Oughourlian, T. C., Wang, C., Raymond, C., Pope, W. B., Salamon, N., Lai, A., Ji, M., Nghiemphu, P. L., Liau, L. M., Cloughesy, T. F., & Ellingson, B. M. (2022). Diagnostic and Prognostic Value of pH- and Oxygen-Sensitive Magnetic Resonance Imaging in Glioma: A Retrospective Study. Cancers, 14(10), 2520. https://doi.org/10.3390/cancers14102520





