The Utilization of Diffusion Tensor Imaging as an Image-Guided Tool in Brain Tumor Resection Surgery: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
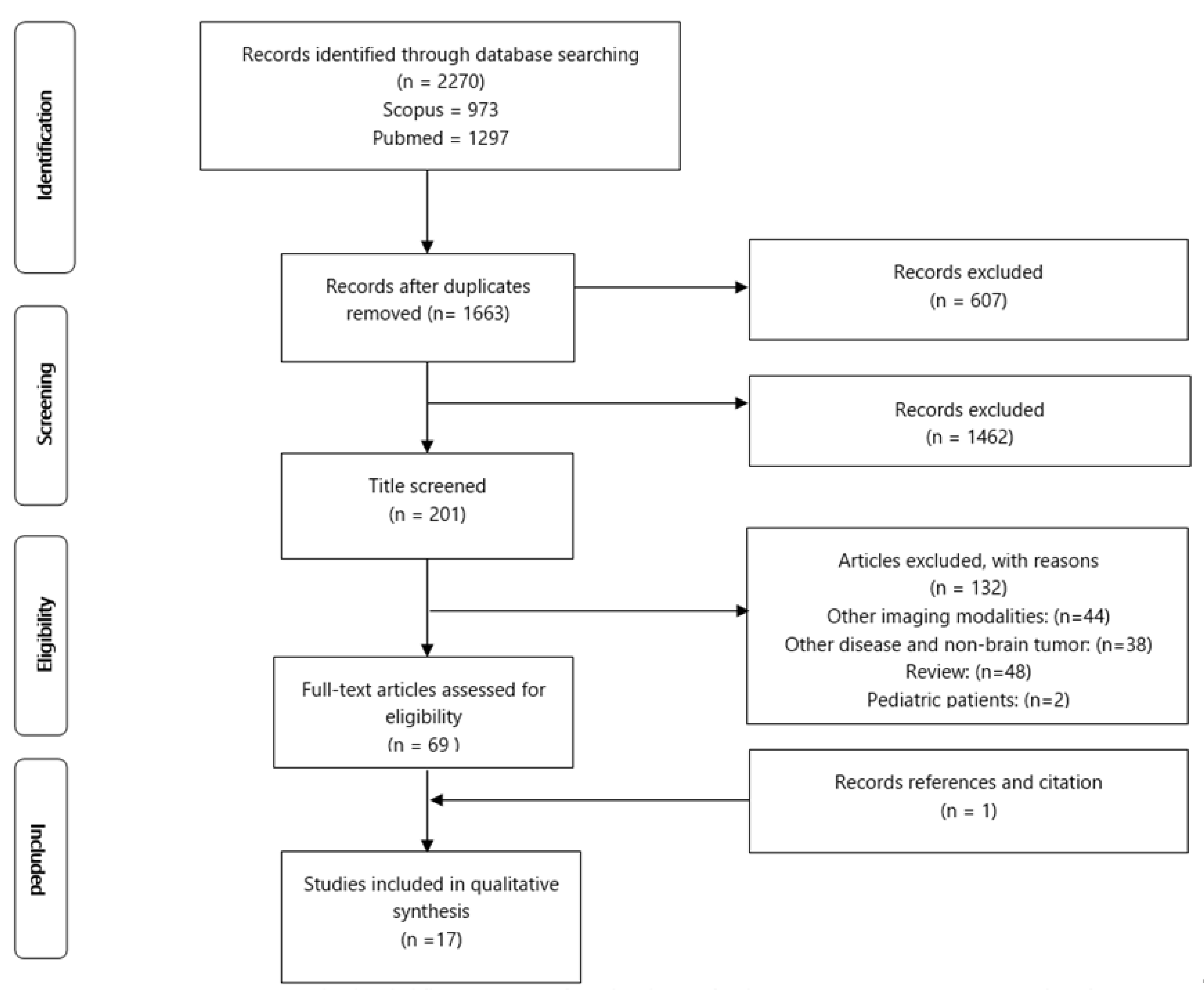
2.1. Search Strategy and Study Selection
2.2. PICOS and Inclusion and Exclusion Criteria
3. Results
3.1. Data Extraction and Study Design
3.2. Participants
3.3. Utilization of DTI in Brain Resection Surgery
3.4. Comparison to the Control Group (n = 6)
3.5. Preoperative and Intraoperative DTI Tractography Evaluation (n = 4)
3.6. Surgical Approach or Preoperative Plan Modification Concerning DTI Data or Tractography (n = 7)
3.7. Evaluation of DTI Tractography Utilization on Postoperative Surgical Outcome
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, C.S.; Li, K.C.; Xuan, Y.; Ji, X.M.; Qin, W. Diffusion tensor tractography in patients with cerebral tumors: A helpful technique for neurosurgical planning and postoperative assessment. Eur. J. Radiol. 2005, 56, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Lin, K.; Liu, Y.; Li, X. Clinical Uses of Diffusion Tensor Imaging Fiber Tracking Merged Neuronavigation with Lesions Adjacent to Corticospinal Tract: A Retrospective Cohort Study. J. Korean Neurosurg. Soc. 2020, 63, 248–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essayed, W.I.; Zhang, F.; Unadkat, P.; Cosgrove, G.R.; Golby, A.J.; O’Donnell, L.J. White matter tractography for neurosurgical planning: A topography-based review of the current state of the art. NeuroImage Clin. 2017, 15, 659–672. [Google Scholar] [CrossRef]
- Witwer, B.P.; Moftakhar, R.; Hasan, K.M.; Deshmukh, P.; Haughton, V.; Field, A.; Arfanakis, K.; Noyes, J.; Moritz, C.; Meyerand, M.E.; et al. Diffusion-tensor imaging of white matter tracts in patients with cerebral neoplasm. J. Neurosurg. 2002, 97, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Elder, J.B. Techniques for Open Surgical Resection of Brain Metastases. Neurosurg. Clin. North Am. 2020, 31, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W. Quantitative evaluation of diffusion tensor imaging for clinical management of glioma. Neurosurg. Rev. 2020, 43, 881–891. [Google Scholar] [CrossRef]
- Romano, A.; D’Andrea, G.; Minniti, G.; Mastronardi, L.; Ferrante, L.; Fantozzi, L.M.; Bozzao, A. Pre-surgical planning and MR-tractography utility in brain tumour resection. Eur. Radiol. 2009, 19, 2798–2808. [Google Scholar] [CrossRef]
- Faust, K.; Vajkoczy, P. Distinct displacements of the optic radiation based on tumor location revealed using preoperative diffusion tensor imaging. J. Neurosurg. 2016, 124, 1343–1352. [Google Scholar] [CrossRef] [Green Version]
- Assaf, Y.; Pasternak, O. Diffusion Tensor Imaging (DTI)-based White Matter Mapping in Brain Research: A Review. J. Mol. Neurosci. 2008, 34, 51–61. [Google Scholar] [CrossRef]
- Soares, J.M.; Marques, P.; Alves, V.; Sousa, N. A hitchhiker’s guide to diffusion tensor imaging. Front. Neurosci. 2013, 7, 31. [Google Scholar] [CrossRef] [Green Version]
- Van Hecke, W.; Emsell, L.; Sunaert, S. Diffusion Tensor Imaging: A Practical Handbook; Springer: New York, NY, USA, 2016; pp. 1–440. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, 332–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manan, H.; Yahya, N. Ageing and Olfactory Dysfunction in Trisomy 21: A Systematic Review. Brain Sci. 2021, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Yahya, N.; Manan, H.A. Utilisation of Diffusion Tensor Imaging in Intracranial Radiotherapy and Radiosurgery Planning for White Matter Dose Optimization: A Systematic Review. World Neurosurg. 2019, 130, e188–e198. [Google Scholar] [CrossRef] [PubMed]
- Manan, H.A.; Franz, E.A.; Yahya, N. The utilisation of resting-state fMRI as a pre-operative mapping tool in patients with brain tumours in comparison to task-based fMRI and intraoperative mapping: A systematic review. Eur. J. Cancer Care 2021, 30, e13428. [Google Scholar] [CrossRef]
- Yahya, N.; Manan, H.A. Diffusion tensor imaging indices to predict cognitive changes following adult radiotherapy. Eur. J. Cancer Care 2021, 30, e13329. [Google Scholar] [CrossRef]
- Voon, N.S.; Manan, H.A.; Yahya, N. Cognitive Decline following Radiotherapy of Head and Neck Cancer: Systematic Review and Meta-Analysis of MRI Correlates. Cancers 2021, 13, 6191. [Google Scholar] [CrossRef]
- Manan, H.A.; Yahya, N.; Han, P.; Hummel, T. A systematic review of olfactory-related brain structural changes in patients with congenital or acquired anosmia. Brain Struct. Funct. 2022, 227, 177–202. [Google Scholar] [CrossRef]
- Okada, T.; Mikuni, N.; Miki, Y.; Kikuta, K.-I.; Urayama, S.-I.; Hanakawa, T.; Fushimi, Y.; Yamamoto, A.; Kanagaki, M.; Fukuyama, H.; et al. Corticospinal Tract Localization: Integration of Diffusion-Tensor Tractography at 3-T MR Imaging with Intraoperative White Matter Stimulation Mapping—Preliminary Results. Radiology 2006, 240, 849–857. [Google Scholar] [CrossRef]
- Bello, L.; Castellano, A.; Fava, E.; Casaceli, G.; Riva, M.; Scotti, G.; Gaini, S.M.; Falini, A. Intraoperative use of diffusion tensor imaging fiber tractography and subcortical mapping for resection of gliomas: Technical considerations. Neurosurg. Focus 2010, 28, E6. [Google Scholar] [CrossRef] [Green Version]
- Hajiabadi, M.; Samii, M.; Fahlbusch, R. A preliminary study of the clinical application of optic pathway diffusion tensor tractography in suprasellar tumor surgery: Preoperative, intraoperative, and postoperative assessment. J. Neurosurg. 2016, 125, 759–765. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Feng, Y.; Cheng, L.; Liu, J.; Li, H.; Jiang, H. Application of diffusion tensor tractography in the surgical treatment of brain tumors located in functional areas. Oncol. Lett. 2020, 19, 615–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aibar-Durán, J.; De Quintana-Schmidt, C.; Holzpafel, M.J..; Hernández, F.M.; Cortés, C.A.; Martínez, G.V.; Bertrán, G.C. Intraoperative Use and Benefits of Tractography in Awake Surgery Patients. World Neurosurg. 2020, 137, e347–e353. [Google Scholar] [CrossRef] [PubMed]
- Nimsky, C.; Ganslandt, O.; Hastreiter, P.; Wang, R.; Benner, T.; Sorensen, A.G.; Fahlbusch, R. Preoperative and Intraoperative Diffusion Tensor Imaging-based Fiber Tracking in Glioma Surgery. Neurosurgery 2005, 56, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.S.; Zhou, L.F.; Tang, W.J.; Mao, Y.; Hu, J.; Song, Y.Y.; Hong, X.N.; Du, G.H. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: A prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery 2007, 61, 935–948. [Google Scholar] [CrossRef]
- Lv, J.; Wei, X.; Quan, W.; Cao, Z. Appliance of preoperative diffusion tensor imaging and fiber tractography in patients with brainstem lesions. Neurol. India 2010, 58, 886–890. [Google Scholar] [CrossRef]
- Maesawa, S.; Fujii, M.; Nakahara, N.; Watanabe, T.; Wakabayashi, T.; Yoshida, J. Intraoperative Tractography and Motor Evoked Potential (MEP) Monitoring in Surgery for Gliomas Around the Corticospinal Tract. World Neurosurg. 2010, 74, 153–161. [Google Scholar] [CrossRef]
- Zakaria, H.; Haider, S.; Lee, I. Automated Whole Brain Tractography Affects Preoperative Surgical Decision Making. Cureus 2017, 9, e1656. [Google Scholar] [CrossRef] [Green Version]
- Alexopoulos, G.; Cikla, U.; El Tecle, N.; Kulkarni, N.; Pierson, M.; Mercier, P.; Kemp, J.; Coppens, J.; Mahmoud, S.; Sehi, M.; et al. The Value of White Matter Tractography by Diffusion Tensor Imaging in Altering a Neurosurgeon’s Operative Plan. World Neurosurg. 2019, 132, e305–e313. [Google Scholar] [CrossRef]
- Xiao, X.; Kong, L.; Pan, C.; Zhang, P.; Chen, X.; Sun, T.; Wang, M.; Qiao, H.; Wu, Z.; Zhang, J.; et al. The role of diffusion tensor imaging and tractography in the surgical management of brainstem gliomas. Neurosurg. Focus 2021, 50, E10. [Google Scholar] [CrossRef]
- Voets, N.L.; Pretorius, P.; Birch, M.D.; Apostolopoulos, V.; Stacey, R.; Plaha, P. Diffusion tractography for awake craniotomy: Accuracy and factors affecting specificity. J. Neuro-Oncol. 2021, 153, 547–557. [Google Scholar] [CrossRef]
- Buchmann, N.; Gempt, J.; Stoffel, M.; Förschler, A.; Meyer, B.; Ringel, F. Utility of diffusion tensor-imaged (DTI) motor fiber tracking for the resection of intracranial tumors near the corticospinal tract. Acta Neurochir. 2011, 153, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Bello, L.; Gambini, A.; Castellano, A.; Carrabba, G.; Acerbi, F.; Fava, E.; Giussani, C.; Cadioli, M.; Blasi, V.; Casarotti, A.; et al. Motor and language DTI Fiber Tracking combined with intraoperative subcortical mapping for surgical removal of gliomas. NeuroImage 2008, 39, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Alam Khan, K.; Jain, S.K.; Sinha, V.D.; Sinha, J. Preoperative Diffusion Tensor Imaging: A Landmark Modality for Predicting the Outcome and Characterization of Supratentorial Intra-Axial Brain Tumors. World Neurosurg. 2019, 124, e540–e551. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Gomaa, M.; Sakr, H.; Elzaher, Y.A. Role of diffusion tensor imaging in characterization and preoperative planning of brain neoplasms. Egypt. J. Radiol. Nucl. Med. 2013, 44, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Shalan, M.E.; Soliman, A.Y.; Nassar, I.A.; Alarabawy, R.A. Surgical planning in patients with brain glioma using diffusion tensor MR imaging and tractography. Egypt. J. Radiol. Nucl. Med. 2021, 52, 110. [Google Scholar] [CrossRef]
- Dubey, A.; Kataria, R.; Sinha, V.D. Role of diffusion tensor imaging in brain tumor surgery. Asian J. Neurosurg. 2018, 13, 302–306. [Google Scholar] [CrossRef]
- Gerard, I.J.; Kersten-Oertel, M.; Petrecca, K.; Sirhan, D.; Hall, J.A.; Collins, D.L. Brain shift in neuronavigation of brain tumors: A review. Med Image Anal. 2017, 35, 403–420. [Google Scholar] [CrossRef]
- Costabile, J.D.; Alaswad, E.; D’Souza, S.; Thompson, J.A.; Ormond, D.R. Current Applications of Diffusion Tensor Imaging and Tractography in Intracranial Tumor Resection. Front. Oncol. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Mikuni, N.; Okada, T.; Enatsu, R.; Miki, Y.; Urayama, S.-I.; Takahashi, J.A.; Nozaki, K.; Fukuyama, H.; Hashimoto, N. Clinical significance of preoperative fibre-tracking to preserve the affected pyramidal tracts during resection of brain tumours in patients with preoperative motor weakness. J. Neurol. Neurosurg. Psychiatry 2007, 78, 716–721. [Google Scholar] [CrossRef] [Green Version]
- Sollmann, N.; Kubitscheck, A.; Maurer, S.; Ille, S.; Hauck, T.; Kirschke, J.S.; Ringel, F.; Meyer, B.; Krieg, S.M. Preoperative language mapping by repetitive navigated transcranial magnetic stimulation and diffusion tensor imaging fiber tracking and their comparison to intraoperative stimulation. Neuroradiology 2016, 58, 807–818. [Google Scholar] [CrossRef]
- Lara-Velazquez, M.; Al-Kharboosh, R.; Jeanneret, S.; Vazquez-Ramos, C.; Mahato, D.; Tavanaiepour, D.; Rahmathulla, G.; Quinones-Hinojosa, A. Advances in Brain Tumor Surgery for Glioblastoma in Adults. Brain Sci. 2017, 7, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wende, T.; Hoffmann, K.-T.; Meixensberger, J. Tractography in Neurosurgery: A Systematic Review of Current Applications. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2020, 81, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Caras, A.; Mugge, L.; Miller, W.; Mansour, T.R.; Schroeder, J.; Medhkour, A. Usefulness and Impact of Intraoperative Imaging for Glioma Resection on Patient Outcome and Extent of Resection: A Systematic Review and Meta-Analysis. World Neurosurg. 2020, 134, 98–110. [Google Scholar] [CrossRef]
- Leclercq, D.; Delmaire, C.; de Champfleur, N.M.; Chiras, J.; Lehéricy, S. Diffusion Tractography: Methods, Validation and Applications in Patients with Neurosurgical Lesions. Neurosurg. Clin. North Am. 2011, 22, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Sąsiadek, M.J.; Szewczyk, P.; Bladowska, J. Application of diffusion tensor imaging (DTI) in pathological changes of the spinal cord. Med Sci. Monit. 2012, 18, RA73–RA79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, P.; Chung, S.; Berman, J.; Hess, C.; Henry, R. Diffusion Tensor MR Imaging and Fiber Tractography: Technical Considerations. Am. J. Neuroradiol. 2008, 29, 843–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| PICOS | Criteria |
|---|---|
| P—Patients | Adult brain tumor patients. |
| I—Intervention | Underwent DTI scanning for preoperative planning. |
| C—Comparison | Re-evaluation of preoperative DTI and intraoperative DTI, modification of the preoperative planning based on DTI data, comparison with the non-DTI control group. |
| O—Outcome | Surgical decision and outcome. |
| S—Study | Only original clinical studies were selected. |
| No | Author (Year): Country [Ref] | No of Patients (Male/Female) | Mean Age, (Age Range) | Tumor Type | Tumor Location | Control Participant |
|---|---|---|---|---|---|---|
| Prospective Study | ||||||
| 1 | Okada et al. (2006): Japan [19] | 8 (4/4) | 41 years, (23–58) | Intracranial space-occupying lesions; Included HGG | Frontal, parietal lobe, pons | NR |
| 2 | Nimsky et al. (2005): Germany [24] | 37 (20/17) | 45.2 ± 21 years, (6–77) | Supratentorial gliomas; Included HGG and LGG | NR | NR |
| 3 | Wu et al. (2007): China [25] | 118 (40/78) | Patients 40.8 ± 15.6 years, (6–75) Control 38.0 ± 16.5 years, (6–70) | Cerebral gliomas; Included HGG and LGG | Frontal, temporal, pariental, insular, central, occipital, basal ganglia, thalamus | 120 (78/42) Control patients underwent craniotomies using 3-D navigational MRI only |
| 4 | Romano et al. (2009): Italy [7] | 28 (19/9) | (38–77) | Intra-axial cerebral tumor; Included HGG, LGG and Metastasis tumor | Thalamus, fronto-parietal, frontal, parietal, temporal, temporooccipital | NR |
| 5 | Bello et al. (2010): Italy [20] | 230 | NR | Gliomas Included HGG and LGG | Precentral, Rolandic, parietal, temporal, insula | NR |
| 6 | Hajiabadi et al. (2015): Germany [21] | 25 (15/10) | 53.08 ± 18.61 years, (11–87) | Suprasellar mass lesion; Included HGG, LGG | Hypothalamus-pituitary | 6 control patients with normal vision |
| 7 | Faust and Vajkoczy (2016): Germany [8] | 113 (70/43) | 54 ± 16 years | Intraaxial tumor; Included HGG, LGG and Metastasis tumor | Temporal | NR |
| 8 | Zhang et al. (2020): China [22] | 21(13/8) | Patients 53.29 years Control 48.24 years | Intracranial tumor: Included HGG, LGG, and Metastasis tumor | Frontal, precentral gyrus, temporal, cerebral falx | 21 (11/10) control patients underwent preoperative MRI only |
| 9 | Aibar-Duran et al. (2020): Spain [23] | 37 (25/12) | 53.8 years, (33–75) | Brain tumor in eloquent areas; Included HGG, LGG, and Metastasis tumor | Temporal or insular, frontal, parietal | 18 control patients with no intraoperative navigated DTI |
| Retrospective Study | ||||||
| 10 | Yu et al. (2005): China [1] | 16 (12/4) | Patients 51.7 years, (20–72) Control 52.5 years (25–68) | Cerebral tumor; Included HGG, LGG and metastasis tumor | Brainstem | 24 (17/7) control patients’ MRI data with suspicion of involvement of the pyramidal tract |
| 11 | Cao et al. (2010): China [26] | 9 (5/4) | 30.1 years, (4–49) | Brainstem lesion; Included HGG, LGG | Brainstem, (pons, medulla oblongata, midbrain) | NR |
| 12 | Maesawa et al. (2010): Japan [27] | 28 (17/11) | 46.5 years, (13–68) | Intracranial tumor; Included HGG, LGG | Deep-seated tumor located 20 mm of CST | NR |
| 13 | Buchmann et al. (2011) Germany [32] | 19 (13/6) | 49 years, (16–72) | Intracranial tumor; Included HGG, LGG, Metastasis tumor | Frontodorsal, frontal, precentral, insular, temporomesial, central, parietal, cingular | NR |
| 14 | Zakaria et al. (2017): USA [28] | 28 (17/11) | Patients 51.75 ± 17.78 years, (26–76) Control 56.11 ± 11.23 years, (27–86) | Brain tumor within eloquent areas; Included HGG, LGG, and Metastasis tumor | Parietal, frontal, temporal, frontal-parietal, frontal-temporal | 45 (30/15) control patients with non-mapping preoperative planning |
| 15 | Alexopoulos et al. (2019): USA [29] | 15 (11/4) | 58.3 years, (45.5–71.5) | Supratentorial tumor | Frontal, parietal, temporal, occipital | NR |
| 16 | Xiao et al. (2021) China [30] | 54 (31/23) | 17.6 years, (1.9–62.2) | Brainstem glioma; Included HGG, LGG | Brainstem | NR |
| 17 | Voets et al. (2021) UK [31] | 91 (48/43) | 49.2 years, (19–74) | Intrinsic Brain tumor; Included HGG, LGG and Metastasis tumor | NR | NR |
| No | Author | Main Findings |
|---|---|---|
| 1 | Yu et al. (2005) [1] | DTI group gave a better GTR outcome and less postoperative deficit in comparison to the control group |
| 2 | Wu et al. (2007) [25] | DTI navigational gave a better GTR in HGG than LGG and a higher KPS score and represented a 43.0% reduction in death risk compared to control |
| 3 | Hajiabadi et al. (2015) [21] | VIS on assessed DTI group was reduced from the compression of optic chiasm, as compared to control which have normal structure of the fiber optic chiasm |
| 4 | Zakaria et al. (2017) [28] | DTI brain mapping group’s postoperative neurology deficits improved in comparison to control |
| 5 | Aibar-Duran et al. (2020) [23] | Intraoperative navigated tractography group had more complete resection, less postoperative neurological damage, and shorter surgery time than the control group |
| 6 | Zhang et al. (2020) [22] | The postoperative KPS score in the DTI group was significantly better than the control group, although there are no significant difference in GTR between the two groups |
| Author (Year) | White Matter Tract of Interest | Assessment of White Matter Tract (WMT) during | Type of Surgery | Modification of Surgical Approach or Plan by DTI Tractography (Yes /No) | Surgery Outcome | Main Finding | |
|---|---|---|---|---|---|---|---|
| Preoperative | Intraoperative | GTR/Postoperative Deficits Assessment | |||||
| Yu et al. (2005) [1] | Pyramidal, Corpus Callosum, Optic Radiation | Preoperative depiction of DTI and WMT characterization evaluation pre-determined surgery approach. | NR | Craniotomy | No | GTR: DTT group patients were higher, compared to the control. Postoperative deficit: locomotive function of the DTT group was improved. | The GTR and surgical approaches were determined by the type of WMT characterization depicted by DTT. |
| Okada et al. (2006) [19] | CST | Preoperative DTI of WMT depicted for surgical planning | DTI tractography used with MEP | Craniotomy | No | No postoperative neurological deficits. | Affective combinations of DTI tractography with MEP. |
| Wu et al. (2007) [25] | Pyramidal Tracts | Preoperative DTI and MRI were used and compared to only MRI scan control. | NR | Craniotomy | No | GTR: Higher chance of HGG in DTI group. Postoperative deficits: KRS score higher in DTI group. | DTI navigational neurosurgery gave reduction in death risk compared to the control group. |
| Romano et al. (2009) [7] | Pyramidal tract, Optic Radiation, Arcuate fasciculus | Preoperative DTI of WMT depicted for surgical planning | Assessment trajectories of fibers, some needed for repeated tractography. | Craniotomy, Corticotomy | Yes, modification of resection margin and surgical approach. | GTR: 64% successful predefined on resection margin, allowed further resection. Postoperative deficits: improved with successful DTI trajectories. | The MR DTI altered preoperational planning and modified the surgical approach to craniotomy in 21% of the patients. |
| Nimsky et al. (2005) [24] | Pyramidal tract, corpus callosum | Preoperative DTI depicted WMT fiber in the vicinity of the tract in error less than 20 mm. | Intraoperative DTI marked inward or outward shifted range of WMT | Craniotomy | No | Postoperative deficits: only one patient encountered new neurological deficits. | Fiber shifts were evaluated by intraoperative DTI, resulting in a shifting pattern inward or outward of WM fibers. |
| Cao et al. (2010) [26] | CST, medial lemnisci | Preoperative DTI tractography used for individualized surgical approach. | One out of eight patients needed to evaluate the DTI tractography. | Craniotomy | Yes, from suboccipital to restomastodial approach. | GTR: total resection was achieved in four patients. Postoperative deficits: neurological examination improved. | MRI scans were sufficient for tumor resection. However, DTI tractography was needed for WMT concerning the lesion. |
| Maesawa et al. (2010) [27] | CST, Pyramidal tract | Preoperative DTI tractography depicted for surgical planning. | Intraoperative DTI tractography illustrated with conditions. | Craniotomy, Microsurgery | Yes, surgical planning needed to revise intraoperatively. | GTR: subtotal and greater in 85.7%, partially in four patients. | Intraoperative tractography gave a more accurate result than preoperative DTI tractography. |
| Bello et al. (2010) [20] | CST, inferior frontal-occipital fasciculus, Inferior longitudinal fasciculus, UNC, SLF | Preoperative DTI tractography was used for surgical approach. | DTI reconstruction was tested intraoperatively, combined with DES. | Craniotomy, Awake surgery | No | Postoperative deficits: neurological examination improved. | DTI tractography reconstruction corresponded with intraoperative subcortical mapping. |
| Buchmann et al. (2011) [32] | CST, Pyramidal tract | Preoperative DTI fiber tracking depicted for surgical planning. | DTI reconstruction was tested intraoperatively, combined with MEP. | Craniotomy | Yes, post hoc reviewed DTI images suggested changes in surgical approach, but only in one case | GTR: incomplete resection in seven patients. Postoperative deficits: temporary impairment after surgery, permanent in two patients. | DTI fiber tracking did not influence the surgical planning or the intraoperative course. |
| Hajiabadi et al. (2015) [21] | Optic Radiation, Visual pathway | Preoperative DTI depicted WMT fiber for surgical planning. | Intraoperative DTI revealed chiasm crossing fibers undetected by preoperative DTI. | Trans-sphenoidal sinus surgery, transcranial surgery. | No | Postoperative deficits: VIS significantly improved, except for one patient. | The intraoperative DTI finding predicted the visual outcome after tumor resection. |
| Faust and Vajkoczy (2016) [8] | Optic Radiation | Preoperative DTI tractography pre-determined fiber shift of OR. | NR | Temporal lobe surgery | Yes, pre-determined by pattern OR fiber shift. | GTR: total of 90% incomplete resection, 9% subtotal, and 1% partially removed. Postoperative deficits: VFD only 4%. | Surgical approaches were pre-determined by the pattern of OR fiber shifts depicted by DTI. |
| Zakaria et al. (2017) [28] | CST, Superior longitudinal fasciculus, and Arcuate Fascicles | Preoperative brain mapping, either for motor or language pathway was compared to non-mapping control. | NR | Craniotomy | No | Postoperative deficits: improved in the brain mapping group compared to the non-mapping group. | Automated whole-brain tractography mapping patients had more significant results in patients’ postoperative recovery. |
| Alexopoulos et al. (2019) [29] | Pyramidal tracts and superior thalamic radiations, SLF, IFOF, ILF, posterior thalamic radiations | Preoperative DTI tractography depicted for surgical approach by type of white matter tract characterization. | NR | Non-surgical | Yes, from total resection decision to subtotal | GTR: total resection in eight patients, and subtotal in seven patients. | DTI WM tractography identified WMT for better surgical outcome, but not operative approach. |
| Aibar-Duran et al. (2020) [23] | Pyramidal tract, inferior frontal-occipital fasciculus, Optic pathway, Inferior longitudinal fasciculus, aslant tract. | Preoperative DTI tractography was performed and compared to non-DTI control. | Evaluation of intraoperative navigated tractography on surgery time. | Awake surgery | No | GTR: more significant complete resection in DTI group compared to non -DTI group. Postoperative deficits: the development of new deficits was doubled in non- DTI group patients. | Intraoperative navigated tractography shortened the awake surgery time. |
| Zhang et al. (2020) [22] | Arcuate fascicles, pyramidal tract | Preoperative DTI tractography used for surgical approach. | NR | Craniotomy | No | GTR: no significant difference between DTI group compared to control group. Postoperative deficits: improved in trial group compared to control group, relating to KPS score. | The MRI scan was sufficient for tumor resection, and DTI tractography was needed for WMT evaluation concerning the tumor. |
| Xiao et al. (2021) [30] | CST | Preoperative DTI tractography was used for surgical approach. | DTI/DTT accuracy validated by DcCS | Craniotomy | Yes, surgical approaches changed based on the DTI finding. | Postoperative deficits: DTI prediction of postoperative deficits correlates to mRS score. | DTT is a valuable tool for surgical management of brainstem glioma. |
| Voets et al. (2021) [31] | CST, Arcuate, SLF, IFOF, Optic radiation, ILF. | Preoperative DTI tractography used for surgical approach. | Intraoperative subcortical stimulation was used | Awake surgery | No | Postoperative deficits: predictions of postoperative deficits were accurate and were preserved. | Preoperative DTI predictions were accurate in localization of tract, and postoperative DTI predicted recovery potential. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manan, A.A.; Yahya, N.; Idris, Z.; Manan, H.A. The Utilization of Diffusion Tensor Imaging as an Image-Guided Tool in Brain Tumor Resection Surgery: A Systematic Review. Cancers 2022, 14, 2466. https://doi.org/10.3390/cancers14102466
Manan AA, Yahya N, Idris Z, Manan HA. The Utilization of Diffusion Tensor Imaging as an Image-Guided Tool in Brain Tumor Resection Surgery: A Systematic Review. Cancers. 2022; 14(10):2466. https://doi.org/10.3390/cancers14102466
Chicago/Turabian StyleManan, Aiman Abdul, Noorazrul Yahya, Zamzuri Idris, and Hanani Abdul Manan. 2022. "The Utilization of Diffusion Tensor Imaging as an Image-Guided Tool in Brain Tumor Resection Surgery: A Systematic Review" Cancers 14, no. 10: 2466. https://doi.org/10.3390/cancers14102466
APA StyleManan, A. A., Yahya, N., Idris, Z., & Manan, H. A. (2022). The Utilization of Diffusion Tensor Imaging as an Image-Guided Tool in Brain Tumor Resection Surgery: A Systematic Review. Cancers, 14(10), 2466. https://doi.org/10.3390/cancers14102466






