HER-2-Targeted Nanoparticles for Breast Cancer Diagnosis and Treatment
Abstract
:Simple Summary
Abstract
1. Introduction
2. HER-2+ BC Current Therapies and Main Drawbacks
2.1. Monoclonal Antibodies and Antibody-Drug Conjugates
2.2. HER-2 Pathway Inhibitors and Immunotherapy
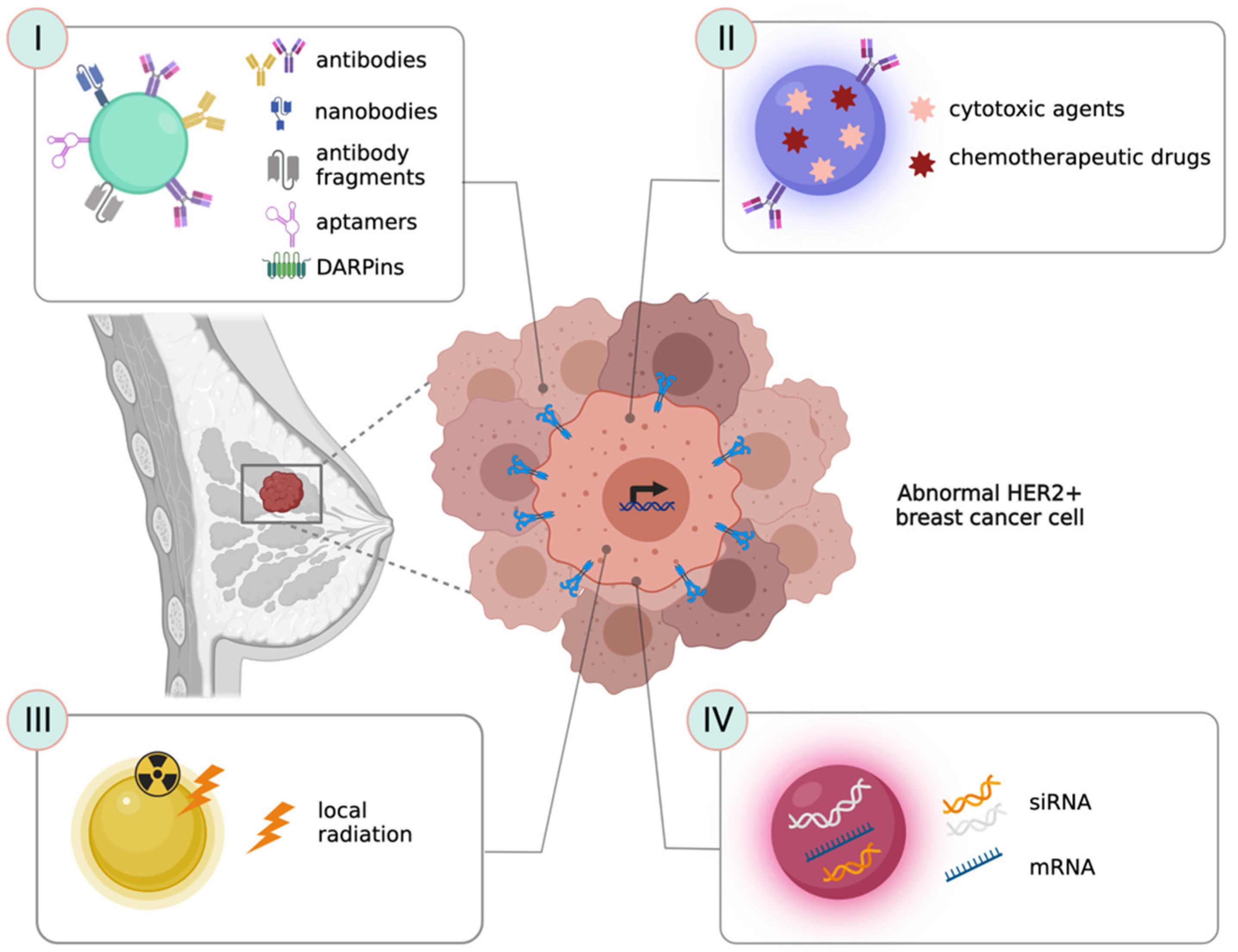
3. Nanotechnological Strategies to Target HER-2 Receptor
3.1. Antibodies as Ligands to Target HER-2+ Cancer Cells
3.2. Nanobodies and Antibody-Fragments as Ligands to Target HER-2+ Cancer Cells
3.3. Peptides, Aptamers and Ankyrins as Ligands to Target HER-2+ Cancer Cells
4. Nanotechnological Strategies for the Treatment of HER-2+ BC
4.1. Strategies to Deliver Chemotherapeutic Drugs and Other Cytotoxic Agents
4.2. Strategies to Vehicle Nucleic Acids or Gene Silencing Molecules
4.3. Nanostrategies to Improve Radiation Efficacy in HER-2+ Tumors
| Mechanism of Action and Targeting Molecule | Achievements | NP Type | Reference | ||
|---|---|---|---|---|---|
| Antibodies | Targeted delivery of anticancer drug. TZ (LuyePharma, Yantai, China/Genentech, South San Francisco, CA, USA) |
| Polymeric/lipid | in vitro | [67,68,69,70,71] |
| Delivery of in vitro-transcribed mRNA coding for TZ |
| Lipid | in vitro—in vivo | [118] | |
| Targeted delivery of anticancer drug. TZ (Roche, Basel, Switzerland) |
| Polymeric | in vitro | [72] | |
| Release of anticancer drug in combination with TZ (Roche, Basel, Switzerland) |
| Albumin-bound Paclitaxel (nab-PTX) | clinical trial | [77] | |
| Targeted delivery of anticancer drug. TZ (Roche, Basel, Switzerland) |
| Polymeric | in vitro | [110] | |
| Nanobodies and antibodies fragments (Fab) | Dual-targeted delivery of chemotherapeutics to HER-2 and EGFR. TZ—Panitumumab Fab fragments (In-house recombinant production) |
| Polymeric | in vitro | [80] |
| Targeted delivery of cytotoxic molecule after photochemical internalization. 11A4-nanobody (In-house recombinant production) |
| Polymeric | in vitro | [84] | |
| Targeted delivery of cytotoxic agent. TZ fragment (Genentech, South San Francisco, CA, USA) |
| Polymeric | in vitro—in vivo | [78] | |
| Induction of tumor specific immune response. anti-HER2-anti-CD3 dual-scFv |
| Cell-derived exosome | in vitro—in vivo | [86] | |
| Peptides | Delivery of antisense oligonucleotide. NH2-PEG200-AHNP |
| Polymeric | in vitro—in vivo | [88] |
| Targeted delivery of cytotoxic agent. HER2pep YCDGFYACY-MDV (In-house recombinant production) |
| Liposome | in vitro—in vivo | [79] | |
| Generation of nanofibers able to disrupt HER2 dimerization. HER2pep BP-FFVLK- YCDGFYACYMDV |
| Peptide-based | in vitro—in vivo | [90] | |
| Targeted delivery of anticancer drug. AHNP (FCDGFYACYADVGGG) |
| Liposome | in vitro—in vivo | [108] | |
| Aptamers | Targeted delivery of anticancer molecule. HB5 DNA aptamer |
| Albumin-based | in vitro | [92] |
| XBP1 deletion by therapeutic siRNA delivery. 3WJ-HER2 aptamer (In-house recombinant production) |
| RNA-based | in vitro—in vivo | [117] | |
| Lysosomal degradation of membrane protein HER-2. HApt aptamer (TaKaRa Ostu, Japan) |
| DNA nanorobot | in vitro—in vivo | [95] | |
| Mechanism of Action and Targeting Molecule | Achievements | NP Type | Reference | ||
|---|---|---|---|---|---|
| Antibodies | Intratumor retention leading to an immune response activation. TZ (Genentech, South San Francisco, CA, USA) |
| Iron oxide | in vitro—in vivo | [74] |
| Targeted photothermal ablation by near-infrared laser. TZ (Roche, Basel, Switzerland) |
| Gold nanorods | in vitro—in vivo | [66] | |
| Selective targeting of HER-2 Increase of pro-apoptotic proteins. TZ (Roche, Basel, Switzerland) |
| Gold nanospheres | in vitro | [63] | |
| HER-2 gene silencing by siRNA delivery. TZ (Roche, Basel, Switzerland) |
| Carbon dots/mesoporous silica | in vitro | [76,114] | |
| Radio-immunotherapy Magnetic hyperthermia. TZ (Roche, Basel, Switzerland) |
| Superparamagnetic iron oxide | in vitro—in vivo | [73] | |
| Antitumor local radiation. TZ (Roche, Basel, Switzerland) |
| Gold NPs | in vitro—in vivo | [64,65,119] | |
| Targeted delivery of anticancer molecule. TZ (Roche, Basel, Switzerland) |
| Carbon-based | in vitro—in vivo | [75] | |
| Nanobodies and antibodies fragments (Fab) | Local irradiation (PDT). sdAb C7b (In-house recombinant production) |
| Silica | in vitro—in vivo | [81] |
| Improved targeting due to the reduced protein corona formation. Anti-HER2 Fab-6His (Hangzhou HealSun Biopharm Co., Ltd., Zhejiang, China) |
| Silica | in vitro | [85] | |
| Peptides | Targeted delivery of anticancer molecule Inhibition of kinase activity. AHNP (GenScript Inc., Piscataway, NJ, USA) |
| Iron oxide | in vitro—in vivo | [89] |
| Aptamers | Targeted delivery of anticancer molecule Downregulation of HER-2 Hapt is also an antagonist Synergic mechanism. HApt aptamer (Sangon Biotech Co., Ltd., Shanghai, China) |
| Mesoporous silica nanocarrier | in vitro | [94] |
| Downregulation of HER-2. (HApt aptamer IDA Inc., Coralville, IA, USA) |
| Gold nanostars | in vivo | [93] | |
| DARPins | Targeted delivery of 90Y radionuclides and toxins. DARPin_9.29 (in-house recombinant production) |
| Upcoversion NPs | in vitro—in vivo | [120] |
| Photothermal therapy. DARPin_9.29 (in-house recombinant production) |
| Upcoversion NPs | in vitro—in vivo | [121] | |
5. Detection of HER-2+ BC: State of the Art and Clinical Needs
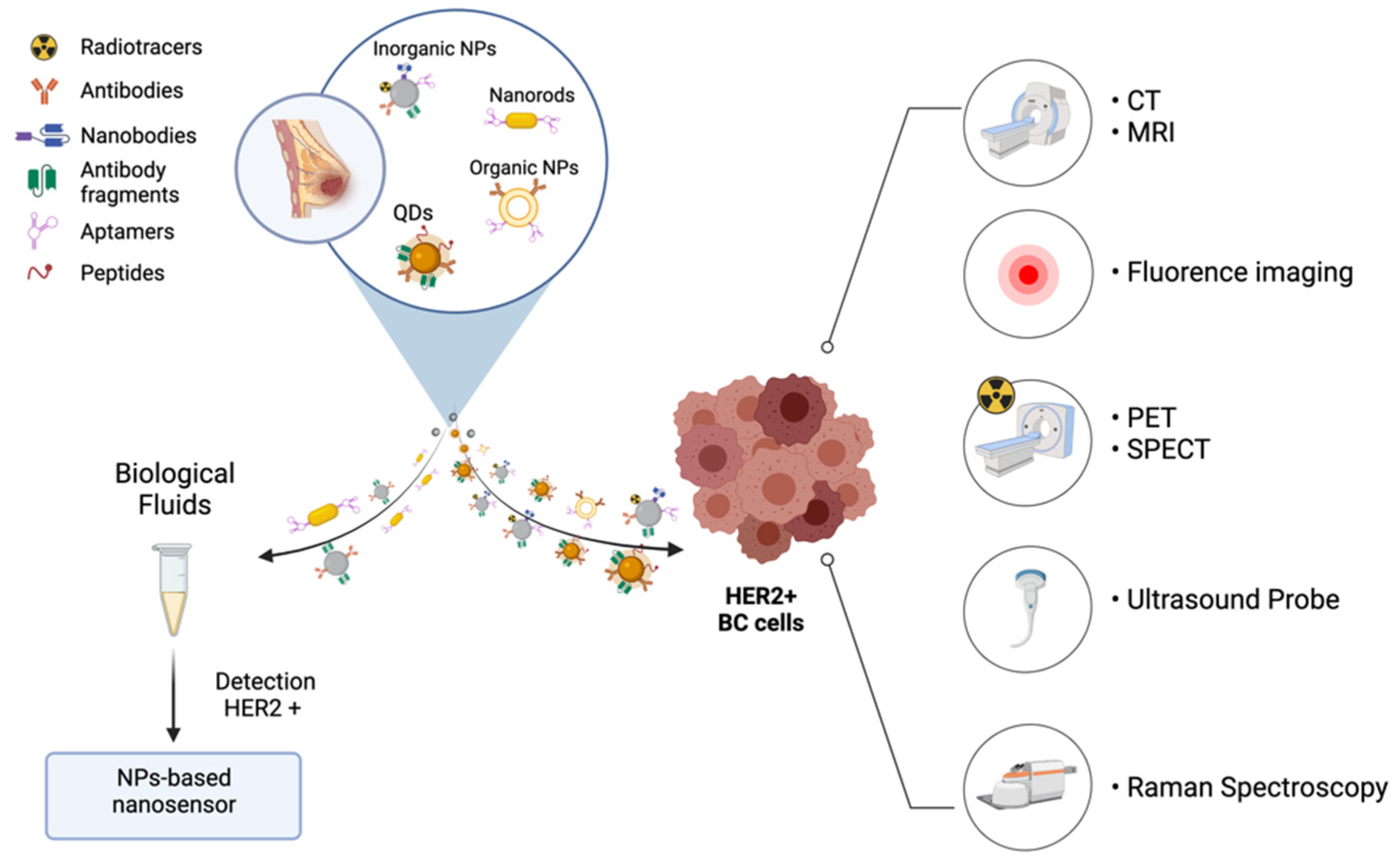
6. Nanotechnological Approaches for HER-2+ BC Diagnosis
6.1. Imaging Agents for Early Diagnosis and Tailored Therapy
6.1.1. Magnetic Resonance Imaging and Computerized Tomography
6.1.2. In Vivo Fluorescence Imaging
6.1.3. Nuclear Medicine
6.1.4. Multimodal Hybrid Approaches
6.2. Nano-Biosensors for the Detection of HER-2 Levels in Biological Fluids and Tissues
6.2.1. Inorganic NPs-Based Nanosensors
6.2.2. Aptamers Based Nanosensors
7. Theranostics
| Technique | NP Type | Achievements | Reference | |
|---|---|---|---|---|
| Magnetic Resonance Imaging | Herceptin-dextran iron oxide nanoparticles (Roche, Basel, Switzerland) |
| in vitro—in vivo | [135] |
| SPIONs-Cy-PEG-scFv (Recombinant scFv 4D5-Cys) |
| in vitro—in vivo | [136] | |
| Magnetosomes functionalized with an anti-HER-2 affibody (Recombinant MamC (GenBank: CDK99608.1) and anti-HER-2 affibody) |
| in vitro—in vivo | [137] | |
| In Vivo Fluorescence Imaging | MnCuInS/ZnS@BSA-Anti-HER-2 bioconjugates (Anti-HER-2 antibody, Sino Biological Inc., Beijing, China) |
| in vitro | [140] |
| Anti-HER-2-QD-antiboy conjugate (Anti-HER-2 antibody, Invitrogen, Carlsbad, CA, USA) |
| in vitro | [141] | |
| Peptide Nanoprobes |
| in vivo—ex vivo | [142] | |
| sdAb-HER-2-QD |
| ex vivo | [143] | |
| Nuclear Medicine | [18F]FB-anti-HER-2 nanobody (In-house recombinant anti-HER-2 nb 2Rs15d) |
| in vivo | [144] |
| 99mTc-ZHER2:2395-Cysc (In-house recombinant production) |
| in vitro—in vivo | [145] | |
| 99mTc-radiolabeled nanosilica system, functionalized with a TZ half-chain (Genentech, South San Francisco, CA, USA) |
| in vitro—ex vivo | [148] | |
| Multimodal Hybrid Approaches | TZ-conjugated Lipo[MNP@m-SiO2]-HER-2Ab (Genentech, Inc., South San Francisco, CA, USA) |
| In vitro | [151] |
| DARPin G3 coated fluorecently labelled SPIONs |
| In vitro—in vivo | [104] | |
| HS-Fe-PEG-HER-2 (Anti-HER-2 antibody, Abcam, Cambridge, UK) |
| in vitro—in vivo | [153] | |
| G5-AuNP-Gd-TZ (Genetech, San Francisco, CA, USA) |
| in vitro—in vivo | [154] | |
| SPIONs-Cy-PEG-scFv (Recombinant scFv 4D5-Cys) |
| In vitro—in vivo | [136] | |
| 89Zr-DFO-scFv-PEG-Cy5-C’ dots (In-house recombinant anti-HER-2 scFv fragments-TZ) |
| in vitro—in vivo | [155] | |
| Inorganic NPs | Pegylated iron oxide NPs conjugated with anti-HER-2 antibodies (Herceptin, F. Hoffmann-La Roche Ltd., Basel, Switzerland) |
| in vitro | [157] |
| Dx-M and As-M were conjugated with a monoclonal scFv (In-house recombinant scFvs) |
| in vitro | [158] | |
| Anti-HER-2 antibody-conjugated silver nanoparticles (Anti-HER-2 antibody, Fuzhou Maxim Biotech, Inc., Fuzhou, China) |
| in vitro | [159] | |
| BSA-AuNCs-LPs-anti-HER-2 (Anti-HER-2 antibody, R&D Systems, Minneapolis, MN, USA) |
| in vitro | [160] | |
| anti HER-2 antibody-biotin conjugate labelled with commercially available QDs (QD525) |
| in vitro | [161] | |
| Aptamers | HeA2_1 and HeA2_3 |
| in vitro—ex vivo | [162] |
| HB5 |
| in vitro | [163] | |
| APlaS to detect ECD-HER-2 protein |
| in vitro | [164] | |
| Antibodies | HER2-DOX-SPIOs@PLGA@A (Herceptin) |
| in vitro—in vivo | [165] |
| 99mTc-SiNPs-TZ/DOX-SiNPs-TZ |
| in vitro—ex vivo—in vivo | [150] | |
| IONP/DOX-MFNC (Herceptin, Chonnam National University Hwasun Hospital) |
| in vitro—in vivo | [166] |
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Breast cancer |
| HER-2+ BC | Human epidermal growth factor receptor-2 overexpressing breast cancer |
| NPs | Nanoparticles |
| EGFR | Epidermal growth factor receptor |
| AKT | Protein kinase B, PKB |
| PI3K | Phosphoinositide 3-kinase |
| MAPK | Mitogen-activated protein kinase |
| IHC | Immunohistochemistry |
| FISH | Fluorescence in situ hybridization |
| mAb | Monoclonal antibodies |
| TZ | Trastuzumab |
| DFS | Disease-free survival |
| OS | Overall survival |
| ADCC | Antibody-Dependent Cellular Cytotoxicity |
| T-DM1, Kadcyla® | Trastuzumab emtansine |
| ADCs | Antibody-drug conjugates, anticancer drugs |
| PFS | Progression-free survival |
| PTEN | Phosphatase and tensin homolog |
| TKIs | Tyrosine kinase inhibitors |
| pCR | Pathological complete response |
| TILs | Tumor-infiltrating lymphocytes |
| PEI | Polyethylenimine |
| PLGA | Poly (D,L-lactide-co-glycolide) |
| sdAb | Single-domain antibodies |
| VH | Human immunoglobulin heavy chain |
| Fab | Antibody fragments |
| TmAb | Fab obtained by papain cleavage of TZ |
| PmAb | Fab obtained by papain cleavage of panitumumab |
| scFv | Single chain variable fragments |
| DOX | Doxorubicin |
| PTX | Paclitaxel |
| PCI | Photochemical Internalization |
| siRNA | Short interfering RNA |
| PTT | Photothermal therapy |
| GNRs | Gold nanorods |
| AuNPs | Gold nanoparticles |
| PK | Pharmacokinetic |
| XBP1 | X-box binding protein 1 |
| PDT | Photodynamic therapy |
| Hapt | Aptamer |
| IHC | Immunohistochemistry |
| CISH | Chromogenic in-situ hybridization |
| CT | Computerized tomography |
| PET | Positron emission tomography |
| SPECT | Single photon emission computed tomography |
| US | Ultrasounds |
| MRI | Magnetic resonance imaging |
| SPIONs | Super paramagnetic iron oxide nanoparticles |
| FGS | Fluorescence guided surgery |
| QDs | Quantum dots |
| Cy | Cyanine |
| BSA | Bovine serum albumin |
| EPR | Enhanced Permeation and Retention |
| PAMAM | Polyamidoamine |
| SERS | Surface-enhanced Raman scattering |
| SELEX | Systematic evolution of ligands by exponential enrichment |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Cronin, K.A.; Kurian, A.W.; Andridge, R. Differences in Breast Cancer Survival by Molecular Subtypes in the United States. Cancer Epidemiol. Biomark. Prev. 2018, 27, 619–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittendorf, E.A.; Wu, Y.; Scaltriti, M.; Meric-Bernstam, F.; Hunt, K.K.; Dawood, S.; Esteva, F.J.; Buzdar, A.U.; Chen, H.; Eksambi, S.; et al. Loss of HER2 Amplification Following Trastuzumab-Based Neoadjuvant Systemic Therapy and Survival Outcomes. Clin. Cancer Res. 2009, 15, 7381–7388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niikura, N.; Liu, J.; Hayashi, N.; Mittendorf, E.A.; Gong, Y.; Palla, S.L.; Tokuda, Y.; Gonzalez-Angulo, A.M.; Hortobagyi, G.N.; Ueno, N.T. Loss of Human Epidermal Growth Factor Receptor 2 (HER2) Expression in Metastatic Sites of HER2-Overexpressing Primary Breast Tumors. J. Clin. Oncol. 2012, 30, 593–599. [Google Scholar] [CrossRef] [Green Version]
- Mohsin, S.K.; Weiss, H.L.; Gutierrez, M.C.; Chamness, G.C.; Schiff, R.; DiGiovanna, M.P.; Wang, C.-X.; Hilsenbeck, S.G.; Osborne, C.K.; Allred, D.C.; et al. Neoadjuvant Trastuzumab Induces Apoptosis in Primary Breast Cancers. J. Clin. Oncol. 2005, 23, 2460–2468. [Google Scholar] [CrossRef]
- Gallardo, A.; Lerma, E.; Escuin, D.; Tibau, A.; Muñoz, J.; Ojeda, B.; Barnadas, A.; Adrover, E.; Sánchez-Tejada, L.; Giner, D.; et al. Increased Signalling of EGFR and IGF1R, and Deregulation of PTEN/PI3K/Akt Pathway Are Related with Trastuzumab Resistance in HER2 Breast Carcinomas. Br. J. Cancer 2012, 106, 1367–1373. [Google Scholar] [CrossRef] [Green Version]
- Arvold, N.D.; Oh, K.S.; Niemierko, A.; Taghian, A.G.; Lin, N.U.; Abi-Raad, R.F.; Sreedhara, M.; Harris, J.R.; Alexander, B.M. Brain Metastases after Breast-Conserving Therapy and Systemic Therapy: Incidence and Characteristics by Biologic Subtype. Breast Cancer Res Treat. 2012, 136, 153–160. [Google Scholar] [CrossRef]
- Kennecke, H.; Yerushalmi, R.; Woods, R.; Cheang, M.C.U.; Voduc, D.; Speers, C.H.; Nielsen, T.O.; Gelmon, K. Metastatic Behavior of Breast Cancer Subtypes. J. Clin. Oncol. 2010, 28, 3271–3277. [Google Scholar] [CrossRef]
- Schlessinger, J. Common and Distinct Elements in Cellular Signaling via EGF and FGF Receptors. Science 2004, 306, 1506–1507. [Google Scholar] [CrossRef]
- Jiang, N.; Dai, Q.; Su, X.; Fu, J.; Feng, X.; Peng, J. Role of PI3K/AKT Pathway in Cancer: The Framework of Malignant Behavior. Mol. Biol. Rep. 2020, 47, 4587–4629. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Saenz, A.; Dreyer, C.; Campbell, M.R.; Steri, V.; Gulizia, N.P.; Moasser, M.M. HER2 Amplification in Tumors Activates PI3K/Akt Signaling Independent of HER3. Cancer Res. 2018, 78, 3645–3658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheen, M.R.; Marotti, J.D.; Allegrezza, M.J.; Rutkowski, M.; Conejo-Garcia, J.R.; Fiering, S. Constitutively Activated PI3K Accelerates Tumor Initiation and Modifies Histopathology of Breast Cancer. Oncogenesis 2016, 5, e267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP Kinase Signalling Pathways in Cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [Green Version]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch. Pathol. Lab. Med. 2018, 142, 1364–1382. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, F.; Senkus, E.; Costa, A.; Papadopoulos, E.; Aapro, M.; André, F.; Harbeck, N.; Aguilar Lopez, B.; Barrios, C.H.; Bergh, J.; et al. 4th ESO–ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Ann. Oncol. 2018, 29, 1634–1657. [Google Scholar] [CrossRef]
- Swain, S.M.; Miles, D.; Kim, S.-B.; Im, Y.-H.; Im, S.-A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Loi, S.; Monturus, E.; et al. Pertuzumab, Trastuzumab, and Docetaxel for HER2-Positive Metastatic Breast Cancer (CLEOPATRA): End-of-Study Results from a Double-Blind, Randomised, Placebo-Controlled, Phase 3 Study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [Green Version]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N. Engl. J. Med 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Piccart-Gebhart, M.J.; Procter, M.; Leyland-Jones, B.; Goldhirsch, A.; Untch, M.; Smith, I.; Gianni, L.; Baselga, J.; Bell, R.; Jackisch, C.; et al. Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Breast Cancer. N. Engl. J. Med 2005, 353, 1659–1672. [Google Scholar] [CrossRef] [Green Version]
- Smith, I.; Procter, M.; Gelber, R.D.; Guillaume, S.; Feyereislova, A.; Dowsett, M.; Goldhirsch, A.; Untch, M.; Mariani, G.; Baselga, J.; et al. 2-Year Follow-up of Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Breast Cancer: A Randomised Controlled Trial. Lancet 2007, 369, 29–36. [Google Scholar] [CrossRef]
- Vogel, C.L.; Cobleigh, M.A.; Tripathy, D.; Gutheil, J.C.; Harris, L.N.; Fehrenbacher, L.; Slamon, D.J.; Murphy, M.; Novotny, W.F.; Burchmore, M.; et al. Efficacy and Safety of Trastuzumab as a Single Agent in First-Line Treatment of HER2-Overexpressing Metastatic Breast Cancer. J. Clin. Oncol. 2002, 20, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zheng, L.; Yang, Y.; Wang, H.; Dong, J.; Wang, C.; Zhang, Y.; Yu, X.; Wang, L.; Xia, T.; et al. A Monoclonal Antibody Targeting ErbB2 Domain III Inhibits ErbB2 Signaling and Suppresses the Growth of ErbB2-Overexpressing Breast Tumors. Oncogenesis 2016, 5, e211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Zhang, G.; Wang, Y.; Xu, N.; He, S.; Zhang, W.; Chen, M.; Liu, M.; Quan, L.; Bai, J.; et al. Inhibition of ErbB2 by Herceptin Reduces Survivin Expression via the ErbB2-β-Catenin/TCF4-Survivin Pathway in ErbB2-Overexpressed Breast Cancer Cells. Cancer Sci. 2010, 101, 1156–1162. [Google Scholar] [CrossRef]
- Davis, N.M.; Sokolosky, M.; Stadelman, K.; Abrams, S.L.; Libra, M.; Candido, S.; Nicoletti, F.; Polesel, J.; Maestro, R.; D’Assoro, A.; et al. Deregulation of the EGFR/PI3K/PTEN/Akt/MTORC1 Pathway in Breast Cancer: Possibilities for Therapeutic Intervention. Oncotarget 2014, 5, 4603–4650. [Google Scholar] [CrossRef] [Green Version]
- Fiszman, G.L.; Jasnis, M.A. Molecular Mechanisms of Trastuzumab Resistance in HER2 Overexpressing Breast Cancer. Int. J. Breast Cancer 2011, 2011, 352182. [Google Scholar] [CrossRef] [Green Version]
- Capietto, A.-H.; Martinet, L.; Fournié, J.-J. Stimulated Γδ T Cells Increase the In Vivo Efficacy of Trastuzumab in HER-2+ Breast Cancer. J. Immunol. 2011, 187, 1031–1038. [Google Scholar] [CrossRef] [Green Version]
- Nagata, Y.; Lan, K.-H.; Zhou, X.; Tan, M.; Esteva, F.J.; Sahin, A.A.; Klos, K.S.; Li, P.; Monia, B.P.; Nguyen, N.T.; et al. PTEN Activation Contributes to Tumor Inhibition by Trastuzumab, and Loss of PTEN Predicts Trastuzumab Resistance in Patients. Cancer Cell 2004, 6, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Nahta, R.; Esteva, F.J. HER2 Therapy: Molecular Mechanisms of Trastuzumab Resistance. Breast Cancer Res. 2006, 8, 215. [Google Scholar] [CrossRef]
- Scaltriti, M.; Rojo, F.; Ocana, A.; Anido, J.; Guzman, M.; Cortes, J.; Di Cosimo, S.; Matias-Guiu, X.; Ramon y Cajal, S.; Arribas, J.; et al. Expression of P95HER2, a Truncated Form of the HER2 Receptor, and Response to Anti-HER2 Therapies in Breast Cancer. JNCI J. Natl. Cancer Inst. 2007, 99, 628–638. [Google Scholar] [CrossRef] [Green Version]
- Hurvitz, S.A.; Martin, M.; Jung, K.H.; Huang, C.-S.; Harbeck, N.; Valero, V.; Stroyakovskiy, D.; Wildiers, H.; Campone, M.; Boileau, J.-F.; et al. Neoadjuvant Trastuzumab Emtansine and Pertuzumab in Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: Three-Year Outcomes from the Phase III KRISTINE Study. J. Clin. Oncol. 2019, 37, 2206–2216. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant Trastuzumab in HER2-Positive Breast Cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef] [Green Version]
- Rugo, H.S.; Im, S.-A.; Cardoso, F.; Cortés, J.; Curigliano, G.; Musolino, A.; Pegram, M.D.; Wright, G.S.; Saura, C.; Escrivá-de-Romaní, S.; et al. Efficacy of Margetuximab vs Trastuzumab in Patients with Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 573. [Google Scholar] [CrossRef]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.J.; Lutz, R.J.; et al. Targeting HER2-Positive Breast Cancer with Trastuzumab-DM1, an Antibody–Cytotoxic Drug Conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diéras, V.; Miles, D.; Verma, S.; Pegram, M.; Welslau, M.; Baselga, J.; Krop, I.E.; Blackwell, K.; Hoersch, S.; Xu, J.; et al. Trastuzumab Emtansine versus Capecitabine plus Lapatinib in Patients with Previously Treated HER2-Positive Advanced Breast Cancer (EMILIA): A Descriptive Analysis of Final Overall Survival Results from a Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2017, 18, 732–742. [Google Scholar] [CrossRef]
- Loibl, S.; Majewski, I.; Guarneri, V.; Nekljudova, V.; Holmes, E.; Bria, E.; Denkert, C.; Schem, C.; Sotiriou, C.; Loi, S.; et al. PIK3CA Mutations Are Associated with Reduced Pathological Complete Response Rates in Primary HER2-Positive Breast Cancer: Pooled Analysis of 967 Patients from Five Prospective Trials Investigating Lapatinib and Trastuzumab. Ann. Oncol. 2016, 27, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.A.; Codony-Servat, J.; Albanell, J.; Rojo, F.; Arribas, J.; Baselga, J. Trastuzumab (Herceptin), a Humanized Anti-Her2 Receptor Monoclonal Antibody, Inhibits Basal and Activated Her2 Ectodomain Cleavage in Breast Cancer Cells. Cancer Res. 2001, 61, 4744–4749. [Google Scholar]
- Ghosh, R.; Narasanna, A.; Wang, S.E.; Liu, S.; Chakrabarty, A.; Balko, J.M.; González-Angulo, A.M.; Mills, G.B.; Penuel, E.; Winslow, J.; et al. Trastuzumab Has Preferential Activity against Breast Cancers Driven by HER2 Homodimers. Cancer Res. 2011, 71, 1871–1882. [Google Scholar] [CrossRef] [Green Version]
- Ríos-Luci, C.; García-Alonso, S.; Díaz-Rodríguez, E.; Nadal-Serrano, M.; Arribas, J.; Ocaña, A.; Pandiella, A. Resistance to the Antibody–Drug Conjugate T-DM1 Is Based in a Reduction in Lysosomal Proteolytic Activity. Cancer Res. 2017, 77, 4639–4651. [Google Scholar] [CrossRef] [Green Version]
- Sauveur, J.; Matera, E.-L.; Chettab, K.; Valet, P.; Guitton, J.; Savina, A.; Dumontet, C. Esophageal Cancer Cells Resistant to T-DM1 Display Alterations in Cell Adhesion and the Prostaglandin Pathway. Oncotarget 2018, 9, 21141–21155. [Google Scholar] [CrossRef] [Green Version]
- Columbus, G. Trastuzumab Deruxtecan Receives Accelerated Approval by FDA for HER2+ Breast Cancer. 2019. Available online: https://www.targetedonc.com/view/trastuzumab-deruxtecan-receives-accelerated-approval-by-fda-for-her2-breast-cancer (accessed on 10 March 2022).
- Rinnerthaler, G.; Gampenrieder, S.; Greil, R. HER2 Directed Antibody-Drug-Conjugates beyond T-DM1 in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Sáez, O.; Chic, N.; Pascual, T.; Adamo, B.; Vidal, M.; González-Farré, B.; Sanfeliu, E.; Schettini, F.; Conte, B.; Brasó-Maristany, F.; et al. Frequency and Spectrum of PIK3CA Somatic Mutations in Breast Cancer. Breast Cancer Res. 2020, 22, 45. [Google Scholar] [CrossRef] [PubMed]
- Verret, B.; Cortes, J.; Bachelot, T.; Andre, F.; Arnedos, M. Efficacy of PI3K Inhibitors in Advanced Breast Cancer. Ann. Oncol. 2019, 30, x12–x20. [Google Scholar] [CrossRef] [PubMed]
- André, F.; Hurvitz, S.; Fasolo, A.; Tseng, L.-M.; Jerusalem, G.; Wilks, S.; O’Regan, R.; Isaacs, C.; Toi, M.; Burris, H.A.; et al. Molecular Alterations and Everolimus Efficacy in Human Epidermal Growth Factor Receptor 2–Overexpressing Metastatic Breast Cancers: Combined Exploratory Biomarker Analysis From BOLERO-1 and BOLERO-3. J. Clin. Oncol. 2016, 34, 2115–2124. [Google Scholar] [CrossRef]
- Segovia-Mendoza, M.; González-González, M.E.; Barrera, D.; Díaz, L.; García-Becerra, R. Efficacy and Mechanism of Action of the Tyrosine Kinase Inhibitors Gefitinib, Lapatinib and Neratinib in the Treatment of HER2-Positive Breast Cancer: Preclinical and Clinical Evidence. Am. J. Cancer Res. 2015, 5, 2531–2561. [Google Scholar]
- Esparís-Ogando, A.; Montero, J.; Arribas, J.; Ocaña, A.; Pandiella, A. Targeting the EGF/HER Ligand-Receptor System in Cancer. Curr. Pharm. Des. 2016, 22, 5887–5898. [Google Scholar] [CrossRef]
- Chan, A.; Delaloge, S.; Holmes, F.A.; Moy, B.; Iwata, H.; Harvey, V.J.; Robert, N.J.; Silovski, T.; Gokmen, E.; Von Minckwitz, G.; et al. Neratinib after Trastuzumab-Based Adjuvant Therapy in Patients with HER2-Positive Breast Cancer (ExteNET): A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 2016, 17, 367–377. [Google Scholar] [CrossRef]
- Singh, H.; Walker, A.J.; Amiri-Kordestani, L.; Cheng, J.; Tang, S.; Balcazar, P.; Barnett-Ringgold, K.; Palmby, T.R.; Cao, X.; Zheng, N.; et al. U.S. Food and Drug Administration Approval: Neratinib for the Extended Adjuvant Treatment of Early-Stage HER2-Positive Breast Cancer. Clin. Cancer Res. 2018, 24, 3486–3491. [Google Scholar] [CrossRef] [Green Version]
- Leo, C.P.; Hentschel, B.; Szucs, T.D.; Leo, C. FDA and EMA Approvals of New Breast Cancer Drugs—A Comparative Regulatory Analysis. Cancers 2020, 12, 437. [Google Scholar] [CrossRef] [Green Version]
- De Azambuja, E.; Holmes, A.P.; Piccart-Gebhart, M.; Holmes, E.; Di Cosimo, S.; Swaby, R.F.; Untch, M.; Jackisch, C.; Lang, I.; Smith, I.; et al. Lapatinib with Trastuzumab for HER2-Positive Early Breast Cancer (NeoALTTO): Survival Outcomes of a Randomised, Open-Label, Multicentre, Phase 3 Trial and Their Association with Pathological Complete Response. Lancet Oncol. 2014, 15, 1137–1146. [Google Scholar] [CrossRef] [Green Version]
- Savas, P.; Salgado, R.; Denkert, C.; Sotiriou, C.; Darcy, P.K.; Smyth, M.J.; Loi, S. Clinical Relevance of Host Immunity in Breast Cancer: From TILs to the Clinic. Nat. Rev. Clin. Oncol. 2016, 13, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Nitta, H.; Wei, L.; Banks, P.M.; Lustberg, M.; Wesolowski, R.; Ramaswamy, B.; Parwani, A.V.; Li, Z. PD-L1 Expression and CD8-Positive T Cells Are Associated with Favorable Survival in HER2-Positive Invasive Breast Cancer. Breast J. 2018, 24, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.M. Pembrolizumab: First Global Approval. Drugs 2014, 74, 1973–1981. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Atezolizumab: First Global Approval. Drugs 2016, 76, 1227–1232. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, H.; Chen, B. Nivolumab as Programmed Death-1 (PD-1) Inhibitor for Targeted Immunotherapy in Tumor. J. Cancer 2017, 8, 410–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieto, C.; Vega, M.A.; Martín del Valle, E.M. Trastuzumab: More than a Guide in HER2-Positive Cancer Nanomedicine. Nanomaterials 2020, 10, 1674. [Google Scholar] [CrossRef] [PubMed]
- White, B.E.; White, M.K.; Adhvaryu, H.; Makhoul, I.; Nima, Z.A.; Biris, A.S.; Ali, N. Nanotechnology Approaches to Addressing HER2-Positive Breast Cancer. Cancer Nano 2020, 11, 12. [Google Scholar] [CrossRef]
- Kumar, M.; Rajnikanth, P.S. A Mini-Review on HER2 Positive Breast Cancer and Its Metastasis: Resistance and Treatment Strategies. Curr. Nanomed. (Former. Recent Pat. Nanomed.) 2020, 10, 36–47. [Google Scholar] [CrossRef]
- Marques, A.C.; Costa, P.J.; Velho, S.; Amaral, M.H. Functionalizing Nanoparticles with Cancer-Targeting Antibodies: A Comparison of Strategies. J. Control. Release 2020, 320, 180–200. [Google Scholar] [CrossRef]
- Juan, A.; Cimas, F.J.; Bravo, I.; Pandiella, A.; Ocaña, A.; Alonso-Moreno, C. An Overview of Antibody Conjugated Polymeric Nanoparticles for Breast Cancer Therapy. Pharmaceutics 2020, 12, 802. [Google Scholar] [CrossRef]
- Lin, X.; O’Reilly Beringhs, A.; Lu, X. Applications of Nanoparticle-Antibody Conjugates in Immunoassays and Tumor Imaging. AAPS J. 2021, 23, 43. [Google Scholar] [CrossRef]
- Bloise, N.; Massironi, A.; Della Pina, C.; Alongi, J.; Siciliani, S.; Manfredi, A.; Biggiogera, M.; Rossi, M.; Ferruti, P.; Ranucci, E.; et al. Extra-Small Gold Nanospheres Decorated with a Thiol Functionalized Biodegradable and Biocompatible Linear Polyamidoamine as Nanovectors of Anticancer Molecules. Front. Bioeng. Biotechnol. 2020, 8, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Z.; Chattopadhyay, N.; Yang, K.; Kwon, Y.L.; Yook, S.; Pignol, J.-P.; Reilly, R.M. 111In-Labeled Trastuzumab-Modified Gold Nanoparticles Are Cytotoxic in Vitro to HER2-Positive Breast Cancer Cells and Arrest Tumor Growth in Vivo in Athymic Mice after Intratumoral Injection. Nucl. Med. Biol. 2016, 43, 818–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziawer, Ł.; Majkowska-Pilip, A.; Gaweł, D.; Godlewska, M.; Pruszyński, M.; Jastrzębski, J.; Wąs, B.; Bilewicz, A. Trastuzumab-Modified Gold Nanoparticles Labeled with 211At as a Prospective Tool for Local Treatment of HER2-Positive Breast Cancer. Nanomaterials 2019, 9, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, X.; Guo, X.; Niu, X.; An, W.; Li, S.; Liu, Z.; Yang, Y.; Wang, N.; Jiang, Q.; Yan, C.; et al. Photothermal Therapeutic Application of Gold Nanorods-Porphyrin-Trastuzumab Complexes in HER2-Positive Breast Cancer. Sci Rep. 2017, 7, 42069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Liu, J.; Li, X.; Li, F.; Lee, R.J.; Sun, F.; Li, Y.; Liu, Z.; Teng, L. Trastuzumab-Coated Nanoparticles Loaded with Docetaxel for Breast Cancer Therapy. Dose-Response 2019, 17, 1559325819872583. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Zhao, J.; Zhang, Z.; Gao, Y.; Zhou, Y.; Teng, L.; Li, Y. Enhanced Delivery of Paclitaxel Using Electrostatically-Conjugated Herceptin-Bearing PEI/PLGA Nanoparticles against HER-Positive Breast Cancer Cells. Int. J. Pharm. 2016, 497, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Naruphontjirakul, P.; Viravaidya-Pasuwat, K. Development of Anti-HER2-Targeted Doxorubicin–Core-Shell Chitosan Nanoparticles for the Treatment of Human Breast Cancer. Int. J. Nanomed. 2019, 14, 4105–4121. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Chen, J.; Xie, F.; Bao, W.; Xu, H.; Wang, H.; Xu, Y.; Du, Z. Herceptin-Conjugated Paclitaxel Loaded PCL-PEG Worm-like Nanocrystal Micelles for the Combinatorial Treatment of HER2-Positive Breast Cancer. Biomaterials 2019, 222, 119420. [Google Scholar] [CrossRef]
- Varshosaz, J.; Ghassami, E.; Noorbakhsh, A.; Minaiyan, M.; Jahanian-Najafabadi, A. Trastuzumab-conjugated Nanoparticles Composed of Poly(Butylene Adipate-Co-butylene Terephthalate) Prepared by Electrospraying Technique for Targeted Delivery of Docetaxel. IET Nanobiotechnol. 2019, 13, 829–833. [Google Scholar] [CrossRef]
- Domínguez-Ríos, R.; Sánchez-Ramírez, D.R.; Ruiz-Saray, K.; Oceguera-Basurto, P.E.; Almada, M.; Juárez, J.; Zepeda-Moreno, A.; del Toro-Arreola, A.; Topete, A.; Daneri-Navarro, A. Cisplatin-Loaded PLGA Nanoparticles for HER2 Targeted Ovarian Cancer Therapy. Colloids Surf. B Biointerfaces 2019, 178, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Cędrowska, E.; Pruszyński, M.; Gawęda, W.; Żuk, M.; Krysiński, P.; Bruchertseifer, F.; Morgenstern, A.; Karageorgou, M.-A.; Bouziotis, P.; Bilewicz, A. Trastuzumab Conjugated Superparamagnetic Iron Oxide Nanoparticles Labeled with 225Ac as a Perspective Tool for Combined α-Radioimmunotherapy and Magnetic Hyperthermia of HER2-Positive Breast Cancer. Molecules 2020, 25, 1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korangath, P.; Barnett, J.D.; Sharma, A.; Henderson, E.T.; Stewart, J.; Yu, S.-H.; Kandala, S.K.; Yang, C.-T.; Caserto, J.S.; Hedayati, M.; et al. Nanoparticle Interactions with Immune Cells Dominate Tumor Retention and Induce T Cell–Mediated Tumor Suppression in Models of Breast Cancer. Sci. Adv. 2020, 6, eaay1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, N.R.; Van, S.Y.; Hong, S.H.; Kim, S.-Y.; Kim, M.; Lee, J.S.; Lee, S.J.; Lee, Y.; Kwon, I.K.; Oh, S.J. Dual PH- and GSH-Responsive Degradable PEGylated Graphene Quantum Dot-Based Nanoparticles for Enhanced HER2-Positive Breast Cancer Therapy. Nanomaterials 2020, 10, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, M.; Gao, F.; Yu, C.; Zeng, M.; He, G.; Wu, Y.; Su, Y.; Hu, N.; Zhou, Z.; Yang, Z.; et al. Dual-Targeted Therapy in HER2-Positive Breast Cancer Cells with the Combination of Carbon Dots/HER3 SiRNA and Trastuzumab. Nanotechnology 2020, 31, 335102. [Google Scholar] [CrossRef]
- Tanaka, S.; Matsunami, N.; Morishima, H.; Oda, N.; Takashima, T.; Noda, S.; Kashiwagi, S.; Tauchi, Y.; Asano, Y.; Kimura, K.; et al. De-Escalated Neoadjuvant Therapy with Nanoparticle Albumin-Bound Paclitaxel and Trastuzumab for Low-Risk Pure HER2 Breast Cancer. Cancer Chemother Pharm. 2019, 83, 1099–1104. [Google Scholar] [CrossRef]
- Duan, D.; Wang, A.; Ni, L.; Zhang, L.; Yan, X.; Jiang, Y.; Mu, H.; Wu, Z.; Sun, K.; Li, Y. Nanoparticle Interactions with Immune Cells Dominate Tumor Retention and Induce T Cell–Mediated Tumor Suppression in Models of Breast Cancer. Int. J. Nanomed. 2018, 13, 1831–1840. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.; Shin, J.; Wu, J.; Omstead, D.T.; Kiziltepe, T.; Littlepage, L.E.; Bilgicer, B. Engineering Peptide-Targeted Liposomal Nanoparticles Optimized for Improved Selectivity for HER2-Positive Breast Cancer Cells to Achieve Enhanced in Vivo Efficacy. J. Control. Release 2020, 322, 530–541. [Google Scholar] [CrossRef]
- Houdaihed, L.; Evans, J.C.; Allen, C. Dual-Targeted Delivery of Nanoparticles Encapsulating Paclitaxel and Everolimus: A Novel Strategy to Overcome Breast Cancer Receptor Heterogeneity. Pharm. Res. 2020, 37, 39. [Google Scholar] [CrossRef]
- Vorotnikov, Y.A.; Novikova, E.D.; Solovieva, A.O.; Shanshin, D.V.; Tsygankova, A.R.; Shcherbakov, D.N.; Efremova, O.A.; Shestopalov, M.A. Single-Domain Antibody C7b for Address Delivery of Nanoparticles to HER2-Positive Cancers. Nanoscale 2020, 12, 21885–21894. [Google Scholar] [CrossRef]
- Holliger, P.; Hudson, P.J. Engineered Antibody Fragments and the Rise of Single Domains. Nat. Biotechnol. 2005, 23, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Van Audenhove, I.; Gettemans, J. Nanobodies as Versatile Tools to Understand, Diagnose, Visualize and Treat Cancer. EBioMedicine 2016, 8, 40–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Jothar, L.; Beztsinna, N.; van Nostrum, C.F.; Hennink, W.E.; Oliveira, S. Selective Cytotoxicity to HER2 Positive Breast Cancer Cells by Saporin-Loaded Nanobody-Targeted Polymeric Nanoparticles in Combination with Photochemical Internalization. Mol. Pharm. 2019, 16, 1633–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Dong, J.; Cheng, F.; Li, C.; Wang, H.; Sun, T.; He, W.; Wang, Q. Controlling Conjugated Antibodies at the Molecular Level for Active Targeting Nanoparticles toward HER2-Positive Cancer Cells. Mol. Pharm. 2021, 18, 1196–1207. [Google Scholar] [CrossRef]
- Shi, X.; Cheng, Q.; Hou, T.; Han, M.; Smbatyan, G.; Lang, J.E.; Epstein, A.L.; Lenz, H.-J.; Zhang, Y. Genetically Engineered Cell-Derived Nanoparticles for Targeted Breast Cancer Immunotherapy. Mol. Ther. 2020, 28, 536–547. [Google Scholar] [CrossRef]
- Okarvi, S.M.; AlJammaz, I. Development of the Tumor-Specific Antigen-Derived Synthetic Peptides as Potential Candidates for Targeting Breast and Other Possible Human Carcinomas. Molecules 2019, 24, 3142. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Gangalum, P.R.; Galstyan, A.; Fox, I.; Patil, R.; Hubbard, P.; Murali, R.; Ljubimova, J.Y.; Holler, E. HER2-Positive Breast Cancer Targeting and Treatment by a Peptide-Conjugated Mini Nanodrug. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Mu, Q.; Kievit, F.M.; Kant, R.J.; Lin, G.; Jeon, M.; Zhang, M. Anti-HER2/Neu Peptide-Conjugated Iron Oxide Nanoparticles for Targeted Delivery of Paclitaxel to Breast Cancer Cells. Nanoscale 2015, 7, 18010–18014. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Jing, D.; Jiang, N.; Rojalin, T.; Baehr, C.M.; Zhang, D.; Xiao, W.; Wu, Y.; Cong, Z.; Li, J.J.; et al. Transformable Peptide Nanoparticles Arrest HER2 Signalling and Cause Cancer Cell Death in Vivo. Nat. Nanotechnol. 2020, 15, 145–153. [Google Scholar] [CrossRef]
- Zhu, G.; Chen, X. Aptamer-Based Targeted Therapy. Adv. Drug Deliv. Rev. 2018, 134, 65–78. [Google Scholar] [CrossRef]
- Saleh, T.; Soudi, T.; Shojaosadati, S.A. Aptamer Functionalized Curcumin-Loaded Human Serum Albumin (HSA) Nanoparticles for Targeted Delivery to HER-2 Positive Breast Cancer Cells. Int. J. Biol. Macromol. 2019, 130, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dam, D.H.M.; Ha, J.W.; Yue, J.; Odom, T.W. Enhanced Human Epidermal Growth Factor Receptor 2 Degradation in Breast Cancer Cells by Lysosome-Targeting Gold Nanoconstructs. ACS Nano 2015, 9, 9859–9867. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, M.; Liu, T.; Liu, J.; Xie, Y.; Zhang, J.; Xu, S.; Liu, H. A Dual-Functional HER2 Aptamer-Conjugated, PH-Activated Mesoporous Silica Nanocarrier-Based Drug Delivery System Provides In Vitro Synergistic Cytotoxicity in HER2-Positive Breast Cancer Cells. Int. J. Nanomed. 2019, 14, 4029–4044. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Zhan, Y.; Zhang, Y.; Shao, X.; Xie, X.; Mao, C.; Cui, W.; Li, Q.; Shi, J.; Li, J.; et al. An Intelligent DNA Nanorobot with in Vitro Enhanced Protein Lysosomal Degradation of HER2. Nano Lett. 2019, 19, 4505–4517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, V.; Baines, A.J. Spectrin and Ankyrin-Based Pathways: Metazoan Inventions for Integrating Cells into Tissues. Physiol. Rev. 2001, 81, 1353–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binz, H.K.; Amstutz, P.; Plückthun, A. Engineering Novel Binding Proteins from Nonimmunoglobulin Domains. Nat. Biotechnol. 2005, 23, 1257–1268. [Google Scholar] [CrossRef]
- Stumpp, M.T.; Dawson, K.M.; Binz, H.K. Beyond Antibodies: The DARPin® Drug Platform. BioDrugs 2020, 34, 423–433. [Google Scholar] [CrossRef]
- Moisseiev, E.; Loewenstein, A. Abicipar Pegol—A Novel Anti-VEGF Therapy with a Long Duration of Action. Eye 2020, 34, 605–606. [Google Scholar] [CrossRef] [Green Version]
- Zahnd, C.; Pecorari, F.; Straumann, N.; Wyler, E.; Plückthun, A. Selection and Characterization of Her2 Binding-Designed Ankyrin Repeat Proteins. J. Biol. Chem. 2006, 281, 35167–35175. [Google Scholar] [CrossRef] [Green Version]
- Theurillat, J.-P.; Dreier, B.; Nagy-Davidescu, G.; Seifert, B.; Behnke, S.; Zürrer-Härdi, U.; Ingold, F.; Plückthun, A.; Moch, H. Designed Ankyrin Repeat Proteins: A Novel Tool for Testing Epidermal Growth Factor Receptor 2 Expression in Breast Cancer. Mod. Pathol. 2010, 23, 1289–1297. [Google Scholar] [CrossRef] [Green Version]
- Zahnd, C.; Kawe, M.; Stumpp, M.T.; de Pasquale, C.; Tamaskovic, R.; Nagy-Davidescu, G.; Dreier, B.; Schibli, R.; Binz, H.K.; Waibel, R.; et al. Efficient Tumor Targeting with High-Affinity Designed Ankyrin Repeat Proteins: Effects of Affinity and Molecular Size. Cancer Res. 2010, 70, 1595–1605. [Google Scholar] [CrossRef] [Green Version]
- Shipunova, V.O.; Kotelnikova, P.A.; Aghayeva, U.F.; Stremovskiy, O.A.; Novikov, I.A.; Schulga, A.A.; Nikitin, M.P.; Deyev, S.M. Self-Assembling Nanoparticles Biofunctionalized with Magnetite-Binding Protein for the Targeted Delivery to HER2/Neu Overexpressing Cancer Cells. J. Magn. Magn. Mater. 2019, 469, 450–455. [Google Scholar] [CrossRef]
- Li, D.-L.; Tan, J.-E.; Tian, Y.; Huang, S.; Sun, P.-H.; Wang, M.; Han, Y.-J.; Li, H.-S.; Wu, H.-B.; Zhang, X.-M.; et al. Multifunctional Superparamagnetic Nanoparticles Conjugated with Fluorescein-Labeled Designed Ankyrin Repeat Protein as an Efficient HER2-Targeted Probe in Breast Cancer. Biomaterials 2017, 147, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Guryev, E.L.; Shilyagina, N.Y.; Kostyuk, A.B.; Sencha, L.M.; Balalaeva, I.V.; Vodeneev, V.A.; Kutova, O.M.; Lyubeshkin, A.V.; Yakubovskaya, R.I.; Pankratov, A.A.; et al. Preclinical Study of Biofunctional Polymer-Coated Upconversion Nanoparticles. Toxicol. Sci. 2019, 170, 123–132. [Google Scholar] [CrossRef]
- Dong, Y.; Li, W.; Gu, Z.; Xing, R.; Ma, Y.; Zhang, Q.; Liu, Z. Inhibition of HER2-Positive Breast Cancer Growth by Blocking the HER2 Signaling Pathway with HER2-Glycan-Imprinted Nanoparticles. Angew. Chem. Int. Ed. 2019, 58, 10621–10625. [Google Scholar] [CrossRef]
- Yezhelyev, M.V.; Gao, X.; Xing, Y.; Al-Hajj, A.; Nie, S.; O’Regan, R.M. Emerging Use of Nanoparticles in Diagnosis and Treatment of Breast Cancer. Lancet Oncol. 2006, 7, 657–667. [Google Scholar] [CrossRef]
- Zahmatkeshan, M.; Gheybi, F.; Rezayat, S.M.; Jaafari, M.R. Improved Drug Delivery and Therapeutic Efficacy of PEgylated Liposomal Doxorubicin by Targeting Anti-HER2 Peptide in Murine Breast Tumor Model. Eur. J. Pharm. Sci. 2016, 86, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Akhtari, J.; Rezayat, S.M.; Teymouri, M.; Alavizadeh, S.H.; Gheybi, F.; Badiee, A.; Jaafari, M.R. Targeting, Bio Distributive and Tumor Growth Inhibiting Characterization of Anti-HER2 Affibody Coupling to Liposomal Doxorubicin Using BALB/c Mice Bearing TUBO Tumors. Int. J. Pharm. 2016, 505, 89–95. [Google Scholar] [CrossRef]
- Niza, E.; Noblejas-López, M.D.M.; Bravo, I.; Nieto-Jiménez, C.; Castro-Osma, J.A.; Canales-Vázquez, J.; Lara-Sanchez, A.; Galán Moya, E.M.; Burgos, M.; Ocaña, A.; et al. Trastuzumab-Targeted Biodegradable Nanoparticles for Enhanced Delivery of Dasatinib in HER2+ Metastasic Breast Cancer. Nanomaterials 2019, 9, 1793. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.; Zheng, X.; Pang, X.; Zhang, Z.; Zhang, Q. Incorporation of Lapatinib into Human Serum Albumin Nanoparticles with Enhanced Anti-Tumor Effects in HER2-Positive Breast Cancer. Colloids Surf. B Biointerfaces 2015, 136, 817–827. [Google Scholar] [CrossRef]
- Dana, H.; Chalbatani, G.M.; Mahmoodzadeh, H.; Karimloo, R.; Rezaiean, O.; Moradzadeh, A.; Mehmandoost, N.; Moazzen, F.; Mazraeh, A.; Marmari, V.; et al. Molecular Mechanisms and Biological Functions of SiRNA. Int. J. Biomed. Sci 2017, 13, 48–57. [Google Scholar] [PubMed]
- Mainini, F.; Eccles, M.R. Lipid and Polymer-Based Nanoparticle SiRNA Delivery Systems for Cancer Therapy. Molecules 2020, 25, 2692. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Ngamcherdtrakul, W.; Reda, M.; Hu, Z.; Gray, J.W.; Yantasee, W. Lack of Acquired Resistance in HER2-Positive Breast Cancer Cells after Long-Term HER2 SiRNA Nanoparticle Treatment. PLoS ONE 2018, 13, e0198141. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Hu, Z.; Ngamcherdtrakul, W.; Castro, D.J.; Morry, J.; Reda, M.M.; Gray, J.W.; Yantasee, W. Therapeutic SiRNA for Drug-Resistant HER2-Positive Breast Cancer. Oncotarget 2016, 7, 14727–14741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristofolini, T.; Dalmina, M.; Sierra, J.A.; Silva, A.H.; Pasa, A.A.; Pittella, F.; Creczynski-Pasa, T.B. Multifunctional Hybrid Nanoparticles as Magnetic Delivery Systems for SiRNA Targeting the HER2 Gene in Breast Cancer Cells. Mater. Sci. Eng. C 2020, 109, 110555. [Google Scholar] [CrossRef]
- Zhang, L.; Mu, C.; Zhang, T.; Wang, Y.; Wang, Y.; Fan, L.; Liu, C.; Chen, H.; Shen, J.; Wei, K.; et al. Systemic Delivery of Aptamer-Conjugated XBP1 SiRNA Nanoparticles for Efficient Suppression of HER2+ Breast Cancer. ACS Appl. Mater. Interfaces 2020, 12, 32360–32371. [Google Scholar] [CrossRef]
- Rybakova, Y.; Kowalski, P.S.; Huang, Y.; Gonzalez, J.T.; Heartlein, M.W.; DeRosa, F.; Delcassian, D.; Anderson, D.G. MRNA Delivery for Therapeutic Anti-HER2 Antibody Expression In Vivo. Mol. Ther. 2019, 27, 1415–1423. [Google Scholar] [CrossRef]
- Cai, Z.; Yook, S.; Lu, Y.; Bergstrom, D.; Winnik, M.A.; Pignol, J.-P.; Reilly, R.M. Local Radiation Treatment of HER2-Positive Breast Cancer Using Trastuzumab-Modified Gold Nanoparticles Labeled with 177Lu. Pharm. Res. 2017, 34, 579–590. [Google Scholar] [CrossRef]
- Guryev, E.L.; Volodina, N.O.; Shilyagina, N.Y.; Gudkov, S.V.; Balalaeva, I.V.; Volovetskiy, A.B.; Lyubeshkin, A.V.; Sen’, A.V.; Ermilov, S.A.; Vodeneev, V.A.; et al. Radioactive (90 Y) Upconversion Nanoparticles Conjugated with Recombinant Targeted Toxin for Synergistic Nanotheranostics of Cancer. Proc. Natl. Acad. Sci. USA 2018, 115, 9690–9695. [Google Scholar] [CrossRef] [Green Version]
- Mironova, K.E.; Khochenkov, D.A.; Generalova, A.N.; Rocheva, V.V.; Sholina, N.V.; Nechaev, A.V.; Semchishen, V.A.; Deyev, S.M.; Zvyagin, A.V.; Khaydukov, E.V. Ultraviolet Phototoxicity of Upconversion Nanoparticles Illuminated with Near-Infrared Light. Nanoscale 2017, 9, 14921–14928. [Google Scholar] [CrossRef]
- Aman, N.A.; Doukoure, B.; Koffi, K.D.; Koui, B.S.; Traore, Z.C.; Kouyate, M.; Effi, A.B. HER2 Overexpression and Correlation with Other Significant Clinicopathologic Parameters in Ivorian Breast Cancer Women. BMC Clin. Pathol. 2019, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Vi, C.; Mandarano, G.; Shigdar, S. Diagnostics and Therapeutics in Targeting HER2 Breast Cancer: A Novel Approach. Int. J. Mol. Sci. 2021, 22, 6163. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, C.; Schiff, R. HER2: Biology, Detection, and Clinical Implications. Arch. Pathol. Lab. Med. 2011, 135, 55–62. [Google Scholar] [CrossRef]
- Salahandish, R.; Ghaffarinejad, A.; Naghib, S.M.; Majidzadeh-A, K.; Zargartalebi, H.; Sanati-Nezhad, A. Nano-Biosensor for Highly Sensitive Detection of HER2 Positive Breast Cancer. Biosens. Bioelectron. 2018, 117, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.A.; Marshall, D.A.; Haas, J.S.; Elkin, E.B.; Liang, S.-Y.; Hassett, M.J.; Ferrusi, I.; Brock, J.E.; Van Bebber, S.L. Clinical Practice Patterns and Cost Effectiveness of Human Epidermal Growth Receptor 2 Testing Strategies in Breast Cancer Patients. Cancer 2009, 115, 5166–5174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Yu, X.; Chen, Z.; Yang, T.; Yang, D.; Liu, Q.; Du, K.; Li, B.; Wang, Z.; Li, S.; et al. Aptamer Selection and Applications for Breast Cancer Diagnostics and Therapy. J. Nanobiotechnol. 2017, 15, 81. [Google Scholar] [CrossRef] [PubMed]
- Mathenge, E.G.; Dean, C.A.; Clements, D.; Vaghar-Kashani, A.; Photopoulos, S.; Coyle, K.M.; Giacomantonio, M.; Malueth, B.; Nunokawa, A.; Jordan, J.; et al. Core Needle Biopsy of Breast Cancer Tumors Increases Distant Metastases in a Mouse Model. Neoplasia 2014, 16, 950–960. [Google Scholar] [CrossRef] [Green Version]
- Castro-Giner, F.; Aceto, N. Tracking Cancer Progression: From Circulating Tumor Cells to Metastasis. Genome Med. 2020, 12, 31. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Li, X.; Zhu, L.; Liu, J.; Xu, W.; Wang, P. Preclinical and Clinical Applications of Specific Molecular Imaging for HER2-Positive Breast Cancer. Cancer Biol. Med. 2017, 14, 271. [Google Scholar] [CrossRef] [Green Version]
- Gao, D.; Gao, J.; Xu, M.; Cao, Z.; Zhou, L.; Li, Y.; Xie, X.; Jiang, Q.; Wang, W.; Liu, J. Targeted Ultrasound-Triggered Phase Transition Nanodroplets for Her2-Overexpressing Breast Cancer Diagnosis and Gene Transfection. Mol. Pharm. 2017, 14, 984–998. [Google Scholar] [CrossRef]
- Busquets, M.A.; Estelrich, J.; Sánchez-Martín, M.J. Nanoparticles in Magnetic Resonance Imaging: From Simple to Dual Contrast Agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Xu, L.; Liang, C.; Wang, C.; Peng, R.; Liu, Z. Photothermal Therapy with Immune-Adjuvant Nanoparticles Together with Checkpoint Blockade for Effective Cancer Immunotherapy. Nat. Commun. 2016, 7, 13193. [Google Scholar] [CrossRef] [PubMed]
- Pellico, J.; Llop, J.; Fernández-Barahona, I.; Bhavesh, R.; Ruiz-Cabello, J.; Herranz, F. Iron Oxide Nanoradiomaterials: Combining Nanoscale Properties with Radioisotopes for Enhanced Molecular Imaging. Contrast Media Mol. Imaging 2017, 2017, 1549580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.-J.; Cheng, T.-H.; Chen, C.-Y.; Hsu, S.C.N.; Cheng, T.-L.; Liu, G.-C.; Wang, Y.-M. Targeted Herceptin–Dextran Iron Oxide Nanoparticles for Noninvasive Imaging of HER2/Neu Receptors Using MRI. J. Biol. Inorg. Chem. 2009, 14, 253–260. [Google Scholar] [CrossRef]
- Alric, C.; Hervé-Aubert, K.; Aubrey, N.; Melouk, S.; Lajoie, L.; Même, W.; Même, S.; Courbebaisse, Y.; Ignatova, A.A.; Feofanov, A.V.; et al. Targeting HER2-Breast Tumors with ScFv-Decorated Bimodal Nanoprobes. J. Nanobiotechnol. 2018, 16, 18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ni, Q.; Xu, C.; Wan, B.; Geng, Y.; Zheng, G.; Yang, Z.; Tao, J.; Zhao, Y.; Wen, J.; et al. Smart Bacterial Magnetic Nanoparticles for Tumor-Targeting Magnetic Resonance Imaging of HER2-Positive Breast Cancers. ACS Appl. Mater. Interfaces 2019, 11, 3654–3665. [Google Scholar] [CrossRef]
- Lim, E.-K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.-M.; Lee, K. Nanomaterials for Theranostics: Recent Advances and Future Challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef]
- Hardman, R. A Toxicologic Review of Quantum Dots: Toxicity Depends on Physicochemical and Environmental Factors. Environ. Health Perspect. 2006, 114, 165–172. [Google Scholar] [CrossRef]
- Wei, T.; Xing, H.; Wang, H.; Zhang, Y.; Wang, J.; Shen, J.; Dai, Z. Bovine Serum Albumin Encapsulation of near Infrared Fluorescent Nano-Probe with Low Nonspecificity and Cytotoxicity for Imaging of HER2-Positive Breast Cancer Cells. Talanta 2020, 210, 120625. [Google Scholar] [CrossRef]
- Seifalian, A.; Rizvi, S.; Rouhi, S.; Taniguchi, S.; Yang, S.Y.; Green, M.; Keshtgar, M. Near-Infrared Quantum Dots for HER2 Localization and Imaging of Cancer Cells. Int. J. Nanomed. 2014, 9, 1323. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, W.; Bu, X.; Wei, Z.; Geng, L.; Wu, Y.; Dong, C.; Li, L.; Zhang, D.; Yang, S.; et al. Microarray Based Screening of Peptide Nano Probes for HER2 Positive Tumor. Anal. Chem. 2015, 87, 8367–8372. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Gomes, F.; Bode, J.; Sukhanova, A.; Bozrova, S.V.; Saccomano, M.; Mitkovski, M.; Krueger, J.E.; Wege, A.K.; Stuehmer, W.; Samokhvalov, P.S.; et al. Single- and Two-Photon Imaging of Human Micrometastases and Disseminated Tumour Cells with Conjugates of Nanobodies and Quantum Dots. Sci. Rep. 2018, 8, 4595. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.; Blykers, A.; Vaneycken, I.; D’Huyvetter, M.; Heemskerk, J.; Lahoutte, T.; Devoogdt, N.; Caveliers, V. 18F-Nanobody for PET Imaging of HER2 Overexpressing Tumors. Nucl. Med. Biol. 2016, 43, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Ahlgren, S.; Wållberg, H.; Tran, T.A.; Widström, C.; Hjertman, M.; Abrahmsén, L.; Berndorff, D.; Dinkelborg, L.M.; Cyr, J.E.; Feldwisch, J.; et al. Targeting of HER2-Expressing Tumors with a Site-Specifically 99m Tc-Labeled Recombinant Affibody Molecule, ZHER2: 2395, with C-Terminally Engineered Cysteine. J. Nucl Med. 2009, 50, 781–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welch, M.J.; Hawker, C.J.; Wooley, K.L. The Advantages of Nanoparticles for PET. J. Nucl Med. 2009, 50, 1743–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Shields, A.F.; Siegel, B.A.; Miller, K.D.; Krop, I.; Ma, C.X.; LoRusso, P.M.; Munster, P.N.; Campbell, K.; Gaddy, D.F.; et al. 64Cu-MM-302 Positron Emission Tomography Quantifies Variability of Enhanced Permeability and Retention of Nanoparticles in Relation to Treatment Response in Patients with Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 4190–4202. [Google Scholar] [CrossRef] [Green Version]
- Rainone, P.; Riva, B.; Belloli, S.; Sudati, F.; Ripamonti, M.; Verderio, P.; Colombo, M.; Colzani, B.; Gilardi, M.C.; Moresco, R.M.; et al. Development of 99mTc-Radiolabeled Nanosilica for Targeted Detection of HER2-Positive Breast Cancer. Int. J. Nanomed. 2017, 12, 3447–3461. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Zheng, J.; Gaddy, D.; Orcutt, K.D.; Leonard, S.; Geretti, E.; Hesterman, J.; Harwell, C.; Hoppin, J.; Jaffray, D.A.; et al. A Gradient-Loadable 64Cu-Chelator for Quantifying Tumor Deposition Kinetics of Nanoliposomal Therapeutics by Positron Emission Tomography. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 155–165. [Google Scholar] [CrossRef]
- Rainone, P.; De Palma, A.; Sudati, F.; Roffia, V.; Rigamonti, V.; Salvioni, L.; Colombo, M.; Ripamonti, M.; Spinelli, A.E.; Mazza, D.; et al. 99mTc-Radiolabeled Silica Nanocarriers for Targeted Detection and Treatment of HER2-Positive Breast Cancer. Int. J. Nanomed. 2021, 16, 1943–1960. [Google Scholar] [CrossRef]
- Jang, M.; Yoon, Y.I.; Kwon, Y.S.; Yoon, T.-J.; Lee, H.J.; Hwang, S.I.; Yun, B.L.; Kim, S.M. Trastuzumab-Conjugated Liposome-Coated Fluorescent Magnetic Nanoparticles to Target Breast Cancer. Korean J. Radiol. 2014, 15, 411. [Google Scholar] [CrossRef]
- Jiang, Q.; Hao, S.; Xiao, X.; Yao, J.; Ou, B.; Zhao, Z.; Liu, F.; Pan, X.; Luo, B.; Zhi, H. Production and Characterization of a Novel Long-Acting Herceptin-Targeted Nanobubble Contrast Agent Specific for Her-2-Positive Breast Cancers. Breast Cancer 2016, 23, 445–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Xia, S.; Zhou, W.; Ji, R.; Zhan, W. Targeted Fe-Doped Silica Nanoparticles as a Novel Ultrasound–Magnetic Resonance Dual-Mode Imaging Contrast Agent for HER2-Positive Breast Cancer. Int. J. Nanomed. 2019, 14, 2397–2413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.S.; Chen, J.; Bhattacharjee, S.; Cao, Z.; Wang, H.; Swanson, S.D.; Zong, H.; Baker, J.R.; Wang, S.H. Functionalized Nanoparticles with Targeted Antibody to Enhance Imaging of Breast Cancer in Vivo. J. Nanobiotechnol. 2020, 18, 135. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ma, K.; Madajewski, B.; Zhuang, L.; Zhang, L.; Rickert, K.; Marelli, M.; Yoo, B.; Turker, M.Z.; Overholtzer, M.; et al. Ultrasmall Targeted Nanoparticles with Engineered Antibody Fragments for Imaging Detection of HER2-Overexpressing Breast Cancer. Nat. Commun. 2018, 9, 4141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Meng, Q.; Yang, Z.; Shi, L.; Hu, R.; Zhang, P.; Wei, J.; Ren, J.; Leng, B.; Xu, D.; et al. Circulating HER-2 MRNA in the Peripheral Blood as a Potential Diagnostic and Prognostic Biomarker in Females with Breast Cancer. Oncol. Lett. 2018, 16, 3726–3734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emami, M.; Shamsipur, M.; Saber, R.; Irajirad, R. An Electrochemical Immunosensor for Detection of a Breast Cancer Biomarker Based on AntiHER2–Iron Oxide Nanoparticle Bioconjugates. Analyst 2014, 139, 2858–2866. [Google Scholar] [CrossRef]
- Villegas-Serralta, E.; Zavala, O.; Flores-Urquizo, I.A.; García-Casillas, P.E.; Chapa González, C. Detection of HER2 through Antibody Immobilization Is Influenced by the Properties of the Magnetite Nanoparticle Coating. J. Nanomater. 2018, 2018, 7571613. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Z.; Zong, S.; Song, C.; Zhang, R.; Cui, Y. Distinguishing Breast Cancer Cells Using Surface-Enhanced Raman Scattering. Anal. Bioanal. Chem. 2012, 402, 1093–1100. [Google Scholar] [CrossRef]
- Tao, Y.; Li, M.; Kim, B.; Auguste, D.T. Incorporating Gold Nanoclusters and Target-Directed Liposomes as a Synergistic Amplified Colorimetric Sensor for HER2-Positive Breast Cancer Cell Detection. Theranostics 2017, 7, 899–911. [Google Scholar] [CrossRef]
- Tabatabaei-Panah, A.-S.; Jeddi-Tehrani, M.; Ghods, R.; Akhondi, M.-M.; Mojtabavi, N.; Mahmoudi, A.-R.; Mirzadegan, E.; Shojaeian, S.; Zarnani, A.-H. Accurate Sensitivity of Quantum Dots for Detection of HER2 Expression in Breast Cancer Cells and Tissues. J. Fluoresc. 2013, 23, 293–302. [Google Scholar] [CrossRef]
- Gijs, M.; Penner, G.; Blackler, G.; Impens, N.; Baatout, S.; Luxen, A.; Aerts, A. Improved Aptamers for the Diagnosis and Potential Treatment of HER2-Positive Cancer. Pharmaceuticals 2016, 9, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, M.; Kang, J.; Wang, W.; Li, H.; Feng, J.; Chu, Z.; Zhang, M.; Xu, L.; Wang, Y. Evaluation of Human Epidermal Growth Factor Receptor 2 in Breast Cancer with a Novel Specific Aptamer. Cell. Mol. Immunol. 2017, 14, 398–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-H.; Suh, J.-S.; Yang, J. Labeling-Free Detection of ECD-HER2 Protein Using Aptamer-Based Nano-Plasmonic Sensor. Nanotechnology 2020, 31, 175501. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Wan, C.; Yang, H.; Xu, L.; Dong, Q.; Du, C.; Du, J.; Li, F. Her2-Targeted Multifunctional Nano-Theranostic Platform Mediates Tumor Microenvironment Remodeling and Immune Activation for Breast Cancer Treatment. Int. J. Nanomed. 2020, 15, 10007–10028. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.I.; Lee, J.H.; Kim, J.-Y.; Heo, S.U.; Jeong, Y.Y.; Kim, Y.H.; Tae, G. Targeted Antitumor Efficacy and Imaging via Multifunctional Nano-Carrier Conjugated with Anti-HER2 Trastuzumab. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 359–368. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitia, L.; Sevieri, M.; Signati, L.; Bonizzi, A.; Chesi, A.; Mainini, F.; Corsi, F.; Mazzucchelli, S. HER-2-Targeted Nanoparticles for Breast Cancer Diagnosis and Treatment. Cancers 2022, 14, 2424. https://doi.org/10.3390/cancers14102424
Sitia L, Sevieri M, Signati L, Bonizzi A, Chesi A, Mainini F, Corsi F, Mazzucchelli S. HER-2-Targeted Nanoparticles for Breast Cancer Diagnosis and Treatment. Cancers. 2022; 14(10):2424. https://doi.org/10.3390/cancers14102424
Chicago/Turabian StyleSitia, Leopoldo, Marta Sevieri, Lorena Signati, Arianna Bonizzi, Arianna Chesi, Francesco Mainini, Fabio Corsi, and Serena Mazzucchelli. 2022. "HER-2-Targeted Nanoparticles for Breast Cancer Diagnosis and Treatment" Cancers 14, no. 10: 2424. https://doi.org/10.3390/cancers14102424
APA StyleSitia, L., Sevieri, M., Signati, L., Bonizzi, A., Chesi, A., Mainini, F., Corsi, F., & Mazzucchelli, S. (2022). HER-2-Targeted Nanoparticles for Breast Cancer Diagnosis and Treatment. Cancers, 14(10), 2424. https://doi.org/10.3390/cancers14102424









