Treatment Sequences in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Cetuximab Followed by Immunotherapy or Vice Versa
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment Protocols
2.3. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. Survival Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cervenka, B.P.; Rao, S.; Bewley, A.F. Head and Neck Cancer and the Elderly Patient. Otolaryngol. Clin. N. Am. 2018, 51, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Aupérin, A. Epidemiology of head and neck cancers: An update. Curr. Opin. Oncol. 2020, 32, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Guigay, J.; Tahara, M.; Licitra, L.; Keilholz, U.; Friesland, S.; Witzler, P.; Mesía, R. The Evolving Role of Taxanes in Combination with Cetuximab for the Treatment of Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck: Evidence, Advantages, and Future Directions. Front. Oncol. 2019, 9, 668. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.; Yang, W.; Li, K.-Y.; Su, Y.-X. Systemic Therapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma- A Systematic Review and Meta-Analysis. Crit. Rev. Oncol. 2020, 153, 102984. [Google Scholar] [CrossRef] [PubMed]
- Jan, B.; Vermorken, R.M.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.-R.; Cupissol, D. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, A.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Anzai, Y.; Brizel, D.M.; Bruce, J.Y.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; et al. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 873–898. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Bell, R.B.; Bifulco, C.B.; Burtness, B.; Gillison, M.L.; Harrington, K.J.; Le, Q.-T.; Lee, N.Y.; Leidner, R.; Lewis, R.L.; et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J. Immunother. Cancer 2019, 7, 184. [Google Scholar] [CrossRef] [Green Version]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [Green Version]
- Cohen, E.E.W.; Soulières, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.-J.; Soria, A.; Machiels, J.-P.; Mach, N.; Mehra, R.; et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 2018, 393, 156–167. [Google Scholar] [CrossRef]
- Guigay, J.; Aupérin, A.; Fayette, J.; Saada-Bouzid, E.; Lafond, C.; Taberna, M.; Geoffrois, L.; Martin, L.; Capitain, O.; Cupissol, D.; et al. Cetuximab, docetaxel, and cisplatin versus platinum, fluorouracil, and cetuximab as first-line treatment in patients with recurrent or metastatic head and neck squamous-cell carcinoma (GORTEC 2014-01 TPExtreme): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2021, 22, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Hasegawa, Y.; Takahashi, S.; Monden, N.; Homma, A.; Okami, K.; Onozawa, Y.; Fujii, M.; Taguchi, T.; de Blas, B.; et al. Platinum-based Chemotherapy Plus Cetuximab for the First-line Treatment of Japanese Patients with Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck: Results of a Phase II Trial. Jpn. J. Clin. Oncol. 2013, 43, 524–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Mello, R.A.; Gerós, S.; Alves, M.P.; Moreira, F.; Avezedo, I.; Dinis, J. Cetuximab Plus Platinum-Based Chemotherapy in Head and Neck Squamous Cell Carcinoma: A Retrospective Study in a Single Comprehensive European Cancer Institution. PLoS ONE 2014, 9, e86697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depenni, R.; Rocca, M.C.; Ferrari, D.; Azzarello, G.; Baldessari, C.; Alù, M.; Nolé, F.; Codecà, C.; Boscolo, G.; Piccininni, M.; et al. Clinical outcomes and prognostic factors in recurrent and/or metastatic head and neck cancer patients treated with chemotherapy plus cetuximab as first-line therapy in a real-world setting. Eur. J. Cancer 2019, 115, 4–12. [Google Scholar] [CrossRef] [PubMed]
- le Tourneau, C.; Ghiani, M.; Cau, M.; Depenni, R.; Ronzino, G.; Bonomo, P.; Montesarchio, V.; Leo, L.; Schulten, J.; Messinger, D.; et al. Cetuximab + platinum-based therapy (PBT) as a first-line treatment for patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN): An observational study (ENCORE). Ann. Oncol. 2018, 29, viii372–viii399. [Google Scholar] [CrossRef]
- Sano, D.; Fujisawa, T.; Tokuhisa, M.; Shimizu, M.; Sakagami, T.; Hatano, T.; Nishimura, G.; Ichikawa, Y.; Iwai, H.; Oridate, N. Real-world Treatment Outcomes of the EXTREME Regimen as First-line Therapy for Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck: A Multi-center Retrospective Cohort Study in Japan. Anticancer Res. 2019, 39, 6819–6827. [Google Scholar] [CrossRef] [Green Version]
- Lien, M.-Y.; Wang, T.-H.; Hsieh, C.-Y.; Tsai, M.-H.; Hua, C.-H.; Cheng, F.-M.; Chung, W.-H.; Tang, C.-H.; Hsieh, J.C.-H. Both combined or sequential use with immune checkpoint inhibitors on cetuximab-treated patients with recurrent or metastatic head and neck squamous cell carcinoma improve the overall survival. Oral Oncol. 2021, 119, 105380. [Google Scholar] [CrossRef]
- Kariya, S.; Shimizu, Y.; Hanai, N.; Yasumatsu, R.; Yokota, T.; Fujii, T.; Tsukahara, K.; Yoshida, M.; Hanyu, K.; Ueda, T.; et al. Effectiveness of nivolumab affected by prior cetuximab use and neck dissection in Japanese patients with recurrent or metastatic head and neck cancer: Results from a retrospective observational study in a real-world setting. Int. J. Clin. Oncol. 2021, 26, 1049–1056. [Google Scholar] [CrossRef]
- Fuereder, T.; Minichsdorfer, C.; Mittlboeck, M.; Wagner, C.; Heller, G.; Putz, E.M.; Oberndorfer, F.; Müllauer, L.; Aretin, M.-B.; Czerny, C.; et al. Pembrolizumab plus docetaxel for the treatment of recurrent/metastatic head and neck cancer: A prospective phase I/II study. Oral Oncol. 2021, 124, 105634. [Google Scholar] [CrossRef]
- Yuka, I.; Suzuki, N.; Tokumitsu, Y.; Kanekyio, S.; Tomochika, S.; Tsunedomi, R.; Tokuhisa, Y.; Iida, M.; Sakamoto, K.; Takeda, S.; et al. Cetuximab strongly enhances immune cellinfiltration into liver metastatic sites in colorectalcancer. Cancer Sci. 2017, 108, 455–460. [Google Scholar]
- Cramer, J.D.; Burtness, B.; Ferris, R.L. Immunotherapy for head and neck cancer: Recent advances and future directions. Oral Oncol. 2019, 99, 104460. [Google Scholar] [CrossRef] [PubMed]
- Larroquette, M.; Domblides, C.; Lefort, F.; Lasserre, M.; Quivy, A.; Sionneau, B.; Bertolaso, P.; Gross-Goupil, M.; Ravaud, A.; Daste, A. Combining immune checkpoint inhibitors with chemotherapy in advanced solid tumours: A review. Eur. J. Cancer 2021, 158, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Camarero, S.; Cabrera-Martín, M.N.; Merino-Menéndez, S.; Paz-Cabezas, M.; García-Barberán, V.; Sanz, M.S.-P.; Iglesias-Moreno, M.; Alonso-Ovies, A.; Pérez-Segura, P. Safety and Efficacy of Cetuximab-Based Salvage Chemotherapy After Checkpoint Inhibitors in Head and Neck Cancer. Oncologist 2021, 26, e1018–e1035. [Google Scholar] [CrossRef] [PubMed]


| IO-Cet | Cet-IO | p | |||
|---|---|---|---|---|---|
| n = 35 | n = 40 | ||||
| Gender | 0.506 | ||||
| Male | 34 | 97% | 36 | 90% | |
| Female | 1 | 3% | 4 | 10% | |
| Age | 0.948 | ||||
| ≤60 | 23 | 66% | 26 | 65% | |
| >60 | 12 | 34% | 14 | 35% | |
| Primary tumor location | 0.812 | ||||
| Oral cavity | 19 | 54% | 18 | 45% | |
| Oropharynx | 11 | 31% | 16 | 40% | |
| Hypopharynx | 4 | 11% | 4 | 10% | |
| Larynx | 1 | 4% | 2 | 5% | |
| p16 status | 0.838 | ||||
| Positive | 4 | 11% | 3 | 8% | |
| Negative | 8 | 23% | 10 | 25% | |
| Unknown | 23 | 66% | 27 | 67% | |
| Initial T stage | 0.279 | ||||
| T1–T2 | 14 | 40% | 21 | 53% | |
| T3–T4 | 21 | 60% | 19 | 47% | |
| Initial N stage | 0.138 | ||||
| N0–N1 | 15 | 43% | 24 | 60% | |
| N2–N3 | 20 | 57% | 16 | 40% | |
| Initial M stage | 0.402 | ||||
| M0 | 31 | 89% | 39 | 97% | |
| M1 | 4 | 11% | 1 | 3% | |
| Initial stage | 0.170 | ||||
| I–II | 8 | 23% | 15 | 38% | |
| III–IV | 27 | 77% | 25 | 62% | |
| Previous surgery | 1.000 | ||||
| Yes | 21 | 60% | 24 | 60% | |
| No | 14 | 40% | 16 | 40% | |
| Previous radiotherapy | 0.189 | ||||
| Yes | 28 | 80% | 27 | 68% | |
| No | 7 | 20% | 13 | 32% | |
| Disease status upon enrollment | 0.195 | ||||
| Local recurrence only | 12 | 34% | 18 | 45% | |
| Distant metastasis | 23 | 66% | 22 | 55% | |
| PD-L1 status | 0.321 | ||||
| High expression | 18 | 51% | 16 | 40% | |
| Low expression | 17 | 49% | 24 | 60% | |
| IO-Cet n = 35 | Cet-IO n = 40 | p | |
|---|---|---|---|
| mPFS1 (m) | 4.5 | 5.1 | 0.777 |
| mPFS2 (m) | 11.4 | 16.5 | 0.566 |
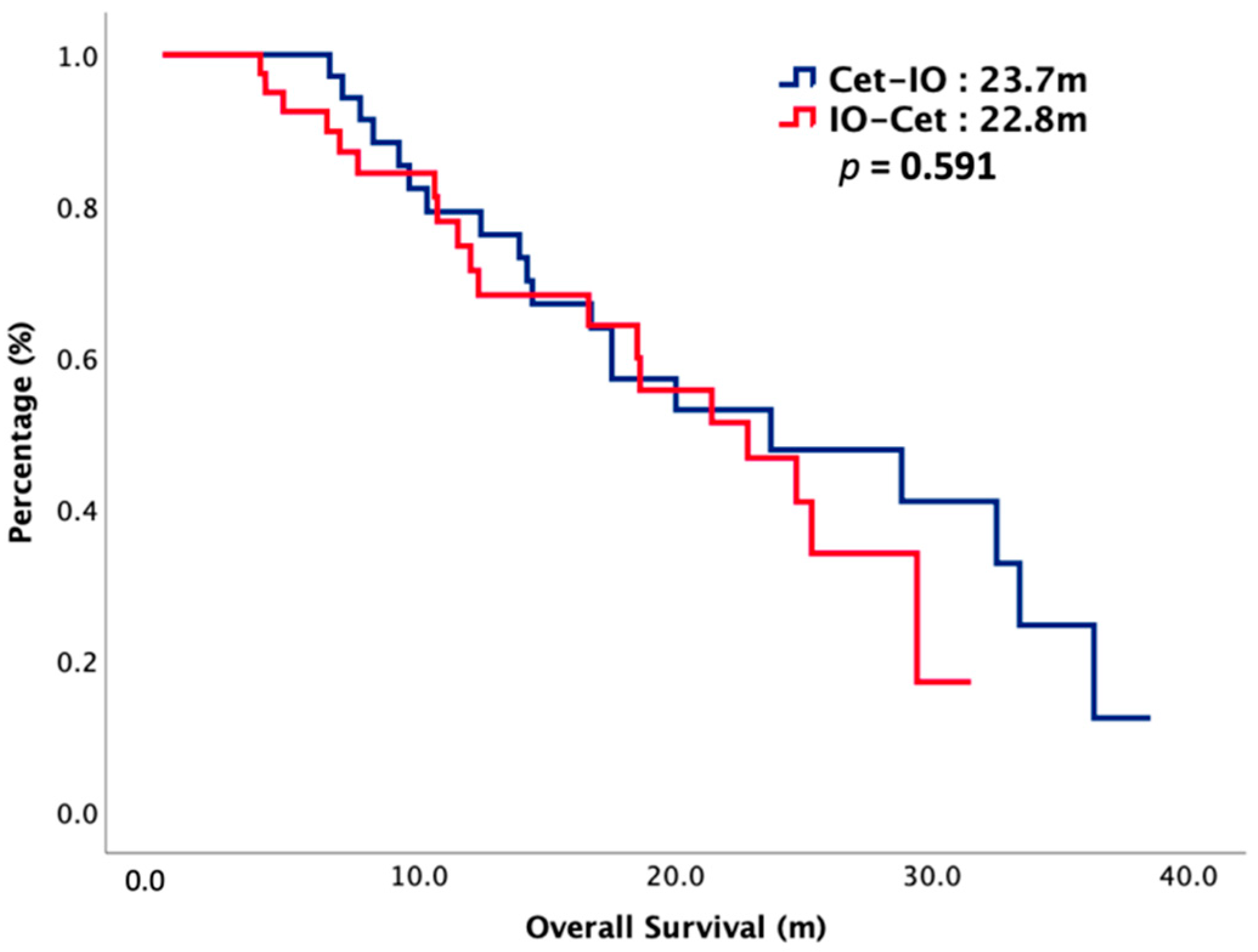
| mOS (m) | 22.8 | 23.7 | 0.484 |
| First-line treatment | |||
| CR (%) | 2 (6) | 8 (20) | |
| PR (%) | 11 (31) | 21 (53) | |
| SD (%) | 9 (26) | 2 (5) | |
| PD (%) | 13 (37) | 9 (22) | |
| ORR (%) | 13 (37) | 29 (73) | 0.002 |
| DCR (%) | 22 (63) | 31 (78) | 0.165 |
| Second-line treatment | |||
| CR (%) | 1 (3) | 3 (8) | |
| PR (%) | 12 (34) | 22 (55) | |
| SD (%) | 5 (14) | 6 (15) | |
| PD (%) | 17 (49) | 9 (22) | |
| ORR (%) | 13 (37) | 25 (63) | 0.028 |
| DCR (%) | 18 (51) | 31 (78) | 0.018 |
| Variables | HR (95% CI) | p Value |
|---|---|---|
| Gender, Male vs. Female | 0.57 (0.22–1.47) | 0.242 |
| Age, ≤60 vs. >60 | 0.91 (0.46–2.82) | 0.789 |
| Primary tumor location, oral cavity vs. others | 0.72 (0.36–0.80) | 0.020 |
| Initial T stage, T1–T2 vs. T3–T4 | 0.72 (0.36–1.41) | 0.338 |
| Initial N stage, N0–N1 vs. N2–N3 | 0.61 (0.31–1.19) | 0.145 |
| Initial M stage, M0 vs. M1 | 0.45 (0.14–1.49) | 0.192 |
| Initial stage, stage 1–2 vs. 3–4 | 0.99 (0.51–1.09) | 0.969 |
| p16 status, positive vs. negative | 0.95 (0.44–2.03) | 0.886 |
| Previous radical surgery, yes vs. no | 0.87 (0.45–1.67) | 0.667 |
| Previous radiotherapy, yes vs. no | 0.84 (0.44–1.62) | 0.606 |
| Disease status, local only vs. metastasis | 0.71 (0.31–1.36) | 0.299 |
| PD-L1 expression, high vs. low | 0.76 (0.40–1.42) | 0.383 |
| Treatment sequence, Cet-IO vs. IO-Cet | 0.79 (0.41–1.53) | 0.485 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-C.; Lien, C.-F.; Hwang, T.-Z.; Wang, C.-C.; Wang, C.-C.; Shih, Y.-C.; Yeh, S.-A.; Hsieh, M.-C. Treatment Sequences in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Cetuximab Followed by Immunotherapy or Vice Versa. Cancers 2022, 14, 2351. https://doi.org/10.3390/cancers14102351
Yang C-C, Lien C-F, Hwang T-Z, Wang C-C, Wang C-C, Shih Y-C, Yeh S-A, Hsieh M-C. Treatment Sequences in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Cetuximab Followed by Immunotherapy or Vice Versa. Cancers. 2022; 14(10):2351. https://doi.org/10.3390/cancers14102351
Chicago/Turabian StyleYang, Chuan-Chien, Ching-Feng Lien, Tzer-Zen Hwang, Chih-Chun Wang, Chien-Chung Wang, Yu-Chen Shih, Shyh-An Yeh, and Meng-Che Hsieh. 2022. "Treatment Sequences in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Cetuximab Followed by Immunotherapy or Vice Versa" Cancers 14, no. 10: 2351. https://doi.org/10.3390/cancers14102351
APA StyleYang, C.-C., Lien, C.-F., Hwang, T.-Z., Wang, C.-C., Wang, C.-C., Shih, Y.-C., Yeh, S.-A., & Hsieh, M.-C. (2022). Treatment Sequences in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Cetuximab Followed by Immunotherapy or Vice Versa. Cancers, 14(10), 2351. https://doi.org/10.3390/cancers14102351





