The Multiple Myeloma Landscape: Epigenetics and Non-Coding RNAs
Abstract
Simple Summary
Abstract
1. Introduction
2. Methylation
3. Acetylation
4. Non-Coding RNAs
4.1. microRNAs
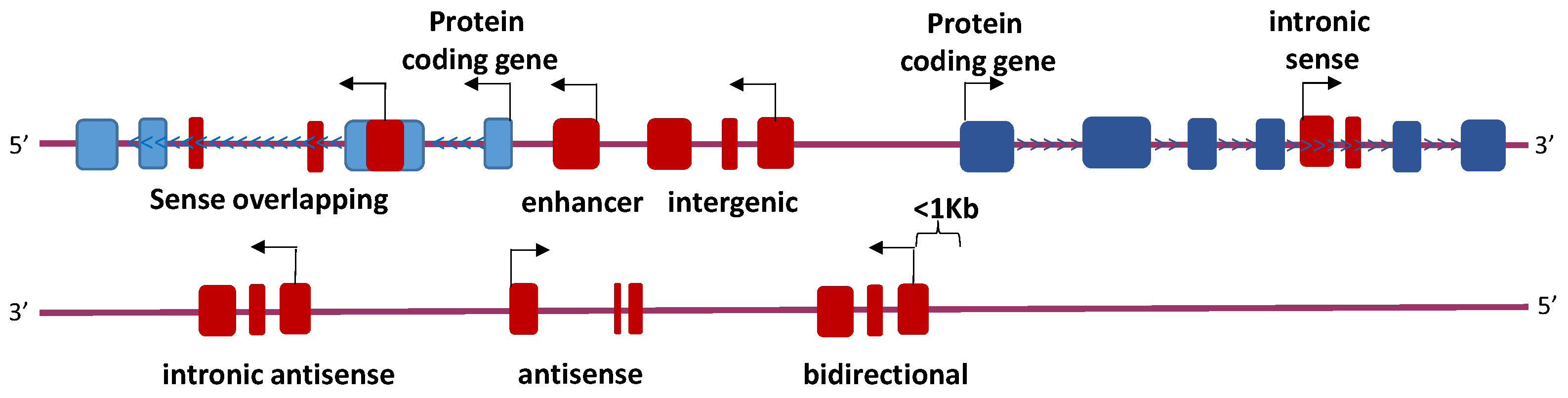
4.2. Long Non-Coding RNAs
4.3. Other ncRNAs
5. Therapies
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kyle, R.A.; Therneau, T.M.; Rajkumar, S.V.; Larson, D.R.; Plevak, M.F.; Offord, J.R.; Dispenzieri, A.; Katzmann, J.A.; Melton, L.J., 3rd. Prevalence of monoclonal gammopathy of undetermined significance. N. Engl. J. Med. 2006, 354, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Laubach, J.P.; Richardson, P.G.; Anderson, K.C. The evolution and impact of therapy in multiple myeloma. Med. Oncol. 2010, 27 (Suppl. S1), 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.H.; Lee, T.-L.; Rennert, O.M.; Chan, W.Y. DNA methylation of cancer genome. Birth Defects Res. Part C Embryo Today Rev. 2009, 87, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, K.; Gimsing, P.; Grønbæk, K. The role of epigenetics in the biology of multiple myeloma. Blood Cancer J. 2014, 4, e207. [Google Scholar] [CrossRef]
- Rasmussen, K.D.; Helin, K. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 2016, 30, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Tsiouplis, N.J.; Bailey, D.W.; Chiou, L.F.; Wissink, F.J.; Tsagaratou, A. TET-mediated epigenetic regulation in immune cell development and disease. Front. Cell Dev. Biol. 2020, 8, 623948. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.A.; Wardell, C.P.; Chiecchio, L.; Smith, E.M.; Boyd, K.D.; Neri, A.; Davies, F.E.; Ross, F.M.; Morgan, G.J. Aberrant global methylation patterns affect the molecular pathogenesis and prognosis of multiple myeloma. Blood 2011, 117, 553–562. [Google Scholar] [CrossRef]
- Agirre, X.; Castellano, G.; Pascual, M.; Heath, S.; Kulis, M.; Segura, V.; Bergmann, A.; Esteve, A.; Merkel, A.; Raineri, E.; et al. Whole-epigenome analysis in multiple myeloma reveals DNA hypermethylation of B cell-specific enhancers. Genome Res. 2015, 25, 478–487. [Google Scholar] [CrossRef]
- Kaiser, M.F.; Johnson, D.C.; Wu, P.; Walker, B.A.; Brioli, A.; Mirabella, F.; Wardell, C.P.; Melchor, L.; Davies, F.E.; Morgan, G.J. Global methylation analysis identifies prognostically important epigenetically inactivated tumor suppressor genes in multiple myeloma. Blood 2013, 122, 219–226. [Google Scholar] [CrossRef]
- Martínez-Baños, D.; Sánchez-Hernández, B.; Jimenez, G.; Barrera-Lumbreras, G.; Barrales-Benítez, O. Global methylation and promoter-specific methylation of the P16, SOCS-1, E-cadherin, P73 and SHP-1 genes and their expression in patients with multiple myeloma during active disease and remission. Exp. Ther. Med. 2017, 13, 2442–2450. [Google Scholar] [CrossRef][Green Version]
- Chong, P.S.Y.; Chng, W.-J.; de Mel, S. STAT3: A promising therapeutic target in multiple myeloma. Cancers 2019, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- Kiziltepe, T.; Hideshima, T.; Catley, L.; Raje, N.; Yasui, H.; Shiraishi, N.; Okawa, Y.; Ikeda, H.; Vallet, S.; Pozzi, S.; et al. 5-azacytidine, a DNA methyltransferase inhibitor, induces ATR-mediated DNA double-strand break responses, apoptosis and synergistic cytotoxicity with doxorubicin and bortezomib against multiple myeloma cells. Mol. Cancer Ther. 2007, 6, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- U.S National Institutes of Health-ClinicalTrials.Gov. Available online: www.clinicaltrials.gov (accessed on 1 September 2020).
- Martinez-Garcia, E.; Popovic, R.; Min, D.-J.; Sweet, S.M.M.; Thomas, P.M.; Zamdborg, L.; Heffner, A.; Will, C.; Lamy, L.; Staudt, L.M.; et al. The MMSET histone methyl transferase switches global histone methylation and alters gene expression in t(4;14) multiple myeloma cells. Blood 2011, 117, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Bannister, A.J.; Schneider, R.; Myers, F.A.; Thorne, A.W.; Crane-Robinson, C.; Kouzarides, T. Spatial distribution of di- and tri-methyl lysine 36 of histone H3 at active genes. J. Biol. Chem. 2005, 280, 17732–17736. [Google Scholar] [CrossRef] [PubMed]
- Kuo, A.J.; Cheung, P.; Chen, K.; Zee, B.M.; Kioi, M.; Lauring, J.; Xi, Y.; Park, B.H.; Shi, X.; Garcia, B.A.; et al. NSD2 links dimethylation of histone H3 at lysine 36 to oncogenic programming. Mol. Cell 2011, 44, 609–620. [Google Scholar] [CrossRef]
- Xie, Z.; Chng, W.J. MMSET: Role and therapeutic opportunities in multiple myeloma. BioMed Res. Int. 2014, 2014, 636514. [Google Scholar] [CrossRef]
- Alzrigat, M.; Jernberg-Wiklund, H.; Licht, J.D. Targeting EZH2 in multiple myeloma-multifaceted anti-tumor activity. Epigenomes 2018, 2, 16. [Google Scholar] [CrossRef]
- Goldsmith, S.R.; Fiala, M.A.; O’Neal, J.; Souroullas, G.P.; Toama, W.; Vij, R.; Schroeder, M.A. EZH2 overexpression in multiple myeloma: Prognostic value, correlation with clinical characteristics, and possible mechanisms. Clin. Lymphoma Myeloma Leuk. 2019, 19, 744–750. [Google Scholar] [CrossRef]
- Kikuchi, J.; Koyama, D.; Wada, T.; Izumi, T.; Hofgaard, P.O.; Bogen, B.; Furukawa, Y. Phosphorylation-mediated EZH2 inactivation promotes drug resistance in multiple myeloma. J. Clin. Investig. 2015, 125, 4375–4390. [Google Scholar] [CrossRef]
- Dutta, R.; Tiu, B.; Sakamoto, K.M. CBP/p300 acetyltransferase activity in hematologic malignancies. Mol. Genet. Metab. 2016, 119, 37–43. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Shi, C.-X.; Bruins, L.A.; Wang, X.; Riggs, D.L.; Porter, B.; Ahmann, J.M.; de Campos, C.B.; Braggio, E.; Bergsagel, P.L.; et al. Identification of lenalidomide resistance pathways in myeloma and targeted resensitization using cereblon replacement, inhibition of STAT3 or targeting of IRF4. Blood Cancer J. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Falkenberg, K.J.; Johnstone, R.W. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat. Rev. Drug Discov. 2014, 13, 673–691. [Google Scholar] [CrossRef] [PubMed]
- Bradner, J.E.; West, N.; Grachan, M.L.; Greenberg, E.F.; Haggarty, S.J.; Warnow, T.; Mazitschek, R. Chemical phylogenetics of histone deacetylases. Nat. Chem. Biol. 2010, 6, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Hirano, M.; Kobayashi, M.; Futami, M.; Tojo, A. HDAC inhibitors exert anti-myeloma effects through multiple modes of action. Cancers 2019, 11, 475. [Google Scholar] [CrossRef] [PubMed]
- Hideshima, T.; Bradner, J.E.; Wong, J.; Chauhan, D.; Richardson, P.; Schreiber, S.L.; Anderson, K.C. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc. Natl. Acad. Sci. USA 2005, 102, 8567–8572. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Tripathi, Y.N. Potential of long non-coding RNAs in cancer patients: From biomarkers to therapeutic targets. Int. J. Cancer 2017, 140, 1955–1967. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Xiao, Y.; Ma, J.; Tang, Y.; Tian, B.; Zhang, Y.; Li, X.; Wu, Z.; Yang, D.; Zhou, Y.; et al. Circular RNAs in cancer: Emerging functions in hallmarks, stemness, resistance and roles as potential biomarkers. Mol. Cancer 2019, 18, 90. [Google Scholar] [CrossRef]
- Wang, T.; Tao, W.; Zhang, L.; Li, S. Oncogenic role of microRNA-20a in human multiple myeloma. Onco Targets Ther. 2017, 10, 4465–4474. [Google Scholar] [CrossRef][Green Version]
- Jiang, Y.; Chang, H.; Chen, G. Effects of microRNA-20a on the proliferation, migration and apoptosis of multiple myeloma via the PTEN/PI3K/AKT signaling pathway. Oncol. Lett. 2018, 15, 10001–10007. [Google Scholar] [CrossRef]
- Xiong, Q.; Zhong, Q.; Zhang, J.; Yang, M.; Li, C.; Zheng, P.; Bi, L.-J.; Ge, F. Identification of novel miR-21 target proteins in multiple myeloma cells by quantitative proteomics. J. Proteome Res. 2012, 11, 2078–2090. [Google Scholar] [CrossRef]
- Zi, Y.; Zhang, Y.; Wu, Y.; Zhang, L.; Yang, R.; Huang, Y. Downregulation of microRNA-25-3p inhibits the proliferation and promotes the apoptosis of multiple myeloma cells via targeting the PTEN/PI3K/AKT signaling pathway. Int. J. Mol. Med. 2021, 47, 8. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ouyang, Y.; Che, J.; Li, X.; Zhao, Y.; Yang, K.; Zhao, X.; Chen, Y.; Fan, C.; Yuan, W. Potential value of miR-221/222 as diagnostic, prognostic, and therapeutic biomarkers for diseases. Front. Immunol. 2017, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Chen, J.; Zhang, H.; Wang, X.; Yao, H.; Peng, Y.; Zhang, W. LncRNA OIP5-AS1 loss-induced microRNA-410 accumulation regulates cell proliferation and apoptosis by targeting KLF10 via activating PTEN/PI3K/AKT pathway in multiple myeloma. Cell Death Dis. 2017, 8, e2975. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, C.; Zhang, R.; Gao, X.; Qu, X.; Zhao, M.; Qiao, C.; Xu, J.; Li, J. miR-17-92 cluster microRNAs confers tumorigenicity in multiple myeloma. Cancer Lett. 2011, 309, 62–70. [Google Scholar] [CrossRef]
- Wang, N.; Liang, X.; Yu, W.; Zhou, S.; Fang, M. Differential expression of microRNA-19b promotes proliferation of cancer stem cells by regulating the TSC1/mTOR signaling pathway in multiple myeloma. Cell. Physiol. Biochem. 2018, 50, 1804–1814. [Google Scholar] [CrossRef]
- Quwaider, D.; Corchete, L.A.; Misiewicz-Krzeminska, I.; Sarasquete, M.E.; Pérez, J.J.; Krzeminski, P.; Puig, N.; Mateos, M.V.; García-Sanz, R.; Herrero, A.B.; et al. DEPTOR maintains plasma cell differentiation and favorably affects prognosis in multiple myeloma. J. Hematol. Oncol. 2017, 10, 92. [Google Scholar] [CrossRef]
- Saba, F.; Soleimani, M.; Abroun, S. New role of hypoxia in pathophysiology of multiple myeloma through miR-210. EXCLI J. 2018, 17, 647–662. [Google Scholar] [CrossRef]
- Ikeda, S.; Kitadate, A.; Abe, F.; Saitoh, H.; Michishita, Y.; Hatano, Y.; Kawabata, Y.; Kitabayashi, A.; Teshima, K.; Kume, M.; et al. Hypoxia-inducible microRNA-210 regulates the DIMT1-IRF4 oncogenic axis in multiple myeloma. Cancer Sci. 2017, 108, 641–652. [Google Scholar] [CrossRef]
- Lee, J.Y.; Ryu, D.; Lim, S.W.; Ryu, K.J.; Choi, M.E.; Yoon, S.E.; Kim, K.; Park, C.; Kim, S.J. Exosomal miR-1305 in the oncogenic activity of hypoxic multiple myeloma cells: A biomarker for predicting prognosis. J. Cancer 2021, 12, 2825–2834. [Google Scholar] [CrossRef]
- Takanlu, J.S.; Fard, A.A.; Mohammdi, S.; Rad, S.M.A.H.; Abroun, S.; Nikbakht, M. Indirect tumor inhibitory effects of microRNA-124 through targeting EZH2 in the multiple myeloma cell line. Cell J. 2020, 22, 23–29. [Google Scholar] [CrossRef]
- De Veirman, K.; Wang, J.; Xu, S.; Leleu, X.; Himpe, E.; Maes, K.; De Bruyne, E.; Van Valckenborgh, E.; Vanderkerken, K.; Menu, E.; et al. Induction of miR-146a by multiple myeloma cells in mesenchymal stromal cells stimulates their pro-tumoral activity. Cancer Lett. 2016, 377, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhou, J.; Wang, J.; Dong, X.; Chang, Y.; Jin, Y. Mechanism of exosomal miR-155 derived from bone marrow mesenchymal stem cells on stemness maintenance and drug resistance in myeloma cells. J. Orthop. Surg. Res. 2021, 16, 637. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, N.C.; Sarasquete, M.E.; Misiewicz-Krzeminska, I.; Delgado, M.; De Las Rivas, J.; Ticona, F.V.; Fermiñán, E.; Martín-Jiménez, P.; Chillon, C.; Risueño, A.; et al. Deregulation of microRNA expression in the different genetic subtypes of multiple myeloma and correlation with gene expression profiling. Leukemia 2010, 24, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Zang, M.; Zhao, L.; Deng, S.; Xu, Y.; Qi, F.; An, G.; Qin, Y.; Sui, W.; Li, F.; et al. Serum high expression of miR-214 and miR-135b as novel predictor for myeloma bone disease development and prognosis. Oncotarget 2016, 7, 19589–19600. [Google Scholar] [CrossRef]
- Umezu, T.; Tadokoro, H.; Azuma, K.; Yoshizawa, S.; Ohyashiki, K.; Ohyashiki, J.H. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood 2014, 124, 3748–3757. [Google Scholar] [CrossRef]
- Bao, Y.; Wei, M.; Ji, X. microRNA-146b overexpression associates with deteriorated clinical characteristics, increased International Staging System stage, cacoethic chromosome abnormality, and unfavorable prognosis in multiple myeloma patients. J. Clin. Lab. Anal. 2020, 34, e23168. [Google Scholar] [CrossRef]
- Peng, J.; Thakur, A.; Zhang, S.; Dong, Y.; Wang, X.; Yuan, R.; Zhang, K.; Guo, X. Expressions of miR-181a and miR-20a in RPMI8226 cell line and their potential as biomarkers for multiple myeloma. Tumor Biol. 2015, 36, 8545–8552. [Google Scholar] [CrossRef]
- Papadimitriou, M.-A.; Papanota, A.-M.; Adamopoulos, P.G.; Pilala, K.-M.; Liacos, C.-I.; Malandrakis, P.; Mavrianou-Koutsoukou, N.; Patseas, D.; Eleutherakis-Papaiakovou, E.; Gavriatopoulou, M.; et al. miRNA-seq and clinical evaluation in multiple myeloma: miR-181a overexpression predicts short-term disease progression and poor post-treatment outcome. Br. J. Cancer 2022, 126, 79–90. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, P.; Li, W.-J.; Zhang, J.; Wang, G.-P.; Jiang, D.-F.; Chen, F.-P. LncRNA NEAT1 sponges miR-214 to regulate M2 macrophage polarization by regulation of B7-H3 in multiple myeloma. Mol. Immunol. 2020, 117, 20–28. [Google Scholar] [CrossRef]
- Gao, D.; Xiao, Z.; Li, H.-P.; Han, D.-H.; Zhang, Y.-P. The mechanism study of miR-125b in occurrence and progression of multiple myeloma. Cancer Med. 2018, 7, 134–145. [Google Scholar] [CrossRef]
- Misso, G.; Zarone, M.R.; Lombardi, A.; Grimaldi, A.; Cossu, A.M.; Ferri, C.; Russo, M.; Vuoso, D.C.; Luce, A.; Kawasaki, H.; et al. miR-125b upregulates miR-34a and sequentially activates stress adaption and cell death mechanisms in multiple myeloma. Mol. Ther.-Nucleic Acids 2019, 16, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-L.; Liu, X.-L.; Guo, S.-F.; Yang, Y.; Zhu, Y.-L.; Li, J.-Z. Long noncoding RNA UCA1 regulates proliferation and apoptosis in multiple myeloma by targeting miR-331-3p/IL6R axis for the activation of JAK2/STAT3 pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9238–9250. [Google Scholar] [CrossRef] [PubMed]
- Tatekawa, S.; Chinen, Y.; Ri, M.; Narita, T.; Shimura, Y.; Matsumura-Kimoto, Y.; Tsukamoto, T.; Kobayashi, T.; Kawata, E.; Uoshima, N.; et al. Epigenetic repression of miR-375 is the dominant mechanism for constitutive activation of the PDPK1/RPS6KA3 signalling axis in multiple myeloma. Br. J. Haematol. 2017, 178, 534–546. [Google Scholar] [CrossRef] [PubMed]
- David, A.; Zocchi, S.; Talbot, A.; Choisy, C.; Ohnona, A.; Lion, J.; Cuccuini, W.; Soulier, J.; Arnulf, B.; Bories, J.-C.; et al. The long non-coding RNA CRNDE regulates growth of multiple myeloma cells via an effect on IL6 signalling. Leukemia 2021, 35, 1710–1721. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, C.; Zhang, Y.; Guo, X.; Liu, Z.; Luo, Z.; Chang, Y.; Liu, S.; Sun, Z.; Wang, X. Let-7b-5p regulates proliferation and apoptosis in multiple myeloma by targeting IGF1R. Acta Biochim. Biophys. Sin. 2014, 46, 965–972. [Google Scholar] [CrossRef]
- Xu, Y.-Y.; Song, Y.-Q.; Huang, Z.-M.; Zhang, H.-B.; Chen, M. microRNA-26a inhibits multiple myeloma cell growth by suppressing cyclin-dependent kinase 6 expression. Kaohsiung J. Med. Sci. 2019, 35, 277–283. [Google Scholar] [CrossRef]
- Li, Z.; Wong, K.Y.; Chan, G.C.-F.; Chim, C.S. Epigenetic silencing of LPP/miR-28 in multiple myeloma. J. Clin. Pathol. 2018, 71, 253–258. [Google Scholar] [CrossRef]
- Nian, F.; Zhu, J.; Chang, H. Long non-coding RNA ANGPTL1-3 promotes multiple myeloma bortezomib resistance by sponging miR-30a-3p to activate c-Maf expression. Biochem. Biophys. Res. Commun. 2019, 514, 1140–1146. [Google Scholar] [CrossRef]
- Cao, Y.; Shi, X.; Liu, Y.; Xu, R.; Ai, Q. microRNA-338-3p inhibits proliferation and promotes apoptosis of multiple myeloma cells through targeting cyclin-dependent kinase 4. Oncol. Res. 2018, 27, 117–124. [Google Scholar] [CrossRef]
- Li, Z.; Wong, K.Y.; Calin, G.A.; Chng, W.-J.; Chan, G.C.-F.; Chim, C.S. Epigenetic silencing of miR-340-5p in multiple myeloma: Mechanisms and prognostic impact. Clin. Epigenetics 2019, 11, 71. [Google Scholar] [CrossRef]
- Caracciolo, D.; Riillo, C.; Juli, G.; Scionti, F.; Todoerti, K.; Polerà, N.; Grillone, K.; Fiorillo, L.; Arbitrio, M.; Di Martino, M.T.; et al. miR-22 modulates lenalidomide activity by counteracting MYC addiction in multiple myeloma. Cancers 2021, 13, 4365. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.N.; Abdi, J.; Yang, Y.; Chang, H. miRNA-29a as a tumor suppressor mediates PRIMA-1Met-induced anti-myeloma activity by targeting c-Myc. Oncotarget 2016, 7, 7149–7160. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, M.T.; Leone, E.; Amodio, N.; Foresta, U.; Lionetti, M.; Pitari, M.R.; Cantafio, M.E.G.; Gullà, A.; Conforti, F.; Morelli, E.; et al. Synthetic miR-34a mimics as a novel therapeutic agent for multiple myeloma: In Vitro and In Vivo evidence. Clin. Cancer Res. 2012, 18, 6260–6270. [Google Scholar] [CrossRef] [PubMed]
- Misso, G.; Di Martino, M.T.; De Rosa, G.; Farooqi, A.A.; Lombardi, A.; Campani, V.; Zarone, M.R.; Gullà, A.; Tagliaferri, P.; Tassone, P.; et al. miR-34: A new weapon against cancer? Mol. Ther.-Nucleic Acids 2014, 3, e194. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, H. LncRNA NEAT1 promotes dexamethasone resistance in multiple myeloma by targeting miR-193a/MCL1 pathway. J. Biochem. Mol. Toxicol. 2018, 32, e22008. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Zhan, Y.; Zhu, W.; Li, J.; Tang, M.; Chen, X.; Jiang, J. microRNA-497 inhibits multiple myeloma growth and increases susceptibility to bortezomib by targeting Bcl-2. Int. J. Mol. Med. 2019, 43, 1058–1066. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, L.; Wu, J.; Khadka, B.; Fang, Z.; Gu, J.; Tang, B.; Xiao, R.; Pan, G.; Liu, J. CircRNA circ_0000190 inhibits the progression of multiple myeloma through modulating miR-767-5p/MAPK4 pathway. J. Exp. Clin. Cancer Res. 2019, 38, 54. [Google Scholar] [CrossRef]
- Tian, F.-Q.; Chen, Z.-R.; Zhu, W.; Tang, M.-Q.; Li, J.-H.; Zhang, X.-C.; Jiang, J.; Cheng, X.-H. Inhibition of hsa_circ_0003489 shifts balance from autophagy to apoptosis and sensitizes multiple myeloma cells to bortezomib via miR-874-3p/HDAC1 axis. J. Gene Med. 2021, 23, e3329. [Google Scholar] [CrossRef]
- Chen, F.; Wang, X.; Fu, S.; Wang, S.; Fu, Y.; Zhang, J.; Liu, Z. circular RNA circ-CDYL sponges miR-1180 to elevate yes-associated protein in multiple myeloma. Exp. Biol. Med. 2020, 245, 925–932. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.; Li, W.; Wang, L.; Yan, Z.; Li, H.; Yao, Y.; Yao, R.; Xu, K.; Li, Z. miR-15a/16 regulates the growth of myeloma cells, angiogenesis and antitumor immunity by inhibiting Bcl-2, VEGF-A and IL-17 expression in multiple myeloma. Leuk. Res. 2016, 49, 73–79. [Google Scholar] [CrossRef]
- Kwon, J.J.; Factora, T.D.; Dey, S.; Kota, J. A systematic review of miR-29 in cancer. Mol. Ther.-Oncolytics 2019, 12, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Xiao, X.; Yang, S. LncRNA MALAT1 acts as an oncogene in multiple myeloma through sponging miR-509-5p to modulate FOXP1 expression. Oncotarget 2017, 8, 101984–101993. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Feng, S.; Li, H.; Chen, X.; Bai, S.; Liu, Y. Long non-coding RNA MALAT1 facilitates the tumorigenesis, invasion and glycolysis of multiple myeloma via miR-1271-5p/SOX13 axis. J. Cancer Res. Clin. Oncol. 2020, 146, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, L. Downregulation of lncRNA UCA1 facilitates apoptosis and reduces proliferation in multiple myeloma via regulation of the miR-1271-5p/HGF axis. J. Chin. Med. Assoc. 2019, 82, 699–709. [Google Scholar] [CrossRef]
- Raimondi, L.; Amodio, N.; Di Martino, M.T.; Altomare, E.; Leotta, M.; Caracciolo, D.; Gullà, A.; Neri, A.; Taverna, S.; D’Aquila, P.; et al. Targeting of multiple myeloma-related angiogenesis by miR-199a-5p mimics: In Vitro and In Vivo anti-tumor activity. Oncotarget 2014, 5, 3039–3054. [Google Scholar] [CrossRef]
- Gowda, P.S.; Wildman, B.J.; Trotter, T.N.; Xu, X.; Hao, X.; Hassan, M.Q.; Yang, Y. Runx2 suppression by miR-342 and miR-363 inhibits multiple myeloma progression. Mol. Cancer Res. 2018, 16, 1138–1148. [Google Scholar] [CrossRef]
- Inomata, M.; Tagawa, H.; Guo, Y.-M.; Kameoka, Y.; Takahashi, N.; Sawada, K. microRNA-17-92 down-regulates expression of distinct targets in different B-cell lymphoma subtypes. Blood 2009, 113, 396–402. [Google Scholar] [CrossRef]
- Leone, E.; Morelli, E.; Di Martino, M.T.; Amodio, N.; Foresta, U.; Gullà, A.; Rossi, M.; Neri, A.; Giordano, A.; Munshi, N.C.; et al. Targeting miR-21 inhibits in vitro and in vivo multiple myeloma cell growth. Clin. Cancer Res. 2013, 19, 2096–2106. [Google Scholar] [CrossRef]
- Memari, F.; Joneidi, Z.; Taheri, B.; Aval, S.F.; Roointan, A.; Zarghami, N. Epigenetics and epi-miRNAs: Potential markers/therapeutics in leukemia. Biomed. Pharmacother. 2018, 106, 1668–1677. [Google Scholar] [CrossRef]
- Agarwal, P.; Alzrigat, M.; Párraga, A.A.; Enroth, S.; Singh, U.; Ungerstedt, J.; Österborg, A.; Brown, P.J.; Ma, A.; Jin, J.; et al. Genome-wide profiling of histone H3 lysine 27 and lysine 4 trimethylation in multiple myeloma reveals the importance of Polycomb gene targeting and highlights EZH2 as a potential therapeutic target. Oncotarget 2016, 7, 6809–6823. [Google Scholar] [CrossRef]
- Amodio, N.; Stamato, M.A.; Gullà, A.M.; Morelli, E.; Romeo, E.; Raimondi, L.; Pitari, M.R.; Ferrandino, I.; Misso, G.; Caraglia, M.; et al. Therapeutic targeting of miR-29b/HDAC4 epigenetic loop in multiple myeloma. Mol. Cancer Ther. 2016, 15, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhang, D.-H.; Wu, N.; Xiao, J.-H.; Wang, X.; Ma, W. ceRNA in cancer: Possible functions and clinical implications. J. Med. Genet. 2015, 52, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kumar, S.; Jin, D.-Y.; Calin, G.A.; Chng, W.-J.; Siu, K.-L.; Poon, M.W.; Chim, C.S. Epigenetic silencing of long non-coding RNA BM742401 in multiple myeloma: Impact on prognosis and myeloma dissemination. Cancer Cell Int. 2020, 20, 403. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.-B.; He, X.; Huang, Y.-F.; Wu, Q.-N.; Zhou, Y.-C.; Hao, D.-J. Long noncoding RNA CRNDE promotes multiple myeloma cell growth by suppressing miR-451. Oncol. Res. 2017, 25, 1207–1214. [Google Scholar] [CrossRef]
- Tong, J.; Xu, X.; Zhang, Z.; Ma, C.; Xiang, R.; Liu, J.; Xu, W.; Wu, C.; Li, J.; Zhan, F.; et al. Hypoxia-induced long non-coding RNA DARS-AS1 regulates RBM39 stability to promote myeloma malignancy. Haematologica 2020, 105, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, Y.; Liu, W.; Huang, Y.; Shen, X.; Jing, R.; Pu, J.; Wang, X.; Ju, S.; Cong, H.; et al. LncRNA H19 overexpression induces bortezomib resistance in multiple myeloma by targeting MCL-1 via miR-29b-3p. Cell Death Dis. 2019, 10, 106. [Google Scholar] [CrossRef]
- Guan, R.; Wang, W.; Fu, B.; Pang, Y.; Lou, Y.; Li, H. Increased lncRNA HOTAIR expression promotes the chemoresistance of multiple myeloma to dexamethasone by regulating cell viability and apoptosis by mediating the JAK2/STAT3 signaling pathway. Mol. Med. Rep. 2019, 20, 3917–3923. [Google Scholar] [CrossRef]
- Hu, Y.; Lin, J.; Fang, H.; Fang, J.; Li, C.; Chen, W.; Liu, S.; Ondrejka, S.; Gong, Z.; Reu, F.; et al. Targeting the MALAT1/PARP1/LIG3 complex induces DNA damage and apoptosis in multiple myeloma. Leukemia 2018, 32, 2250–2262. [Google Scholar] [CrossRef]
- Handa, H.; Kuroda, Y.; Kimura, K.; Masuda, Y.; Hattori, H.; Alkebsi, L.; Matsumoto, M.; Kasamatsu, T.; Kobayashi, N.; Tahara, K.-I.; et al. Long non-coding RNA MALAT1 is an inducible stress response gene associated with extramedullary spread and poor prognosis of multiple myeloma. Br. J. Haematol. 2017, 179, 449–460. [Google Scholar] [CrossRef]
- Benetatos, L.; Dasoula, A.; Hatzimichael, E.; Georgiou, I.; Syrrou, M.; Bourantas, K.L. Promoter hypermethylation of the MEG3 (DLK1/MEG3) imprinted gene in multiple myeloma. Clin. Lymphoma Myeloma 2008, 8, 171–175. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, X.; Zhang, F.; Jiang, S.; Liu, J.; Luo, Y. Bortezomib-inducible long non-coding RNA myocardial infarction associated transcript is an oncogene in multiple myeloma that suppresses miR-29b. Cell Death Dis. 2019, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- Taiana, E.; Favasuli, V.; Ronchetti, D.; Todoerti, K.; Pelizzoni, F.; Manzoni, M.; Barbieri, M.; Fabris, S.; Silvestris, I.; Cantafio, M.E.G.; et al. Long non-coding RNA NEAT1 targeting impairs the DNA repair machinery and triggers anti-tumor activity in multiple myeloma. Leukemia 2020, 34, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Geng, W.; Guo, X.; Zhang, L.; Ma, Y.; Wang, L.; Liu, Z.; Ji, H.; Xiong, Y. Resveratrol inhibits proliferation, migration and invasion of multiple myeloma cells via NEAT1-mediated Wnt/beta-catenin signaling pathway. Biomed. Pharmacother. 2018, 107, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Jiang, S.; Fu, Y.; Luo, Y.; Gui, R.; Liu, J. Upregulation of lncRNA NR_046683 serves as a prognostic biomarker and potential drug target for multiple myeloma. Front. Pharmacol. 2019, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ye, H.; He, M.; Zhou, X.; Sun, N.; Guo, W.; Lin, X.; Huang, H.; Lin, Y.; Yao, R.; et al. LncRNA PDIA3P interacts with c-Myc to regulate cell proliferation via induction of pentose phosphate pathway in multiple myeloma. Biochem. Biophys. Res. Commun. 2018, 498, 207–213. [Google Scholar] [CrossRef]
- Li, B.; Xu, H.; Han, H.; Song, S.; Zhang, X.; Ouyang, L.; Qian, C.; Hong, Y.; Qiu, Y.; Zhou, W.; et al. Exosome-mediated transfer of lncRUNX2-AS1 from multiple myeloma cells to MSCs contributes to osteogenesis. Oncogene 2018, 37, 5508–5519. [Google Scholar] [CrossRef]
- Carrasco-Leon, A.; Ezponda, T.; Meydan, C.; Valcarcel, L.V.; Ordoñez, R.; Kulis, M.; Garate, L.; Miranda, E.; Segura, V.; Guruceaga, E.; et al. Characterization of complete lncRNAs transcriptome reveals the functional and clinical impact of lncRNAs in multiple myeloma. Leukemia 2021, 35, 1438–1450. [Google Scholar] [CrossRef]
- Yang, X.; Huang, H.; Wang, X.; Liu, H.; Liu, H.; Lin, Z. Knockdown of lncRNA SNHG16 suppresses multiple myeloma cell proliferation by sponging miR-342-3p. Cancer Cell Int. 2020, 20, 38. [Google Scholar] [CrossRef]
- Pu, J.; Huang, H.; Su, J.; Yuan, J.; Cong, H.; Wang, X.; Ju, S. Decreased expression of long noncoding RNA XLOC_013703 promotes cell growth via NF-kappaB pathway in multiple myeloma. IUBMB Life 2019, 71, 1240–1251. [Google Scholar] [CrossRef]
- Gao, D.; Lv, A.-E.; Li, H.-P.; Han, D.-H.; Zhang, Y.-P. LncRNA MALAT-1 elevates HMGB1 to promote autophagy resulting in inhibition of tumor cell apoptosis in multiple myeloma. J. Cell. Biochem. 2017, 118, 3341–3348. [Google Scholar] [CrossRef]
- Ikeda, S.; Kitadate, A.; Abe, F.; Takahashi, N.; Tagawa, H. Hypoxia-inducible KDM3A addiction in multiple myeloma. Blood Adv. 2018, 2, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Bhan, A.; Mandal, S.S. LncRNA HOTAIR: A master regulator of chromatin dynamics and cancer. Biochim. Biophys. Acta 2015, 1856, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hong, J.; Hong, M.; Wang, Y.; Yu, T.; Zang, S.; Wu, Q. piRNA-823 delivered by multiple myeloma-derived extracellular vesicles promoted tumorigenesis through re-educating endothelial cells in the tumor environment. Oncogene 2019, 38, 5227–5238. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wu, Q.-L.; Sun, C.-Y.; Ai, L.-S.; Deng, J.; Zhang, L.; Chen, L.; Chu, Z.-B.; Tang, B.; Wang, K.; et al. piRNA-823 contributes to tumorigenesis by regulating de novo DNA methylation and angiogenesis in multiple myeloma. Leukemia 2015, 29, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Taulli, R.; Pandolfi, P.P. “Snorkeling” for missing players in cancer. J. Clin. Investig. 2012, 122, 2765–2768. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Su, M.Y.; Maggi, L.B., Jr.; Lu, L.; Mullins, C.; Crosby, S.; Huang, G.; Chng, W.J.; Vij, R.; Tomasson, M.H. Multiple myeloma-associated chromosomal translocation activates orphan snoRNA ACA11 to suppress oxidative stress. J. Clin. Investig. 2012, 122, 2793–2806. [Google Scholar] [CrossRef]
- Oliveira, V.; Mahajan, N.; Bates, M.L.; Tripathi, C.; Kim, K.Q.; Zaher, H.S.; Maggi, L.B., Jr.; Tomasson, M.H. The snoRNA target of t(4;14) in multiple myeloma regulates ribosome biogenesis. FASEB BioAdvances 2019, 1, 404–414. [Google Scholar] [CrossRef]
- Zhou, Y.; Goodenbour, J.M.; Godley, L.A.; Wickrema, A.; Pan, T. High levels of tRNA abundance and alteration of tRNA charging by bortezomib in multiple myeloma. Biochem. Biophys. Res. Commun. 2009, 385, 160–164. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, D.; Wei, W.; Chen, H.; Shi, H.; Zhou, N.; Wu, L.; Peng, R. Comprehensive profiling of circular RNA expressions reveals potential diagnostic and prognostic biomarkers in multiple myeloma. BMC Cancer 2020, 20, 40. [Google Scholar] [CrossRef]
- Gao, M.; Li, C.; Xiao, H.; Dong, H.; Jiang, S.; Fu, Y.; Gong, L. hsa_circ_0007841: A novel potential biomarker and drug resistance for multiple myeloma. Front. Oncol. 2019, 9, 1261. [Google Scholar] [CrossRef]
- Palumbo, A.; Anderson, K. Multiple myeloma. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Oggu, G.S.; Sasikumar, S.; Reddy, N.; Ella, K.K.R.; Rao, C.M.; Bokara, K.K. Gene delivery approaches for mesenchymal stem cell therapy: Strategies to increase efficiency and specificity. Stem Cell Rev. Rep. 2017, 13, 725–740. [Google Scholar] [CrossRef] [PubMed]
- Chira, S.; Gulei, D.; Hajitou, A.; Zimta, A.A.; Cordelier, P.; Berindan-Neagoe, I. CRISPR/Cas9: Transcending the reality of genome editing. Mol. Ther.-Nucleic Acids 2017, 7, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Hirosawa, M.; Fujita, Y.; Parr, C.J.C.; Hayashi, K.; Kashida, S.; Hotta, A.; Woltjen, K.; Saito, H. Cell-type-specific genome editing with a microRNA-responsive CRISPR-Cas9 switch. Nucleic Acids Res. 2017, 45, e118. [Google Scholar] [CrossRef] [PubMed]
- Brocken, D.J.W.; Tark-Dame, M.; Dame, R.T. dCas9: A versatile tool for epigenome editing. Curr. Issues Mol. Biol. 2018, 26, 15–32. [Google Scholar] [CrossRef]
- Hilton, I.B.; D’Ippolito, A.M.; Vockley, C.M.; Thakore, P.I.; Crawford, G.E.; Reddy, T.E.; Gersbach, C.A. Epigenome editing by a CRISPR-Cas9-based acetyltransferase activates genes from promoters and enhancers. Nat. Biotechnol. 2015, 33, 510–517. [Google Scholar] [CrossRef]
- Vojta, A.; Dobrinić, P.; Tadić, V.; Bockor, L.; Korać, P.; Julg, B.; Klasić, M.; Zoldoš, V. Repurposing the CRISPR-Cas9 system for targeted DNA methylation. Nucleic Acids Res. 2016, 44, 5615–5628. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Zhao, Y.-T.; Lamonica, J.M.; Zhou, Z. Locus-specific histone deacetylation using a synthetic CRISPR-Cas9-based HDAC. Nat. Commun. 2017, 8, 15315. [Google Scholar] [CrossRef]
- Granados-Riveron, J.T.; Aquino-Jarquin, G. CRISPR-Cas13 precision transcriptome engineering in cancer. Cancer Res. 2018, 78, 4107–4113. [Google Scholar] [CrossRef]
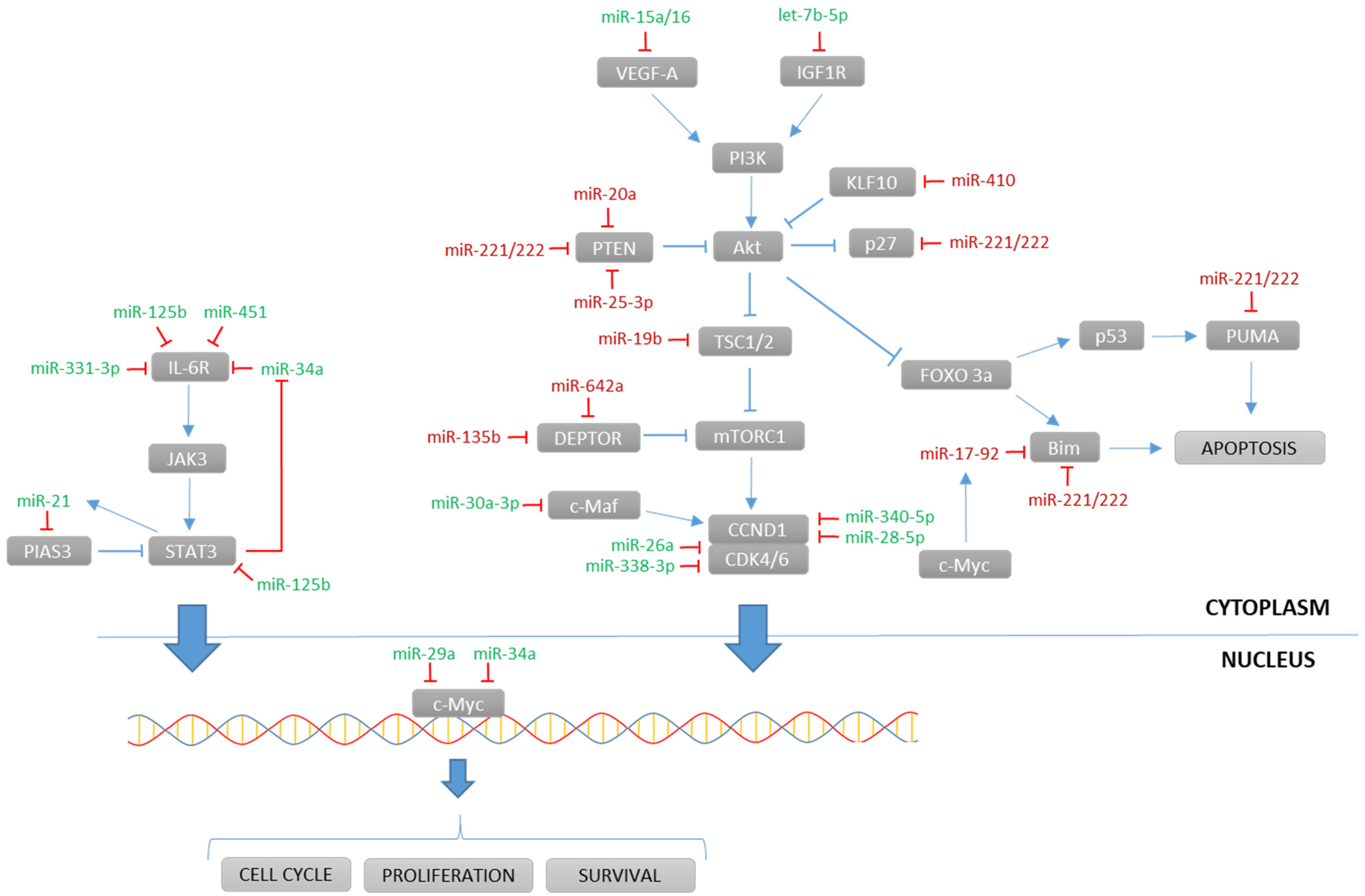
| Activity/Pathway Affected | miRNA | Status 1 | Target | References |
|---|---|---|---|---|
| Enhances PI3K/Akt pathway | miR-20a |  | EGR2, PTEN | [29,30] |
| miR-21 |  | PIAS3 | [31] | |
| miR-25-3p |  | PTEN | [32] | |
| miR-221/222 |  | PUMA, PTEN, CDKN1B, p27 | [33] | |
| miR-410 |  | KLF10 | [34] | |
| Enhances mTOR pathway | miR-19b |  | TSC1 | [35,36] |
| miR-135b, miR-642a |  | DEPTOR | [37] | |
| Related to a hypoxia phenotype | miR-210 |  | DIMT1 | [38,39] |
| miR-1305 |  | MDM2, IGF1, FGF2 | [40] | |
| Disrupts PRC2 activity | miR-124 |  | EZH2 | [41] |
| Modulates microenvironment | miR-146a |  | Not described | [42] |
| miR-155 |  | Not described | [43] | |
| Promotes proliferation, circulating miRNAs | miR-17-92 |  | BIM | [35] |
| miR-221/222 |  | [33] | ||
| Circulating miRNA | miR-1 |  | Not described | [44] |
| miR-133a/b |  | Not described | [44] | |
| miR-135b |  | HIF1A | [45,46] | |
| miR-146b |  | Not described | [47] | |
| miR-181a |  | BCL2L11 | [48,49] | |
| miR-214 |  | CD276 | [50] | |
| Represses JAK/STAT pathway | miR-125b |  | IL6R, STAT3, MALAT1 | [51,52] |
| miR-331-3p |  | IL6R | [53] | |
| miR-375 |  | PDPK1 | [54] | |
| miR-451 |  | IL6R | [55] | |
| let-7b-5p |  | IGF1R | [56] | |
| Regulates cyclin activity | miR-26a |  | CDK6 | [57] |
| miR-28-5p |  | CCND1 | [58] | |
| miR-30a-3p |  | MAF | [59] | |
| miR-338-3p |  | CDK4 | [60] | |
| miR-340-5p |  | CCND1, NRAS | [61] | |
| miR-196a/b |  | CCND2 | [44] | |
| Regulates proliferation | miR-22 |  | c-Myc | [62] |
| miR-29a |  | c-Myc | [63] | |
| miR-34a |  | BCL2, CDK6, NOTCH1, c-Myc, MET, IL6R | [52,64,65] | |
| miR-193a |  | MCL1 | [66] | |
| miR-497 |  | BCL2 | [67] | |
| miR-767-5p |  | MAPK4 | [68] | |
| miR-874-3p |  | HDAC1 | [69] | |
| miR-1180 |  | YAP | [70] | |
| Prevents angiogenesis | miR-15a/16 |  | BCL2, VEGF, IL17 | [71] |
| Regulates acetylation | miR-29b |  | HDAC4, MCL1 | [72] |
| Regulates transcriptional activity | miR-509-5p |  | FOXP1 | [73] |
| miR-1271-5p |  | SOX13, HGF | [74,75] | |
| Prevents hypoxia phenotype | miR-199a-5p |  | HIF1A, VEGFA | [76] |
| Prevents osteolytic activity | miR-342 |  | RUNX2 | [77] |
| miR-363 |  | RUNX2 | [77] |
| lncRNA | Status 1 | Target | Activity/Pathway Affected | References |
|---|---|---|---|---|
| ANGPLT1-3 |  | miR-30a-3p | ceRNA | [59] |
| BM742401 |  | Not described | Inhibit myeloma cell migration, biomarker | [84] |
| CRNDE |  | miR-451 | ceRNA | [55,85] |
| DARS-AS1 |  | RBM39 | Enhances mTOR pathway, hypoxia phenotype | [86] |
| H19 |  | miR-29b | ceRNA, biomarker | [87] |
| HOTAIR |  | Not described | Enhances JAK/STAT pathway | [88] |
| MALAT1 |  | HMGB1, miR-509-5p, miR-1271 | Contributes to genomic stability, ceRNA, biomarker | [73,74,89,90] |
| MEG3 |  | miR-181a | Promotes osteogenic differentiation, biomarker, ceRNA | [91] |
| MIAT |  | miR-29b | Inducible by bortezomib, ceRNA, biomarker | [92] |
| NEAT1 |  | miR-214, miR-193a | Downregulates genes involved in DNA repair, enhances Wnt/β-catenin pathway, ceRNA | [50,66,93,94] |
| NR_046683 |  | Not described | Biomarker | [95] |
| OPI5-AS1 |  | miR-410 | ceRNA | [34] |
| PDIA3P |  | c-Myc | Regulates proliferation | [96] |
| RUNX2-AS1 |  | RUNX2 pre-mRNA | Promotes osteogenesis | [97] |
| SMILO |  | Not described | Regulates proliferation | [98] |
| SNHG16 |  | miR-342 | ceRNA | [99] |
| UCA1 |  | miR-1271-5p, miR-331-3p | ceRNA | [61,75] |
| XLOC_013703 |  | IKKA | Represses NF-κB pathway | [100] |
| lncRNA | miRNA | Gene | References |
|---|---|---|---|
| ANGPLT1-3 | miR-30a-3p | MAF | [59] |
| CRNDE | miR-451 | IL6R | [55,85] |
| H19 | miR-29b | HDAC4 and MCL1 | [72,87] |
| MALAT1 | miR-509-5p | FOXP1 | [73] |
| miR-1271-5p | SOX13 | [74] | |
| MEG3 | miR-181a | BCL2L11 | [91] |
| MIAT | miR-29b | HDAC4 and MCL1 | [72,92] |
| NEAT1 | miR-214 | CD276 | [50] |
| miR-193a | MCL1 | [66] | |
| OPI5-AS1 | miR-410 | KLF10 | [34] |
| PRAL | miR-210 | DIMT1 | [38,39] |
| SNHG16 | miR-342 | RUNX2 | [99] |
| UCA1 | miR-331-3p | IL6R | [61] |
| miR-1271-5p | SOX13 and HGF | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coira, I.F.; Rincón, R.; Cuendet, M. The Multiple Myeloma Landscape: Epigenetics and Non-Coding RNAs. Cancers 2022, 14, 2348. https://doi.org/10.3390/cancers14102348
Coira IF, Rincón R, Cuendet M. The Multiple Myeloma Landscape: Epigenetics and Non-Coding RNAs. Cancers. 2022; 14(10):2348. https://doi.org/10.3390/cancers14102348
Chicago/Turabian StyleCoira, Isabel F., Rafael Rincón, and Muriel Cuendet. 2022. "The Multiple Myeloma Landscape: Epigenetics and Non-Coding RNAs" Cancers 14, no. 10: 2348. https://doi.org/10.3390/cancers14102348
APA StyleCoira, I. F., Rincón, R., & Cuendet, M. (2022). The Multiple Myeloma Landscape: Epigenetics and Non-Coding RNAs. Cancers, 14(10), 2348. https://doi.org/10.3390/cancers14102348






