Updates on Molecular Targeted Therapies for Intraparenchymal CNS Metastases
Abstract
:Simple Summary
Abstract
1. Introduction
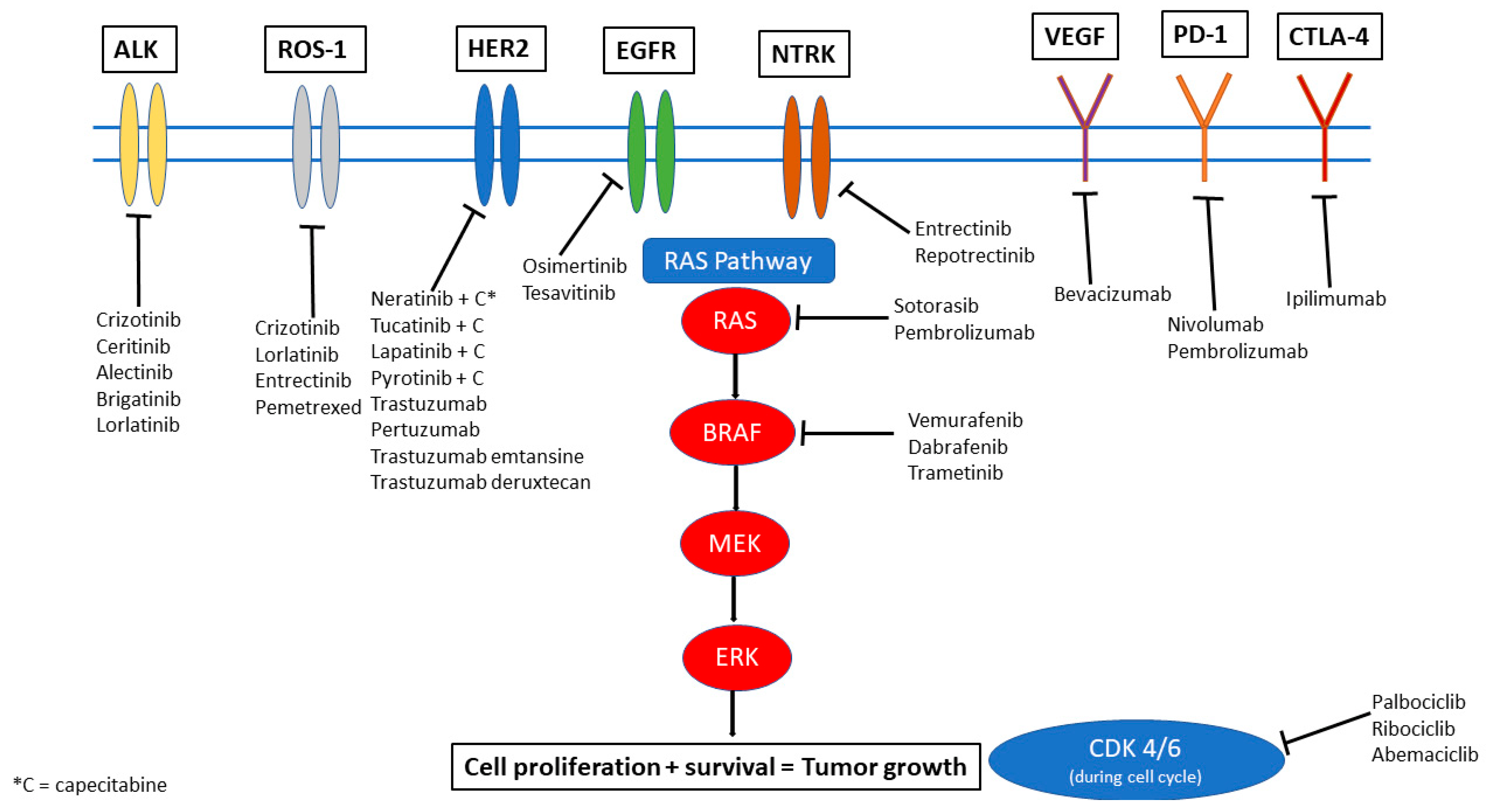
2. ALK-Targeted Therapies
3. EGFR Targeted Therapies
4. ROS-1 Alterations
5. NTRK
6. KRAS
7. CDK4/6
8. Her2+ Targeted Therapies
9. PD-1/PD-L1 Inhibition
10. Other Agents
11. Conclusions/Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Kromer, C.; Xu, J.; Ostrom, Q.; Gittleman, H.; Kruchko, C.; Sawaya, R.; Barnholtz-Sloan, J.S. Estimating the annual frequency of synchronous brain metastasis in the United States 2010–2013: A population-based study. J. Neuro-Oncol. 2017, 134, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Schouten, L.J.; Rutten, J.; Huveneers, H.A.M.; Twijnstra, A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002, 94, 2698–2705. [Google Scholar] [CrossRef] [PubMed]
- Villano, J.L.; Durbin, E.B.; Normandeau, C.; Thakkar, J.P.; Moirangthem, V.; Davis, F.G. Incidence of brain metastasis at initial presentation of lung cancer. Neuro Oncol. 2014, 17, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brastianos, P.K.; Carter, S.L.; Santagata, S.; Cahill, D.; Taylor-Weiner, A.; Jones, R.T.; Van Allen, E.M.; Lawrence, M.S.; Horowitz, P.; Cibulskis, K.; et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov. 2015, 5, 1164–1177. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Ou, Q.; Li, D.; Qin, T.; Bao, H.; Hou, X.; Wang, K.; Wang, F.; Deng, Q.; Liang, J.; et al. Genes associated with increased brain metastasis risk in non–small cell lung cancer: Comprehensive genomic profiling of 61 resected brain metastases versus primary non–small cell lung cancer (Guangdong Association Study of Thoracic Oncology 1036). Cancer 2019, 125, 3535–3544. [Google Scholar] [CrossRef]
- Shaw, A.T.; Kim, T.M.; Crinò, L.; Gridelli, C.; Kiura, K.; Liu, G.; Novello, S.; Bearz, A.; Gautschi, O.; Mok, T.; et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 874–886. [Google Scholar] [CrossRef]
- Nishio, M.; Felip, E.; Orlov, S.; Park, K.; Yu, C.-J.; Tsai, C.-M.; Cobo, M.; McKeage, M.; Su, W.-C.; Mok, T.; et al. Final Overall Survival and Other Efficacy and Safety Results From ASCEND-3: Phase II Study of Ceritinib in ALKi-Naive Patients With ALK-Rearranged NSCLC. J. Thorac. Oncol. 2019, 15, 609–617. [Google Scholar] [CrossRef]
- Chow, L.; Barlesi, F.; Bertino, E.; Bent, M.V.D.; Wakelee, H.; Wen, P.; Chiu, C.-H.; Orlov, S.; Majem, M.; Chiari, R.; et al. Results of the ASCEND-7 phase II study evaluating ALK inhibitor (ALKi) ceritinib in patients (pts) with ALK+ non-small cell lung cancer (NSCLC) metastatic to the brain. Ann. Oncol. 2019, 30, v602–v603. [Google Scholar] [CrossRef]
- Hida, T.; Nokihara, H.; Kondo, M.; Kim, Y.H.; Azuma, K.; Seto, T.; Takiguchi, Y.; Nishio, M.; Yoshioka, H.; Imamura, F.; et al. Alectinib versus crizotinib in patients with ALK -positive non-small-cell lung cancer (J-ALEX): An open-label, randomised phase 3 trial. Lancet 2017, 390, 29–39. [Google Scholar] [CrossRef]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.W.; Ou, S.-H.I.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef]
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.H.; Han, J.-Y.; Hochmair, M.J.; Lee, K.H.; Delmonte, A.; Campelo, M.R.G.; Kim, D.-W.; et al. Brigatinib Versus Crizotinib in Advanced ALK Inhibitor–Naive ALK-Positive Non–Small Cell Lung Cancer: Second Interim Analysis of the Phase III ALTA-1L Trial. J. Clin. Oncol. 2020, 38. [Google Scholar] [CrossRef]
- Shaw, A.T.; Bauer, T.M.; De Marinis, F.; Felip, E.; Goto, Y.; Liu, G.; Mazieres, J.; Kim, D.-W.; Mok, T.; Polli, A.; et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 2020, 383, 2018–2029. [Google Scholar] [CrossRef]
- Johung, K.L.; Yeh, N.; Desai, N.B.; Williams, T.M.; Lautenschlaeger, T.; Arvold, N.D.; Ning, M.S.; Attia, A.; Lovly, C.; Goldberg, S.; et al. Extended Survival and Prognostic Factors for Patients With ALK-Rearranged Non–Small-Cell Lung Cancer and Brain Metastasis. J. Clin. Oncol. 2016, 34, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.-L.; Ahn, M.-J.; Garassino, M.C.; Han, J.-Y.; Katakami, N.; Kim, H.R.; Hodge, R.; Kaur, P.; Brown, A.P.; Ghiorghiu, D.; et al. CNS Efficacy of Osimertinib in Patients With T790M-Positive Advanced Non–Small-Cell Lung Cancer: Data From a Randomized Phase III Trial (AURA3). J. Clin. Oncol. 2018, 36, 2702–2709. [Google Scholar] [CrossRef]
- Reungwetwattana, T.; Nakagawa, K.; Cho, B.C.; Cobo, M.; Cho, E.K.; Bertolini, A.; Bohnet, S.; Zhou, C.; Lee, K.H.; Nogami, N.; et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 3290–3297. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef]
- Magnuson, W.J.; Lester-Coll, N.; Wu, A.J.; Yang, T.J.; Lockney, N.; Gerber, N.K.; Beal, K.; Amini, A.; Patil, T.; Kavanagh, B.D.; et al. Management of Brain Metastases in Tyrosine Kinase Inhibitor–Naïve Epidermal Growth Factor Receptor–Mutant Non–Small-Cell Lung Cancer: A Retrospective Multi-Institutional Analysis. J. Clin. Oncol. 2017, 35, 1070–1077. [Google Scholar] [CrossRef]
- Xie, L.; Nagpal, S.; Wakelee, H.A.; Li, G.; Soltys, S.G.; Neal, J.W. Osimertinib for EGFR -Mutant Lung Cancer with Brain Metastases: Results from a Single-Center Retrospective Study. Oncologist 2018, 24, 836–843. [Google Scholar] [CrossRef] [Green Version]
- Lin, N.U.; Freedman, R.A.; Miller, K.; Jhaveri, K.L.; Eiznhamer, D.A.; Berger, M.S.; Hamilton, E.P. Determination of the maximum tolerated dose (MTD) of the CNS penetrant tyrosine kinase inhibitor (TKI) tesevatinib administered in combination with trastuzumab in HER2+ patients with metastatic breast cancer (BC). J. Clin. Oncol. 2016, 34, 514. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Hsieh, M.-S.; Wu, S.-G.; Chang, Y.-L.; Yu, C.-J.; Yang, J.C.-H.; Yang, P.-C.; Shih, J.-Y. Efficacy of Pemetrexed-Based Chemotherapy in Patients with ROS1 Fusion–Positive Lung Adenocarcinoma Compared with in Patients Harboring Other Driver Mutations in East Asian Populations. J. Thorac. Oncol. 2016, 11, 1140–1152. [Google Scholar] [CrossRef] [Green Version]
- Kumthekar, P.; Grimm, S.A.; Avram, M.J.; Kaklamani, V.; Helenowski, I.; Rademaker, A.; Cianfrocca, M.; Gradishar, W.; Patel, J.; Mulcahy, M.; et al. Pharmacokinetics and efficacy of pemetrexed in patients with brain or leptomeningeal metastases. J. Neuro-Oncology 2013, 112, 247–255. [Google Scholar] [CrossRef]
- Shaw, A.T.; Felip, E.; Bauer, T.M.; Besse, B.; Navarro, A.; Postel-Vinay, S.; Gainor, J.F.; Johnson, M.; Dietrich, J.; James, L.P.; et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: An international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017, 18, 1590–1599. [Google Scholar] [CrossRef]
- Wu, Y.L.; Yang, J.C.; Kim, D.W.; Lu, S.; Zhou, J.; Seto, T.; Yang, J.J.; Yamamoto, N.; Ahn, M.J.; Takahashi, T.; et al. Phase II Study of Crizotinib in East Asian Patients With ROS1-Positive Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1405–1411. [Google Scholar] [CrossRef]
- Drilon, A.E.; Ou, S.-H.I.; Cho, B.C.; Kim, D.-W.; Lee, J.; Lin, J.J.; Zhu, V.W.; Kim, H.; Kim, T.M.; Ahn, M.-J.; et al. A phase 1 study of the next-generation ALK/ROS1/TRK inhibitor ropotrectinib (TPX-0005) in patients with advanced ALK/ROS1/NTRK+ cancers (TRIDENT-1). J. Clin. Oncol. 2018, 36, 2513. [Google Scholar] [CrossRef] [Green Version]
- Yun, M.R.; Kim, D.H.; Kim, S.-Y.; Joo, H.-S.; Lee, Y.W.; Choi, H.M.; Park, C.W.; Heo, S.G.; Kang, H.N.; Lee, S.S.; et al. Repotrectinib Exhibits Potent Antitumor Activity in Treatment-Naïve and Solvent-Front–Mutant ROS1-Rearranged Non–Small Cell Lung Cancer. Clin. Cancer Res. 2020. [Google Scholar] [CrossRef] [Green Version]
- Drilon, A.; Siena, S.; Dziadziuszko, R.; Barlesi, F.; Krebs, M.G.; Shaw, A.T.; de Braud, F.; Rolfo, C.; Ahn, M.-J.; Wolf, J.; et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2019, 21, 261–270. [Google Scholar] [CrossRef]
- Drilon, A.; Siena, S.; Ou, S.-H.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.J.; Kummar, S.; Tan, D.S.-W.; Lassen, U.N.; Leyvraz, S.; Liu, Y.; Moreno, V.; Patel, J.D.; Rosen, L.S.; Solomon, B.M.; et al. Long-term efficacy and safety of larotrectinib in patients with TRK fusion-positive lung cancer. J. Clin. Oncol. 2021, 39, 9109. [Google Scholar] [CrossRef]
- Marabese, M.; Ganzinelli, M.; Garassino, M.C.; Shepherd, F.A.; Piva, S.; Caiola, E.; Macerelli, M.; Bettini, A.; Lauricella, C.; Floriani, I.; et al. KRAS mutations affect prognosis of non-small-cell lung cancer patients treated with first-line platinum containing chemotherapy. Oncotarget 2015, 6, 34014–34022. [Google Scholar] [CrossRef] [Green Version]
- Hong, D.S.; Fakih, M.G.; Strickler, J.H.; Desai, J.; Durm, G.A.; Shapiro, G.I.; Falchook, G.S.; Price, T.J.; Sacher, A.; Denlinger, C.S.; et al. KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. N. Engl. J. Med. 2020, 383, 1207–1217. [Google Scholar] [CrossRef]
- Herbst, R.; Lopes, G.; Kowalski, D.; Kasahara, K.; Wu, Y.-L.; De Castro, G.; Cho, B.; Turna, H.; Cristescu, R.; Aurora-Garg, D.; et al. LBA4 Association of KRAS mutational status with response to pembrolizumab monotherapy given as first-line therapy for PD-L1-positive advanced non-squamous NSCLC in Keynote-042. Ann. Oncol. 2019, 30, xi63–xi64. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Hei, R.; Li, X.; Cai, H.; Wu, X.; Zheng, Q.; Cai, C. CDK inhibitors in cancer therapy, an overview of recent development. Am. J. Cancer Res. 2021, 11, 1913–1935. [Google Scholar] [PubMed]
- Tolaney, S.M.; Sahebjam, S.; Le Rhun, E.; Bachelot, T.; Kabos, P.; Awada, A.; Yardley, D.; Chan, A.; Conte, P.; Diéras, V.; et al. A Phase II Study of Abemaciclib in Patients with Brain Metastases Secondary to Hormone Receptor–Positive Breast Cancer. Clin. Cancer Res. 2020, 26, 5310–5319. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Eierman, W.; Greil, R.; Campone, M.; Kaufman, B.; Steplewski, K.; Lane, S.R.; Zembryki, D.; Rubin, S.D.; Winer, E.P. Randomized phase II study of lapatinib plus capecitabine or lapatinib plus topotecan for patients with HER2-positive breast cancer brain metastases. J. Neuro-Oncol. 2011, 105, 613–620. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Saura, C.; Oliveira, M.; Trudeau, M.E.; Moy, B.; Delaloge, S.; Gradishar, W.; Kim, S.; Haley, B.; Ryvo, L.; et al. Efficacy of Neratinib Plus Capecitabine in the Subgroup of Patients with Central Nervous System Involvement from the NALA Trial. Oncologist 2021, 26, e1327–e1338. [Google Scholar] [CrossRef]
- Lin, N.U.; Borges, V.; Anders, C.; Murthy, R.K.; Paplomata, E.; Hamilton, E.; Hurvitz, S.; Loi, S.; Okines, A.; Abramson, V.; et al. Intracranial Efficacy and Survival With Tucatinib Plus Trastuzumab and Capecitabine for Previously Treated HER2-Positive Breast Cancer With Brain Metastases in the HER2CLIMB Trial. J. Clin. Oncol. 2020, 38, 2610–2619. [Google Scholar] [CrossRef]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020, 382, 597–609, Erratum in 2020, 382, 586. [Google Scholar] [CrossRef]
- Borges, V.F.; Ferrario, C.; Aucoin, N.; Falkson, C.; Khan, Q.; Krop, I.; Welch, S.; Conlin, A.; Chaves, J.; Bedard, P.L.; et al. Tucatinib Combined With Ado-Trastuzumab Emtansine in Advanced ERBB2/HER2-Positive Metastatic Breast Cancer: A Phase 1b Clinical Trial. JAMA Oncol. 2018, 4, 1214–1220. [Google Scholar] [CrossRef] [Green Version]
- Metzger Filho, O.; Leone, J.P.; Li, T.; Tan-Wasielewski, Z.; Trippa, L.; Barry, W.T.; Younger, J.; Lawler, E.; Walker, L.; Freedman, R.A.; et al. Phase I dose-escalation trial of tucatinib in combination with trastuzumab in patients with HER2-positive breast cancer brain metastases. Ann. Oncol. 2020, 31, 1231–1239. [Google Scholar] [CrossRef]
- Anwar, M.; Chen, Q.; Ouyang, D.; Wang, S.; Xie, N.; Ouyang, Q.; Fan, P.; Qian, L.; Chen, G.; Zhou, E.; et al. Pyrotinib Treatment in Patients With HER2-positive Metastatic Breast Cancer and Brain Metastasis: Exploratory Final Analysis of Real-World, Multicenter Data. Clin. Cancer Res. 2021, 27, 4634–4641. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, M.; Zhang, J.; Wang, B.; Tao, Z.; Du, Y.; Zhang, S.; Cao, J.; Wang, L.; Hu, X. Real-World Data of Pyrotinib-Based Therapy in Metastatic HER2-Positive Breast Cancer: Promising Efficacy in Lapatinib-Treated Patients and in Brain Metastasis. Cancer Res. Treat. 2020, 52, 1059–1066. [Google Scholar] [CrossRef]
- Zhou, C.; Li, X.; Wang, Q.; Gao, G.; Zhang, Y.; Chen, J.; Shu, Y.; Hu, Y.; Fan, Y.; Fang, J.; et al. Pyrotinib in HER2-Mutant Advanced Lung Adenocarcinoma After Platinum-Based Chemotherapy: A Multicenter, Open-Label, Single-Arm, Phase II Study. J. Clin. Oncol. 2020, 38, 2753–2761. [Google Scholar] [CrossRef]
- Yan, M.; Ouyang, Q.; Sun, T.; Niu, L.; Yang, J.; Li, L.; Song, Y.; Hao, C.; Chen, Z. Pyrotinib plus capecitabine for HER2-positive metastatic breast cancer patients with brain metastases (PERMEATE): A multicenter, single-arm phase II study. J. Clin. Oncol. 2021, 39, 1037. [Google Scholar] [CrossRef]
- Lin, N.U.; Pegram, M.; Sahebjam, S.; Ibrahim, N.; Fung, A.; Cheng, A.; Nicholas, A.; Kirschbrown, W.; Kumthekar, P. Pertuzumab Plus High-Dose Trastuzumab in Patients With Progressive Brain Metastases and HER2-Positive Metastatic Breast Cancer: Primary Analysis of a Phase II Study. J. Clin. Oncol. 2021, 39, 2667–2675. [Google Scholar] [CrossRef]
- Terrell-Hall, T.B.; Nounou, M.I.; El-Amrawy, F.; Griffith, J.; Lockman, P.R. Trastuzumab distribution in an in-vivo and in-vitro model of brain metastases of breast cancer. Oncotarget 2017, 8, 83734–83744. [Google Scholar] [CrossRef]
- Montemurro, F.; Delaloge, S.; Barrios, C.; Wuerstlein, R.; Anton, A.; Brain, E.; Hatschek, T.; Kelly, C.M.; Peña-Murillo, C.; Yilmaz, M.; et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: Exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial☆. Ann. Oncol. 2020, 31, 1350–1358. [Google Scholar] [CrossRef]
- Jerusalem, G.H.M.; Park, Y.H.; Yamashita, T.; Hurvitz, S.A.; Modi, S.; Andre, F.; Krop, I.E.; Gonzalez, X.; Hall, P.S.; You, B.; et al. Trastuzumab deruxtecan (T-DXd) in patients with HER2+ metastatic breast cancer with brain metastases: A subgroup analysis of the DESTINY-Breast01 trial. J. Clin. Oncol. 2021, 39, 526. [Google Scholar] [CrossRef]
- Bonneau, C.; Paintaud, G.; Tredan, O.; Dubot, C.; Desvignes, C.; Dieras, V.; Taillibert, S.; Tresca, P.; Turbiez, I.; Li, J.; et al. Phase I feasibility study for intrathecal administration of trastuzumab in patients with HER2 positive breast carcinomatous meningitis. Eur. J. Cancer 2018, 95, 75–84. [Google Scholar] [CrossRef]
- Figura, N.B.; Rizk, V.T.; Mohammadi, H.; Evernden, B.; Mokhtari, S.; Yu, H.M.; Robinson, T.J.; Etame, A.B.; Tran, N.D.; Liu, J.; et al. Clinical outcomes of breast leptomeningeal disease treated with intrathecal trastuzumab, intrathecal chemotherapy, or whole brain radiation therapy. Breast Cancer Res. Treat. 2019, 175, 781–788. [Google Scholar] [CrossRef]
- Kumthekar, P.; Lassman, A.B.; Lin, N.; Grimm, S.; Gradishar, W.; Pentsova, E.; Jeyapalan, S.; Groves, M.; Melisko, M.; Raizer, J. LPTO-02. INTRATHECAL (IT) TRASTUZUMAB (T) FOR THE TREATMENT OF LEPTOMENINGEAL DISEASE (LM) IN PATIENTS (PTS) WITH HUMAN EPIDERMAL RECEPTOR-2 POSITIVE (HER2+) CANCER: A MULTICENTER PHASE 1/2 STUDY. Neuro-Oncol. Adv. 2019, 1, i6. [Google Scholar] [CrossRef]
- McArthur, G.A.; Maio, M.; Arance, A.; Nathan, P.; Blank, C.; Avril, M.-F.; Garbe, C.; Hauschild, A.; Schadendorf, D.; Hamid, O.; et al. Vemurafenib in metastatic melanoma patients with brain metastases: An open-label, single-arm, phase 2, multicentre study. Ann. Oncol. 2016, 28, 634–641. [Google Scholar] [CrossRef]
- Long, G.V.; Atkinson, V.; Lo, S.; Guminski, A.D.; Sandhu, S.K.; Brown, M.P.; Gonzalez, M.; Scolyer, R.A.; Emmett, L.; McArthur, G.A.; et al. Five-year overall survival from the anti-PD1 brain collaboration (ABC Study): Randomized phase 2 study of nivolumab (nivo) or nivo+ipilimumab (ipi) in patients (pts) with melanoma brain metastases (mets). J. Clin. Oncol. 2021, 39, 9508. [Google Scholar] [CrossRef]
- Long, G.V.; Atkinson, V.; Lo, S.; Sandhu, S.; Guminski, A.D.; Brown, M.P.; Wilmott, J.S.; Edwards, J.; Gonzalez, M.; Scolyer, R.A.; et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 2018, 19, 672–681. [Google Scholar] [CrossRef]
- A Davies, M.; Saiag, P.; Robert, C.; Grob, J.-J.; Flaherty, K.T.; Arance, A.; Sileni, V.C.; Thomas, L.; Lesimple, T.; Mortier, L.; et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): A multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 863–873. [Google Scholar] [CrossRef]
- Tawbi, H.A.-H.; Forsyth, P.A.J.; Hodi, F.S.; Lao, C.D.; Moschos, S.J.; Hamid, O.; Atkins, M.B.; Lewis, K.D.; Thomas, R.P.; Glaspy, J.A.; et al. Efficacy and safety of the combination of nivolumab (NIVO) plus ipilimumab (IPI) in patients with symptomatic melanoma brain metastases (CheckMate 204). J. Clin. Oncol. 2019, 37, 9501. [Google Scholar] [CrossRef]
- A Tawbi, H.; A Forsyth, P.; Hodi, F.S.; Lao, C.D.; Moschos, S.J.; Hamid, O.; Atkins, M.B.; Lewis, K.; Thomas, R.P.; A Glaspy, J.; et al. Safety and efficacy of the combination of nivolumab plus ipilimumab in patients with melanoma and asymptomatic or symptomatic brain metastases (CheckMate 204). Neuro-Oncol. 2021, 23, 1961–1973. [Google Scholar] [CrossRef]
- Khan, M.; Lin, J.; Liao, G.; Tian, Y.; Liang, Y.; Li, R.; Liu, M.; Yuan, Y. SRS in Combination With Ipilimumab: A Promising New Dimension for Treating Melanoma Brain Metastases. Technol. Cancer Res. Treat. 2018, 17. [Google Scholar] [CrossRef] [Green Version]
- Kiess, A.P.; Wolchok, J.D.; Barker, C.; Postow, M.A.; Tabar, V.; Huse, J.T.; Chan, T.A.; Yamada, Y.; Beal, K. Stereotactic Radiosurgery for Melanoma Brain Metastases in Patients Receiving Ipilimumab: Safety Profile and Efficacy of Combined Treatment. Int. J. Radiat. Oncol. 2015, 92, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Skrepnik, T.; Sundararajan, S.; Cui, H.; Stea, B. Improved time to disease progression in the brain in patients with melanoma brain metastases treated with concurrent delivery of radiosurgery and ipilimumab. OncoImmunology 2017, 6, e1283461. [Google Scholar] [CrossRef] [Green Version]
- Minniti, G.; Anzellini, D.; Reverberi, C.; Cappellini, G.C.A.; Marchetti, L.; Bianciardi, F.; Bozzao, A.; Osti, M.; Gentile, P.C.; Esposito, V. Stereotactic radiosurgery combined with nivolumab or Ipilimumab for patients with melanoma brain metastases: Evaluation of brain control and toxicity. J. Immunother. Cancer 2019, 7, 102. [Google Scholar] [CrossRef] [Green Version]
- Powell, S.F.; Rodríguez-Abreu, D.; Langer, C.J.; Tafreshi, A.; Paz-Ares, L.; Kopp, H.-G.; Rodríguez-Cid, J.; Kowalski, D.M.; Cheng, Y.; Kurata, T.; et al. Outcomes With Pembrolizumab Plus Platinum-Based Chemotherapy for Patients With NSCLC and Stable Brain Metastases: Pooled Analysis of KEYNOTE-021, -189, and -407. J. Thorac. Oncol. 2021, 16, 1883–1892. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Diéras, V.; Weaver, R.; Tolaney, S.M.; Bardia, A.; Punie, K.; Brufsky, A.; Rugo, H.S.; Kalinsky, K.; Traina, T.; Klein, L.; et al. Abstract PD13-07: Subgroup analysis of patients with brain metastases from the phase 3 ASCENT study of sacituzumab govitecan versus chemotherapy in metastatic triple-negative breast cancer. Cancer Res. 2021, 81, PD13-07. [Google Scholar] [CrossRef]
- Litton, J.K.; Scoggins, M.E.; Hess, K.R.; Adrada, B.E.; Murthy, R.K.; Damodaran, S.; DeSnyder, S.M.; Brewster, A.M.; Barcenas, C.H.; Valero, V.; et al. Neoadjuvant Talazoparib for Patients With Operable Breast Cancer With a Germline BRCA Pathogenic Variant. J. Clin. Oncol. 2020, 38, 388–394. [Google Scholar] [CrossRef]
- Kumthekar, P.; Dixit, K.; Grimm, S.A.; Lukas, R.V.; Schwartz, M.A.; Rademaker, A.; Sharp, L.; Nelson, V.; Raizer, J.J. A phase II trial of bevacizumab in patients with recurrent solid tumor brain metastases who have failed whole brain radiation therapy (WBRT). J. Clin. Oncol. 2019, 37, 2070. [Google Scholar] [CrossRef]
- Delishaj, D.; Ursino, S.; Pasqualetti, F.; Cristaudo, A.; Cosottini, M.; Fabrini, M.G.; Paiar, F. Bevacizumab for the Treatment of Radiation-Induced Cerebral Necrosis: A Systematic Review of the Literature. J. Clin. Med. Res. 2017, 9, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Levin, V.A.; Bidaut, L.; Hou, P.; Kumar, A.J.; Wefel, J.S.; Bekele, B.N.; Prabhu, S.; Loghin, M.; Gilbert, M.R.; Jackson, E. Randomized Double-Blind Placebo-Controlled Trial of Bevacizumab Therapy for Radiation Necrosis of the Central Nervous System. Int. J. Radiat. Oncol. 2011, 79, 1487–1495. [Google Scholar] [CrossRef] [Green Version]
| Targeted Mutations | Trial | Phase | Population | Investigational Drug(s) | Total n | Primary Outcome | Comments |
|---|---|---|---|---|---|---|---|
| ALK, ROS1 | NCT02927340 | II | NSCLC | Loratinib | 30 | Intracranial disease control rate | |
| ALK, ROS1 | NCT01970865 | I/II | NSCLC | PF-06463922 vs. Crizotinib monotherapy | 334 | Participants with DLT, percentage of participants with overall and intracranial ORR | PF-0643922—ALK/ROS1 inhibitor |
| ALK, ROS1, or NTRK1-3 | NCT03093116 | I/II | Any IPM | Repotrectinib | 450 | DLT, recommended Phase II dose, ORR | Multiple arms comparing prior TKI and/or chemotherapy and treatment naïve |
| ALK, ROS1, NTRK1-3 | NCT05004116 | I/II | Any IPM | Repotrectinib + Irinotecan + Temozolomide | 50 | Incidence of DLT, MTD | |
| EGFR | NCT03769103 | II | NSCLC | SRS + Osimertinib vs. Osimertinib monotherapy | 76 | Intracranial PFS | Treatment naïve brain mets included |
| ROS1 | NCT04621188 | II | NSCLC | Loratinib | 84 | ORR | Recurrence after failure of first-line TKI |
| ROS1 | NCT03612154 | II | NSCLC | Loratinib | 35 | ORR | |
| ROS1 | NCT04919811 | II | NSCLC or other IPM | Taletrectinib (DS-6051b) | 119 | ORR | |
| ROS1, NTRK | NCT02675491 | I | Any IPM | DS-6051b | 15 | Number and severity of adverse events | |
| CDK, PI3K, NTRK/ROS1 | NCT03994796 | II | Any IPM | Abemaciclib or Paxalisib or Entrectinib | 150 | ORR | CDK population—Ademaciclib, PI3K—Paxalisib, NTRK/ROS1—Entrectinib |
| KRAS, EGFR | NCT01859026 | I/IB | NSCLC | Erlotinib + MEK162 | 43 | MTD | |
| KRAS | NCT03299088 | I | NSCLC | Pembrolizumab + Trametinib | 15 | Incidence of DLT | |
| KRAS | NCT03170206 | I/II | NSCLC | Palbociclib or Binimetinib monotherapy vs. combination therapy | 72 | MTD, safety and tolerability, PFS | CDK4/6 inhibitor + MEK inhibitor |
| KRAS | NCT03808558 | II | NSCLC | TVB-2640 | 12 | Disease control rate and response rate | |
| KRAS | NCT04111458 | I | Any IPM | BI-1701963 monotherapy vs. co-administration with Trametinib | 80 | MTD based on DLT, number of patients with DLT, ORR | |
| KRASG12C | NCT03785249 | I/II | Any IPM | MRTX849 (Adagrasib) monotherapy vs. combination therapy with Pembrolizumab, Cetuximab, or Afatinib | 565 | Safety, pharmacokinetics, and clinical activity/efficacy of MRTX849 | |
| CDK | NCT02896335 | II | Any IPM | Palbociclib | 30 | Clinical benefit rate (intracranial) | |
| HER-2 negative | NCT04647916 | II | Breast cancer | Sacituzumab Govitecan | 44 | ORR | |
| BRAFV600 | NCT03911869 | II | Melanoma | Encorafebib + Binimetinib vs. high dose | 13 | Incidence of DLT, incidence and severity of AE, incidence of dose modifications and discontinuations due to AE, brain metastasis response rate | |
| Checkpoint inhibition | NCT03340129 | II | Melanoma | Ipilimumab + nivolumab w/ RT vs. Ipilimumab + Nivolumab alone | 218 | Neurological specific cause of death |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.; Singer, L.; Kumthekar, P. Updates on Molecular Targeted Therapies for Intraparenchymal CNS Metastases. Cancers 2022, 14, 17. https://doi.org/10.3390/cancers14010017
Sharma A, Singer L, Kumthekar P. Updates on Molecular Targeted Therapies for Intraparenchymal CNS Metastases. Cancers. 2022; 14(1):17. https://doi.org/10.3390/cancers14010017
Chicago/Turabian StyleSharma, Akanksha, Lauren Singer, and Priya Kumthekar. 2022. "Updates on Molecular Targeted Therapies for Intraparenchymal CNS Metastases" Cancers 14, no. 1: 17. https://doi.org/10.3390/cancers14010017
APA StyleSharma, A., Singer, L., & Kumthekar, P. (2022). Updates on Molecular Targeted Therapies for Intraparenchymal CNS Metastases. Cancers, 14(1), 17. https://doi.org/10.3390/cancers14010017






