PAK4 and NAMPT as Novel Therapeutic Targets in Diffuse Large B-Cell Lymphoma, Follicular Lymphoma, and Mantle Cell Lymphoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines, Reagents, and Culture Conditions
2.2. Immunohistochemistry of Tissue Microarray
2.3. Cell Viability Determined by Trypan Blue Assay
2.4. Quantification of Apoptosis by 7-Amino-Actinomycin D (7-AAD)
2.5. ATP and NAD Analyses
2.6. Western Blotting
2.7. Development of Subcutaneous DLBCL Xenograft Model
2.8. Isolation of Tumor Tissue Proteins and Western Blot Analysis
2.9. RNA Isolation and mRNA Real-Time RT-qPCR
2.10. Development of Systemic FL Xenograft Model
2.11. Development of Subcutaneous MCL Xenograft Models
2.12. Residual Tumor Marker Analysis
2.13. Statistical Analysis
3. Results
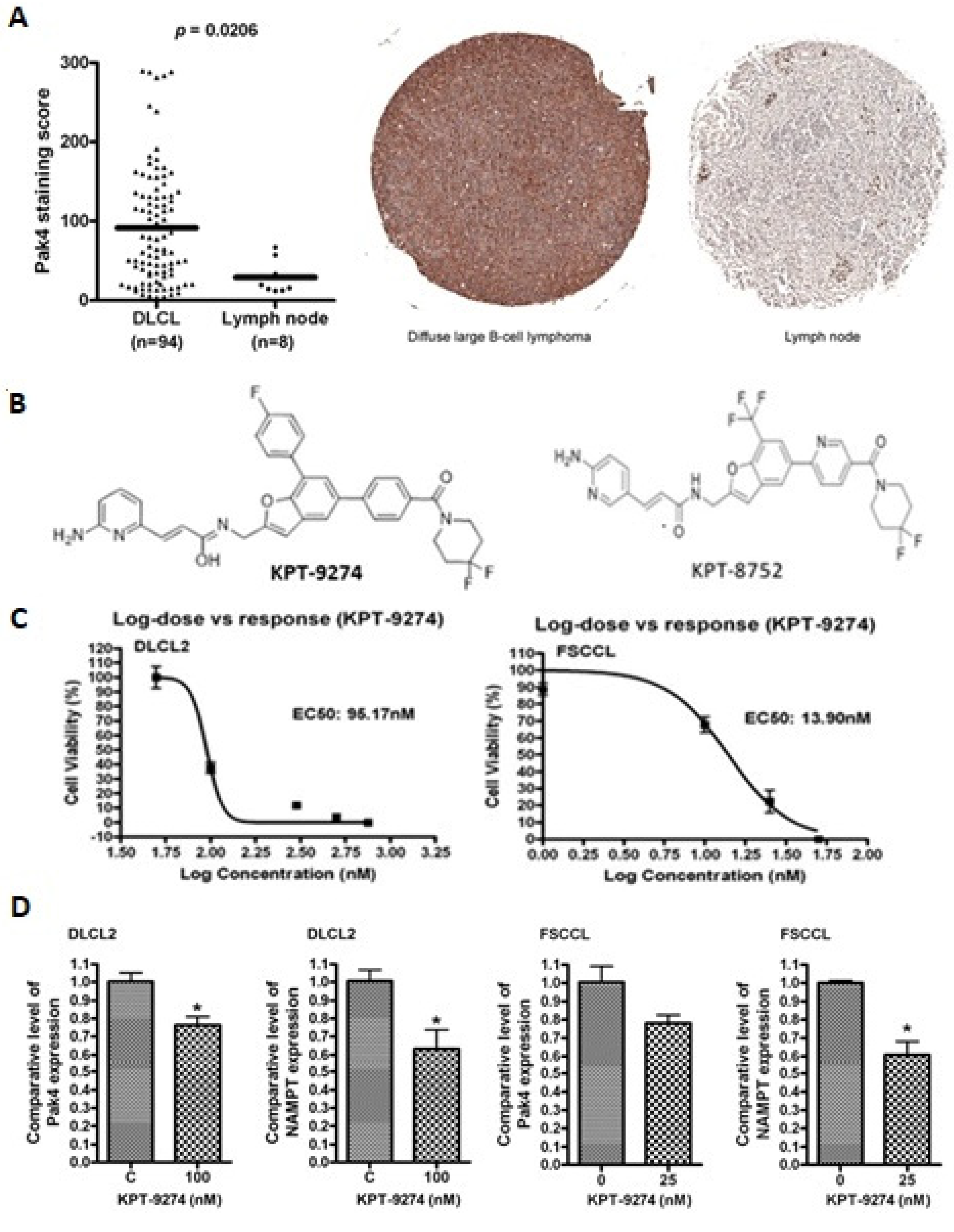
3.1. PAK4-NAMPT Is Essential for Lymphoma Cell Survival
3.2. PAK4-NAMPT Inhibition Induces Apoptosis
3.3. Impact of KPT-9274 on Cellular ATP and NAD Pool
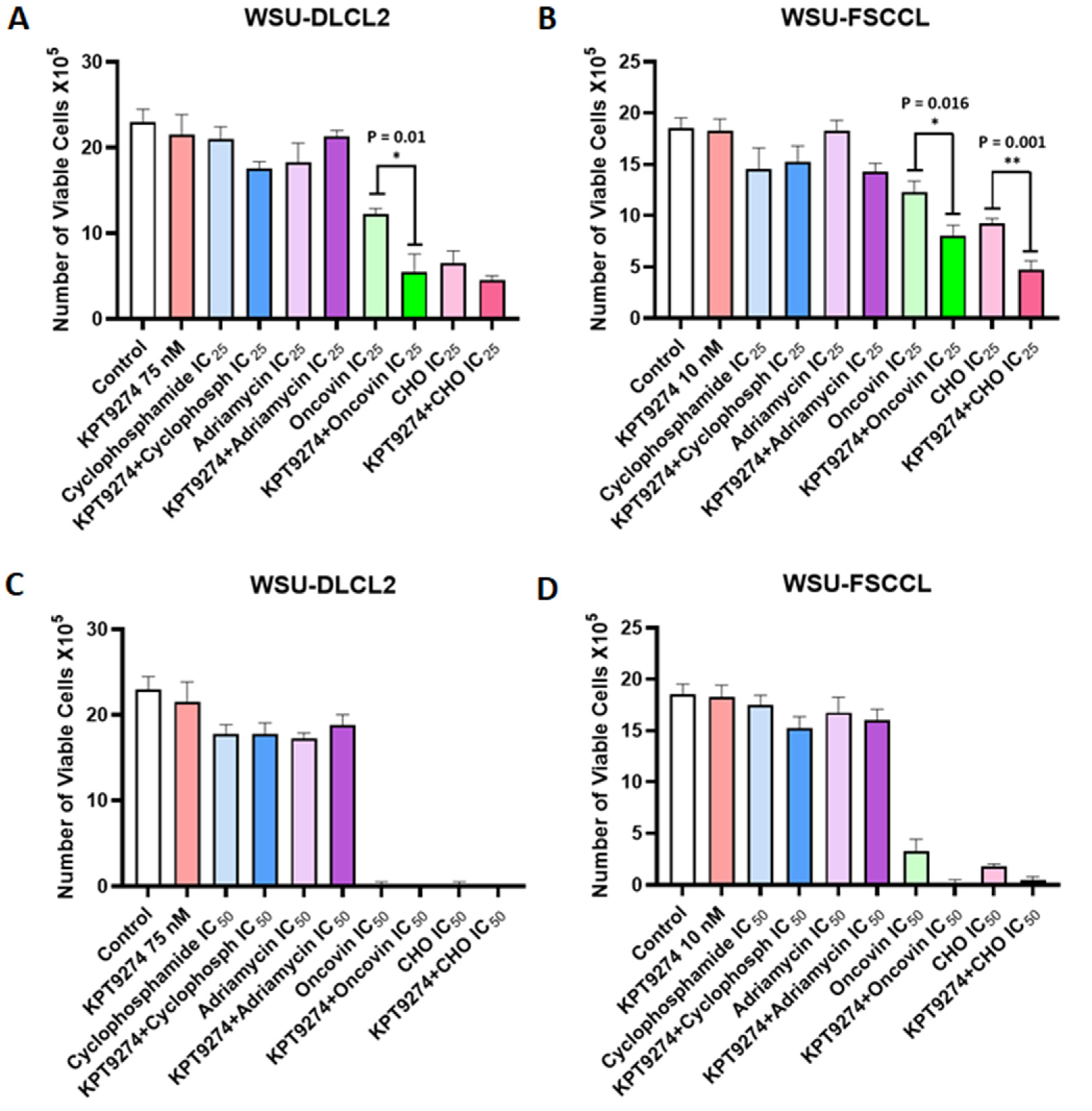
3.4. KPT-9274 Chemotherapy Combination Studies
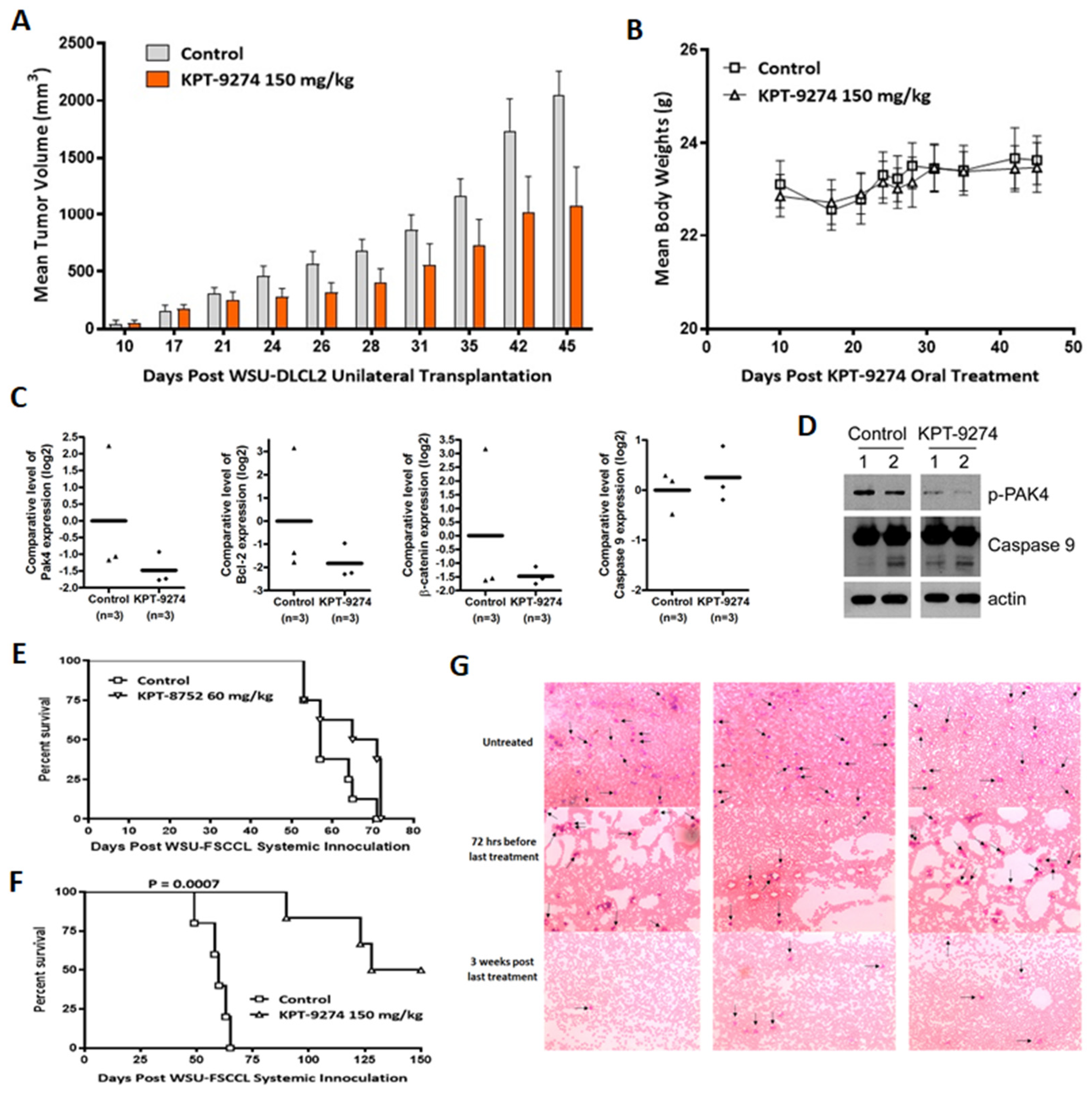
3.5. Preclinical Anti-Tumor Efficacy Trial of KPT-9274 in Lymphoma Xenografts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crump, M.; Neelapu, S.S.; Farooq, U.; Neste, E.V.D.; Kuruvilla, J.; Westin, J.; Link, B.K.; Hay, A.; Cerhan, J.R.; Zhu, L.; et al. Outcomes in refractory diffuse large B-cell lymphoma: Results from the international SCHOLAR-1 study. Blood 2017, 130, 1800–1808. [Google Scholar] [CrossRef]
- Hans, C.P.; Weisenburger, D.D.; Vose, J.M.; Hock, L.M.; Lynch, J.C.; Aoun, P.; Greiner, T.C.; Chan, W.C.; Bociek, R.G.; Bierman, P.J.; et al. A significant diffuse component predicts for inferior survival in grade 3 follicular lymphoma, but cytologic subtypes do not predict survival. Blood 2003, 101, 2363–2367. [Google Scholar] [CrossRef] [PubMed]
- Monga, N.; Garside, J.; Quigley, J.; Hudson, M.; O’Donovan, P.; O’Rourke, J.; Tapprich, C.; Parisi, L.; Davids, M.S.; Tam, C. Systematic literature review of the global burden of illness of mantle cell lymphoma. Curr. Med. Res. Opin. 2020, 36, 843–852. [Google Scholar] [CrossRef]
- Chini, C.C.; Tarragó, M.G.; Chini, E.N. NAD and the aging process: Role in life, death and everything in between. Mol. Cell. Endocrinol. 2017, 455, 62–74. [Google Scholar] [CrossRef]
- Heyes, M.P.; Chen, C.Y.; Major, E.O.; Saito, K. Different kynurenine pathway enzymes limit quinolinic acid formation by various human cell types. Biochem. J. 1997, 326, 351–356. [Google Scholar] [CrossRef]
- Burgos, E.S.; Schramm, V.L. Weak coupling of ATP hydrolysis to the chemical equilibrium of human nicotinamide phosphoribosyltransferase. Biochemistry 2008, 47, 11086–11096. [Google Scholar] [CrossRef] [PubMed]
- Preiss, J.; Handler, P. Biosynthesis of diphosphopyridine nucleotide. II. Enzymatic aspects. J. Biol. Chem. 1958, 233, 493–500. [Google Scholar] [CrossRef]
- Preiss, J.; Handler, P. Enzymatic synthesis of nicotinamide mononucleotide. J. Biol. Chem. 1957, 225, 759–770. [Google Scholar] [CrossRef]
- Canto, C.; Auwerx, J. NAD+ as a Signaling Molecule Modulating Metabolism. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 291–298. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Bheda, P.; Revollo, J.R.; Imai, S.-I.; Wolberger, C. Structure of Nampt/PBEF/visfatin, a mammalian NAD+ biosynthetic enzyme. Nat. Struct. Mol. Biol. 2006, 13, 661–662. [Google Scholar] [CrossRef]
- Shackelford, R.E.; Mayhall, K.; Maxwell, N.M.; Kandil, E.; Coppola, D. Nicotinamide phosphoribosyltransferase in malignancy: A review. Genes Cancer 2013, 4, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Sampath, D.; Zabka, T.S.; Misner, D.L.; O’Brien, T.; Dragovich, P.S. Inhibition of nicotinamide phosphoribosyltransferase (NAMPT) as a therapeutic strategy in cancer. Pharmacol. Ther. 2015, 151, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Cea, M.; Cagnetta, A.; Fulciniti, M.; Tai, Y.T.; Hideshima, T.; Chauhan, D.; Roccaro, A.; Sacco, A.; Calimeri, T.; Cottini, F.; et al. Targeting NAD+ salvage pathway induces autophagy in multiple myeloma cells via mTORC1 and extracellular signal-regulated kinase (ERK1/2) inhibition. Blood 2012, 120, 3519–3529. [Google Scholar] [CrossRef]
- Chini, C.C.; Guerrico, A.M.G.; Nin, V.; Camacho-Pereira, J.; Escande, C.; Barbosa, M.T.; Chini, E.N. Targeting of NAD Metabolism in Pancreatic Cancer Cells: Potential Novel Therapy for Pancreatic Tumors. Clin. Cancer Res. 2014, 20, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, I.; Bouchard, E.; Beiggi, S.; Poeppl, A.G.; Johnston, J.B.; Gibson, S.; Banerji, V. On-Target Effect of FK866, a Nicotinamide Phosphoribosyl Transferase Inhibitor, by Apoptosis-Mediated Death in Chronic Lymphocytic Leukemia Cells. Clin. Cancer Res. 2014, 20, 4861–4872. [Google Scholar] [CrossRef]
- Matheny, C.J.; Wei, M.C.; Bassik, M.C.; Donnelly, A.J.; Kampmann, M.; Iwasaki, M.; Piloto, O.; Solow-Cordero, D.E.; Bouley, D.M.; Rau, R.; et al. Next-Generation NAMPT Inhibitors Identified by Sequential High-Throughput Phenotypic Chemical and Functional Genomic Screens. Chem. Biol. 2013, 20, 1352–1363. [Google Scholar] [CrossRef]
- Vega, G.; Aviles-Salas, A.; Chalapud, J.R.; Martínez-Paniagua, M.; Pelayo, R.; Mayani, H.; Hernández-Pando, R.; Martínez-Maza, O.; Huerta-Yepez, S.; Bonavida, B.; et al. P38 MAPK expression and activation predicts failure of response to CHOP in patients with Diffuse Large B-Cell Lymphoma. BMC Cancer 2015, 15, 722. [Google Scholar] [CrossRef]
- Elenitoba-Johnson, K.S.; Jenson, S.D.; Abbott, R.T.; Palais, R.A.; Bohling, S.D.; Lin, Z.; Tripp, S.; Shami, P.J.; Wang, L.Y.; Coupland, R.W.; et al. Involvement of multiple signaling pathways in follicular lympho-ma transformation: P38-mitogen-activated protein kinase as a target for therapy. Proc. Natl. Acad. Sci. USA 2003, 100, 7259–7264. [Google Scholar] [CrossRef]
- Pomerance, M.; Quillard, J.; Chantoux, F.; Young, J.; Blondeau, J.P. High-level expression, activation, and subcellular localization of p38-MAP kinase in thyroid neoplasms. J. Pathol. 2006, 209, 298–306. [Google Scholar] [CrossRef]
- Loesch, M.; Chen, G. The p38 MAPK stress pathway as a tumor suppressor or more? Front. Biosci. 2008, 13, 3581–3593. [Google Scholar] [CrossRef]
- King, H.; Nicholas, N.S.; Wells, C.M. Role of p-21-Activated Kinases in Cancer Progression. Int. Rev. Cell Mol. Biol. 2014, 309, 347–387. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.Y.; Wang, J.; You, L.H.; Zhou, R.Z.; Zhao, D.M.; Cheng, M.S.; Li, F. GL-1196 Suppresses the Proliferation and Invasion of Gastric Cancer Cells via Tar-geting PAK4 and Inhibiting PAK4-Mediated Signaling Pathways. Int. J. Mol. Sci. 2016, 17, 470. [Google Scholar] [CrossRef] [PubMed]
- Callow, M.G.; Clairvoyant, F.; Zhu, S.; Schryver, B.; Whyte, D.B.; Bischoff, J.R.; Jallal, B.; Smeal, T. Requirement for PAK4 in the Anchorage-independent Growth of Human Cancer Cell Lines. J. Biol. Chem. 2002, 277, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.S.; Aboukameel, A.; Muqbil, I.; Li, Y.; Senapedis, W.; Baloglu, E.; Landesman, Y.; Kauffman, M.; Shacham, S.; Al-Katib, A.; et al. Abstract 1358: P21 activated kinase 4 (pak4) as a novel therapeutic target for non-hodgkin’s lymphoma. Cancer Res. 2017, 77, 1358. [Google Scholar]
- Abu Aboud, O.; Chen, C.-H.; Senapedis, W.; Baloglu, E.; Argueta, C.; Weiss, R.H. Dual and Specific Inhibition of NAMPT and PAK4 by KPT-9274 Decreases Kidney Cancer Growth. Mol. Cancer Ther. 2016, 15, 2119–2129. [Google Scholar] [CrossRef] [PubMed]
- Al-Katib, A.M.; Sun, Y.; Goustin, A.S.; Azmi, A.S.; Chen, B.; Aboukameel, A.; Mohammad, R.M. SMI of Bcl-2 TW-37 is active across a spectrum of B-cell tumors irrespective of their proliferative and differentiation status. J. Hematol. Oncol. 2009, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Baloglu, E.; Shacham, S.; Senapedis, W.; McCauley, D.; Landesman, Y.; Gai, G.; Kalid, O.; Shechter, S. Substituted Benzofuranyl and Benzoxazolyl Compounds as PAK Modulators and Their Preparation. U.S. Patent US20160368904A1, 2 January 2018. [Google Scholar]
- Murray, B.W.; Guo, C.; Piraino, J.; Westwick, J.K.; Zhang, C.; Lamerdin, J.; Dagostino, E.; Knighton, D.; Loi, C.-M.; Zager, M.; et al. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc. Natl. Acad. Sci. USA 2010, 107, 9446–9451. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.B.; Bae, M.-H.; Kim, M.-K.; Im, I.; Kim, Y.-C.; Eom, S.H. Crystal structure of Rattus norvegicus Visfatin/PBEF/Nampt in complex with an FK866-based inhibitor. Mol. Cells 2009, 27, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, R.M.; Li, Y.; Muqbil, I.; Aboukameel, A.; Senapedis, W.; Baloglu, E.; Landesman, Y.; Philip, P.A.; Azmi, A.S. Targeting Rho GTPase effector p21 activated kinase 4 (PAK4) suppresses p-Bad-microRNA drug resistance axis leading to inhibition of pancreatic ductal adenocarcinoma proliferation. Small GTPases 2017, 10, 367–377. [Google Scholar] [CrossRef]
- Aboukameel, A.; Muqbil, I.; Senapedis, W.; Baloglu, E.; Landesman, Y.; Shacham, S.; Kauffman, M.; Philip, P.A.; Mohammad, R.M.; Azmi, A.S. Novel p21-Activated Kinase 4 (PAK4) Allosteric Modulators Overcome Drug Resistance and Stemness in Pancreatic Ductal Adenocarcinoma. Mol. Cancer Ther. 2017, 16, 76–87. [Google Scholar] [CrossRef]
- Fulciniti, M.; Martinez-Lopez, J.; Senapedis, W.; Oliva, S.; Bandi, R.L.; Amodio, N.; Xu, Y.; Szalat, R.; Gulla, A.; Samur, M.K.; et al. Functional role and therapeutic targeting of p21-activated kinase 4 in multiple myeloma. Blood 2017, 129, 2233–2245. [Google Scholar] [CrossRef]
- Li, N.; Lopez, M.A.; Linares, M.; Kumar, S.; Oliva, S.; Martinez-Lopez, J.; Xu, L.; Xu, Y.; Perini, T.; Senapedis, W.; et al. Dual PAK4-NAMPT inhibition impacts growth and survival, and increases sensitivity to DNA-damaging agents in Waldenstrom Macroglobulinemia. Clin. Cancer Res. 2018, 25, 369–377. [Google Scholar] [CrossRef]
- Takao, S.; Chien, W.; Madan, V.; Lin, D.C.; Ding, L.W.; Sun, Q.Y.; Mayakonda, A.; Sudo, M.; Xu, L.; Chen, Y.; et al. Targeting the vulnerability to NAD(+) depletion in B-cell acute lymphoblastic leukemia. Leukemia 2018, 32, 616–625. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.; Oeh, J.; Xiao, Y.; Liang, X.; Vanderbilt, A.; Qin, A.; Yang, L.; Lee, L.B.; Ly, J.; Cosino, E.; et al. Supplementation of nicotinic acid with NAMPT inhibitors results in loss of in vivo effica-cy in NAPRT1-deficient tumor models. Neoplasia 2013, 15, 1314–1329. [Google Scholar] [CrossRef]
- Begum, A.; Imoto, I.; Kozaki, K.-I.; Tsuda, H.; Suzuki, E.; Amagasa, T.; Inazawa, J. Identification ofPAK4as a putative target gene for amplification within 19q13.12-q13.2 in oral squamous-cell carcinoma. Cancer Sci. 2009, 100, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Kanaan, Y.; Baed, Y.-K.; Gabrielson, E. Chromosomal changes in aggressive breast cancers with basal-like features. Cancer Genet. Cytogenet. 2009, 193, 29–37. [Google Scholar] [CrossRef]
- Radu, M.; Semenova, G.; Kosoff, R.; Chernoff, J. PAK signalling during the development and progression of cancer. Nat. Rev. Cancer 2014, 14, 13–25. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, H.; Tian, Y.; Nekrasova, T.; Hao, X.; Lee, H.J.; Suh, N.; Yang, C.S.; Minden, A. The Pak4 Protein Kinase Plays a Key Role in Cell Survival and Tumorigenesis in Athymic Mice. Mol. Cancer Res. 2008, 6, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sanawar, R.; Li, X.; Li, F. Structure, biochemistry, and biology of PAK kinases. Gene 2017, 605, 20–31. [Google Scholar] [CrossRef]
- Ma, Y.; McCarty, S.K.; Kapuriya, N.P.; Brendel, V.J.; Wang, C.; Zhang, X.; Jarjoura, D.; Saji, M.; Chen, C.S.; Ringel, M.D. Development of p21 activated kinase-targeted multikinase inhibitors that in-hibit thyroid cancer cell migration. J. Clin. Endocrinol. Metab. 2013, 98, E1314–E1322. [Google Scholar] [CrossRef]
- Rudolph, J.; Crawford, J.J.; Hoeflich, K.P.; Wang, W. Inhibitors of p21-Activated Kinases (PAKs). J. Med. Chem. 2015, 58, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Rane, C.; Senapedis, W.; Baloglu, E.; Landesman, Y.; Crochiere, M.; Das-Gupta, S.; Minden, A. A novel orally bioavailable compound KPT-9274 inhibits PAK4, and blocks triple negative breast cancer tumor growth. Sci. Rep. 2017, 7, srep42555. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.; Leong, S.; Pishvaian, M.; Razak, A.; Mahipal, A.; Berlin, J.; Cho, D.; Senapedis, W.; Shacham, S.; Kauffman, M.; et al. A first in human phase 1 study of KPT-9274, a first in class dual inhibitor of PAK4 and NAMPT, in patients with advanced solid malignancies or NHL. Ann. Oncol. 2017, 28, v125. [Google Scholar] [CrossRef]



| Primers | Sequences | |
|---|---|---|
| PAK4 | Forward | GTGCAAGAGAGCTGAGGGAG |
| Reverse | ATGCTGGTGGGACAGAAGTG | |
| Bcl-2 | Forward | TGAACTGGGGGAGGATTGTG |
| Reverse | CGTACAGTTCCACAAAGGCA | |
| β-Catenin | Forward | CGCCATTTTAAGCCTCTCGG |
| Reverse | CTCCTCAGACCTTCCTCCGT | |
| Caspase 9 | Forward | TGTTCAGGCCCCATATGATCG |
| Reverse | CAACTTTGCTGCTTGCCTGT | |
| β-actin | Forward | GCACAGAGCCTCGCCTT |
| Reverse | TCATCATCCATGGTGAGCTG | |
| 18S | Forward | GCAATTATTCCCCATGAACG |
| Reverse | GGCCTCACTAAACCATCCAA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, H.Y.; Uddin, M.H.; Balasubramanian, S.K.; Sulaiman, N.; Iqbal, M.; Chaker, M.; Aboukameel, A.; Li, Y.; Senapedis, W.; Baloglu, E.; et al. PAK4 and NAMPT as Novel Therapeutic Targets in Diffuse Large B-Cell Lymphoma, Follicular Lymphoma, and Mantle Cell Lymphoma. Cancers 2022, 14, 160. https://doi.org/10.3390/cancers14010160
Khan HY, Uddin MH, Balasubramanian SK, Sulaiman N, Iqbal M, Chaker M, Aboukameel A, Li Y, Senapedis W, Baloglu E, et al. PAK4 and NAMPT as Novel Therapeutic Targets in Diffuse Large B-Cell Lymphoma, Follicular Lymphoma, and Mantle Cell Lymphoma. Cancers. 2022; 14(1):160. https://doi.org/10.3390/cancers14010160
Chicago/Turabian StyleKhan, Husain Yar, Md. Hafiz Uddin, Suresh Kumar Balasubramanian, Noor Sulaiman, Marium Iqbal, Mahmoud Chaker, Amro Aboukameel, Yiwei Li, William Senapedis, Erkan Baloglu, and et al. 2022. "PAK4 and NAMPT as Novel Therapeutic Targets in Diffuse Large B-Cell Lymphoma, Follicular Lymphoma, and Mantle Cell Lymphoma" Cancers 14, no. 1: 160. https://doi.org/10.3390/cancers14010160
APA StyleKhan, H. Y., Uddin, M. H., Balasubramanian, S. K., Sulaiman, N., Iqbal, M., Chaker, M., Aboukameel, A., Li, Y., Senapedis, W., Baloglu, E., Mohammad, R. M., Zonder, J., & Azmi, A. S. (2022). PAK4 and NAMPT as Novel Therapeutic Targets in Diffuse Large B-Cell Lymphoma, Follicular Lymphoma, and Mantle Cell Lymphoma. Cancers, 14(1), 160. https://doi.org/10.3390/cancers14010160







