Melanoma Progression under Obesity: Focus on Adipokines
Abstract
Simple Summary
Abstract
1. Introduction
2. Leptin
3. Resistin
4. Visfatin
5. Osteopontin
6. Adiponectin
7. Nesfatin-1
8. Chemerin
9. Apelin
10. Chemokines
11. Interleukins
11.1. Interleukin-32
11.2. Interleukin-6
11.3. Leukemia Inhibitory Factor
12. Tumor Necrosis Factor
13. Plasminogen Activator Inhibitor-1
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Clement, E.; Lazar, I.; Muller, C.; Nieto, L. Obesity and melanoma: Could fat be fueling malignancy? Pigment Cell Melanoma Res. 2017, 30, 294–306. [Google Scholar] [CrossRef]
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M. Adipose tissue, obesity and adipokines: Role in cancer promotion. Horm. Mol. Biol. Clin. Investig. 2015, 21, 57–74. [Google Scholar] [CrossRef]
- Saely, C.H.; Geiger, K.; Drexel, H. Brown versus white adipose tissue: A mini-review. Gerontology 2011, 58, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Schoettl, T.; Fischer, I.P.; Ussar, S. Heterogeneity of adipose tissue in development and metabolic function. J. Exp. Biol. 2018, 221, jeb162958. [Google Scholar] [CrossRef]
- Coelho, P.; Almeida, J.; Prudêncio, C.; Fernandes, R.; Soares, R. Effect of Adipocyte Secretome in Melanoma Progression and Vasculogenic Mimicry. J. Cell. Biochem. 2016, 117, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Richard, A.J.; White, U.; Elks, C.M.; Stephens, J.M. Adipose Tissue: Physiology to Metabolic Dysfunction. In Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- De Oliveira Leal, V.; Mafra, D. Adipokines in obesity. Clin. Chim. Acta 2013, 18, 87–94. [Google Scholar] [CrossRef]
- Fasshauer, M.; Blu, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Van Kruijsdijk, R.C.M.; Van Der Wall, E.; Visseren, F.L.J. Obesity and cancer: The role of dysfunctional adipose tissue. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.J.; Ouchi, N.; Gokce, N.; Walsh, K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ. Res. 2016, 118, 1786–1807. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Dossus, L.; Marant-Micallef, C.; His, M. Obesity and Cancer. Bull. Cancer 2019, 106, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kruper, L.; Dieli-Conwright, C.M.; Mortimer, J.E. The Impact of Obesity on Breast Cancer Diagnosis and Treatment. Curr. Oncol. Rep. 2019, 21, 41. [Google Scholar] [CrossRef]
- Roslan, N.H.; Makpol, S.; Mohd Yusof, Y.A. A review on dietary intervention in obesity associated colon cancer. Asian Pac. J. Cancer Prev. 2019, 25, 1309–1319. [Google Scholar] [CrossRef]
- Marengo, A.; Rosso, C.; Bugianesi, E. Liver cancer: Connections with obesity, fatty liver, and cirrhosis. Annu. Rev. Med. 2016, 67, 103–117. [Google Scholar] [CrossRef]
- Miller, A.J.; Mihm, M.C. Melanoma. N. Engl. J. Med. 2006, 355, 51–65. [Google Scholar] [CrossRef]
- Mazurkiewicz, J.; Simiczyjew, A.; Dratkiewicz, E.; Ziętek, M.; Matkowski, R.; Nowak, D. Stromal cells present in the melanoma niche affect tumor invasiveness and its resistance to therapy. Int. J. Mol. Sci. 2021, 22, 529. [Google Scholar] [CrossRef]
- Lahmann, P.H.; Hughes, M.C.B.; Williams, G.M.; Green, A.C. A prospective study of measured body size and height and risk of keratinocyte cancers and melanoma. Cancer Epidemiol. 2016, 40, 119–125. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Præstegaard, C.; Kjær, S.K.; Christensen, J.; Tjønneland, A.; Halkjær, J.; Jensen, A. Obesity and risks for malignant melanoma and non-melanoma skin cancer: Results from a large danish prospective cohort study. J. Investig. Dermatol. 2015, 135, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Rodriguez, C.; Walker-thurmond, K.; Thun, M.J. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults Eugenia. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Sergentanis, T.N.; Antoniadis, A.G.; Gogas, H.J.; Antonopoulos, C.N.; Adami, H.O.; Ekbom, A.; Petridou, E.T. Obesity and risk of malignant melanoma: A meta-analysis of cohort and case-control studies. Eur. J. Cancer 2013, 49, 642–657. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Wang, Y.; Dang, Y.; Gagel, A.; Ross, M.I.; Gershenwald, J.E.; Cormier, J.N.; Wargo, J.; Haydu, L.E.; Davies, M.A.; et al. Association between Body Mass Index, C-Reactive Protein Levels, and Melanoma Patient Outcomes. J. Investig. Dermatol. 2017, 137, 1792–1795. [Google Scholar] [CrossRef]
- Skowron, F.; Bérard, F.; Balme, B.; Maucort-Boulch, D. Role of obesity on the thickness of primary cutaneous melanoma. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 262–269. [Google Scholar] [CrossRef]
- Brandon, E.L.; Gu, J.W.; Cantwell, L.; He, Z.; Wallace, G.; Hall, J.E. Obesity promotes melanoma tumor growth: Role of leptin. Cancer Biol. Ther. 2009, 8, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Vijayakumar, M.V.; Ajay, A.K.; Malvi, P.; Bhat, M.K. Diet-induced obesity increases melanoma progression: Involvement of Cav-1 and FASN. Int. J. Cancer 2012, 130, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.I.; Cho, H.J.; Jung, Y.J.; Kwon, S.H.; Her, S.; Choi, S.S.; Shin, S.H.; Lee, K.W.; Park, J.H.Y. High-fat diet-induced obesity increases lymphangiogenesis and lymph node metastasis in the B16F10 melanoma allograft model: Roles of adipocytes and M2-macrophages. Int. J. Cancer 2015, 136, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhao, J.; Meng, H.; Zhang, X. Adipose tissue-resident immune cells in obesity and type 2 diabetes. Front. Immunol. 2019, 10, 1173. [Google Scholar] [CrossRef]
- Simiczyjew, A.; Dratkiewicz, E.; Mazurkiewicz, J.; Ziętek, M.; Matkowski, R.; Nowak, D. The influence of tumor microenvironment on immune escape of melanoma. Int. J. Mol. Sci. 2020, 21, 8359. [Google Scholar] [CrossRef]
- Smith, L.K.; Arabi, S.; Lelliott, E.J.; McArthur, G.A.; Sheppard, K.E. Obesity and the impact on cutaneous melanoma: Friend or foe? Cancers 2020, 12, 1583. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Nájera, L.E.; Chanona-Pérez, J.J.; Valdivia-Flores, A.; Marrero-Rodríguez, D.; Salcedo-Vargas, M.; García-Ruiz, D.I.; Castro-Reyes, M.A. Morphometric study of adipocytes on breast cancer by means of photonic microscopy and image analysis. Microsc. Res. Tech. 2018, 81, 240–249. [Google Scholar] [CrossRef]
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Majed, B.; Wang, Y.Y.; Meulle, A.; Salles, B.; Le Gonidec, S.; et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011, 71, 2455–2465. [Google Scholar] [CrossRef]
- Zoico, E.; Darra, E.; Rizzatti, V.; Tebon, M.; Franceschetti, G.; Mazzali, G.; Rossi, A.P.; Fantin, F.; Zamboni, M. Role of adipose tissue in melanoma cancer microenvironment and progression. Int. J. Obes. 2018, 42, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, B.; Li, Z.; Li, J.; Sun, S.; Sun, S. Cancer-associated adipocytes: Key players in breast cancer progression. J. Hematol. Oncol. 2019, 6, 1–15. [Google Scholar] [CrossRef]
- Shiga, K.; Hara, M.; Nagasaki, T.; Sato, T.; Takahashi, H.; Takeyama, H. Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers 2015, 7, 2443–2458. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Francisco, V.; Pino, J.; Campos-Cabaleiro, V.; Ruiz-Fernández, C.; Mera, A.; Gonzalez-Gay, M.A.; Gómez, R.; Gualillo, O. Obesity, fat mass and immune system: Role for leptin. Front. Physiol. 2018, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Moreno, C.; Pichel, J.G.; Chesnokova, V.; De Pablo, F. Increased leptin and white adipose tissue hypoplasia are sexually dimorphic in Lif null/Igf-I haploinsufficient mice. FEBS Lett. 2004, 557, 64–68. [Google Scholar] [CrossRef]
- Li, S.; Li, X. Leptin in normal physiology and leptin resistance. Sci. Bull. 2016, 61, 1480–1488. [Google Scholar] [CrossRef]
- Simons, P.J.; Van Den Pangaart, P.S.; Van Roomen, C.P.A.A.; Aerts, J.M.F.G.; Boon, L. Cytokine-mediated modulation of leptin and adiponectin secretion during in vitro adipogenesis: Evidence that tumor necrosis factor-α- and interleukin-1β-treated human preadipocytes are potent leptin producers. Cytokine 2005, 32, 94–103. [Google Scholar] [CrossRef]
- Askarpour, M.; Alizadeh, S.; Hadi, A.; Symonds, M.E.; Miraghajani, M.; Sheikhi, A.; Ghaedi, E. Effect of Bariatric Surgery on the Circulating Level of Adiponectin, Chemerin, Plasminogen Activator Inhibitor-1, Leptin, Resistin, and Visfatin: A Systematic Review and Meta-Analysis. Horm. Metab. Res. 2020, 52, 207–215. [Google Scholar] [CrossRef]
- Chen, K.; Li, F.; Li, J.; Cai, H.; Strom, S.; Bisello, A.; Kelley, D.E.; Friedman-Einat, M.; Skibinski, G.A.; McCrory, M.A.; et al. Induction of leptin resistance through direct interaction of C-reactive protein with leptin. Nat. Med. 2006, 12, 425–432. [Google Scholar] [CrossRef]
- Khazaei, M.; Tahergorabi, Z. Leptin and its cardiovascular effects: Focus on angiogenesis. Adv. Biomed. Res. 2015, 4, 79. [Google Scholar] [CrossRef]
- Friedman, J.M. Leptin and the endocrine control of energy balance. Nat. Metab. 2019, 1, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Kaleta, B. The role of osteopontin in kidney diseases. Inflamm. Res. 2019, 68, 93–102. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Silva, C.; Rotellar, F.; Hernández-Lizoain, J.L.; Baixauli, J.; Valentí, V.; Pardo, F.; et al. Up-regulation of the novel proinflammatory adipokines lipocalin-2, chitinase-3 like-1 and osteopontin as well as angiogenic-related factors in visceral adipose tissue of patients with colon cancer. J. Nutr. Biochem. 2011, 22, 634–641. [Google Scholar] [CrossRef]
- Ahmad, R.; Al-Mass, A.; Al-Ghawas, D.; Shareif, N.; Zghoul, N.; Melhem, M.; Hasan, A.; Al-Ghimlas, F.; Dermime, S.; Behbehani, K. Interaction of Osteopontin with IL-18 in Obese Individuals: Implications for Insulin Resistance. PLoS ONE 2013, 8, e63944. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Catalán, V.; Ramírez, B.; Rodríguez, A.; Colina, I.; Silva, C.; Rotellar, F.; Mugueta, C.; Gil, M.J.; Cienfuegos, J.A.; et al. Plasma osteopontin levels and expression in adipose tissue are increased in obesity. J. Clin. Endocrinol. Metab. 2007, 92, 3719–3727. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Gezmen-karada, M.; Arif, M.; Gezmen-karada, M. The Multiple Functions and Mechanisms of Osteopontin. Clin. Biochem. 2018, 59, 17–24. [Google Scholar]
- Moorman, H.R.; Poschel, D.; Klement, J.D.; Lu, C.; Redd, P.S.; Liu, K. Osteopontin: A key regulator of tumor progression and immunomodulation. Cancers 2020, 12, 3379. [Google Scholar] [CrossRef] [PubMed]
- Budzik, M.P.; Badowska-Kozakiewicz, A.M. The multidirectional role of osteopontin in cancer. J. Oncol. 2018, 68, 176–183. [Google Scholar]
- Wai, P.Y.; Kuo, P.C. Osteopontin: Regulation in tumor metastasis. Cancer Metastasis Rev. 2008, 27, 103–118. [Google Scholar] [CrossRef]
- Fasshauer, M.; Neumann, S.; Eszlinger, M.; Paschke, R.; Klein, J. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2002, 290, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Karimi, K.; Lindgren, T.H.; Koch, C.A.; Brodell, R.T. Obesity as a risk factor for malignant melanoma and non-melanoma skin cancer. Rev. Endocr. Metab. Disord. 2016, 17, 389–403. [Google Scholar] [CrossRef]
- Zhang, H.P.; Zou, J.; Xu, Z.Q.; Ruan, J.; Yang, S.D.; Yin, Y.; Mu, H.J. Association of leptin, visfatin, apelin, resistin and adiponectin with clear cell renal cell carcinoma. Oncol. Lett. 2017, 13, 463–468. [Google Scholar] [CrossRef][Green Version]
- Jonas, M.I.; Kurylowicz, A.; Bartoszewicz, Z.; Lisik, W.; Jonas, M.; Domienik-Karlowicz, J.; Puzianowska-Kuznicka, M. Adiponectin/resistin interplay in serum and in adipose tissue of obese and normal-weight individuals. Diabetol. Metab. Syndr. 2017, 9, 1–9. [Google Scholar] [CrossRef]
- Achari, A.E.; Jain, S.K. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [PubMed]
- Davoudi-Kiakalayeh, A.; Mohammadi, R.; Pourfathollah, A.A.; Siery, Z.; Davoudi-Kiakalayeh, S. Alloimmunization in thalassemia patients: New insight for healthcare. Int. J. Prev. Med. 2017, 8, 101. [Google Scholar]
- Ramanjaneya, M.; Chen, J.; Brown, J.E.; Tripathi, G.; Hallschmid, M.; Patel, S.; Kern, W.; Hillhouse, E.W.; Lehnert, H.; Tan, B.K.; et al. Identification of nesfatin-1 in human and murine adipose tissue: A novel depot-specific adipokine with increased levels in obesity. Endocrinology 2010, 151, 3169–3180. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Zhang, X.; Xiang, X.; Li, Y.; Mulholland, M.W.; Zhang, W. Nesfatin-1 promotes brown adipocyte phenotype. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Yin, C.; Liu, W.; Xu, E.; Zhang, M.; Lv, W.; Lu, Q.; Xiao, Y. Copeptin and Nesfatin-1 Are Interrelated Biomarkers with Roles in the Pathogenesis of Insulin Resistance in Chinese Children with Obesity. Ann. Nutr. Metab. 2020, 76, 223–232. [Google Scholar] [CrossRef]
- Saygin, C.; Reizes, O.; Berger, N.A. Adipocytes, Adipocytokines, and Cancer. In Adipocytokines, Energy Balance, and Cancer; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–19. [Google Scholar]
- Dore, R.; Krotenko, R.; Reising, J.P.; Murru, L.; Sundaram, S.M.; Di Spiezio, A.; Müller-Fielitz, H.; Schwaninger, M.; Jöhren, O.; Mittag, J.; et al. Nesfatin-1 decreases the motivational and rewarding value of food. Neuropsychopharmacology 2020, 45, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Schalla, M.A.; Stengel, A. Current understanding of the role of nesfatin-1. J. Endocr. Soc. 2018, 2, 1188–1206. [Google Scholar] [CrossRef]
- Ma, J.; Sun, F.; Wang, J.; Jiang, H.; Lu, J.; Wang, X.; Zhang, J.; Shi, C.; You, W.; Li, X.; et al. Effects of Aldosterone on Chemerin Expression and Secretion in 3T3-L1 Adipocytes. Exp. Clin. Endocrinol. Diabetes 2018, 126, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Eisenberg, D.; Zhao, L.; Adams, C.; Leib, R.; Morser, J.; Leung, L. Chemerin activation in human obesity. Obesity 2016, 24, 1522–1529. [Google Scholar] [CrossRef]
- Goralski, K.B.; McCarthy, T.C.; Hanniman, E.A.; Zabel, B.A.; Butcher, E.C.; Parlee, S.D.; Muruganandan, S.; Sinal, C.J. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 2007, 282, 28175–28188. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, M.; Pandolfi, A. Chemerin in renal dysfunction and cardiovascular disease. Vascul. Pharmacol. 2016, 77, 28–34. [Google Scholar] [CrossRef]
- Helfer, G.; Wu, Q.F. Chemerin: A multifaceted adipokine involved in metabolic disorders. J. Endocrinol. 2018, 238, R79–R94. [Google Scholar] [CrossRef] [PubMed]
- Lago, F.; Dieguez, C.; Gómez-Reino, J.; Gualillo, O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev. 2007, 18, 313–325. [Google Scholar] [CrossRef]
- Huber, J.; Kiefer, F.W.; Zeyda, M.; Ludvik, B.; Silberhumer, G.R.; Prager, G.; Zlabinger, G.J.; Stulnig, T.M. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J. Clin. Endocrinol. Metab. 2008, 93, 3215–3221. [Google Scholar] [CrossRef] [PubMed]
- Maury, E.; Brichard, S.M. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol. Cell. Endocrinol. 2010, 314, 1–16. [Google Scholar] [CrossRef]
- Kopasov, A.E.; Blokhin, S.N.; Volkova, E.N.; Morozov, S.G. Chemokine Expression in Neutrophils and Subcutaneous Adipose Tissue Cells Obtained during Abdominoplasty from Patients with Obesity and Normal Body Weight. Bull. Exp. Biol. Med. 2019, 167, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Zlotnik, A.; Yoshie, O. The Chemokine Superfamily Revisited. Immunity 2012, 36, 705–716. [Google Scholar] [CrossRef]
- Wysocka, M.B.; Pietraszek-Gremplewicz, K.; Nowak, D. The role of apelin in cardiovascular diseases, obesity and cancer. Front. Physiol. 2018, 9, 557. [Google Scholar] [CrossRef]
- Gholamnejad, M.; Meghrazi, K.; Akhgar, M.; Shaianmehr, M. The Assessment of Serum Apelin-12 Level in a Variety of Pulmonary Malignancies in Smokers. Addict. Health 2019, 11, 93–99. [Google Scholar] [PubMed]
- Boucher, J.; Masri, B.; Daviaud, D.; Gesta, S.; Guigné, C.; Mazzucotelli, A.; Castan-Laurell, I.; Tack, I.; Knibiehler, B.; Carpéné, C.; et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 2005, 146, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Feijóo-Bandín, S.; Aragón-Herrera, A.; Moraña-Fernández, S.; Anido-Varela, L.; Tarazón, E.; Roselló-Lletí, E.; Portolés, M.; Moscoso, I.; Gualillo, O.; González-Juanatey, J.R.; et al. Adipokines and inflammation: Focus on cardiovascular diseases. Int. J. Mol. Sci. 2020, 21, 7711. [Google Scholar] [CrossRef]
- Rodríguez-López, C.P.; González-Torres, M.C.; Cruz-Bautista, I.; Nájera-Medina, O. Visceral obesity, skeletal muscle mass and resistin in metabolic syndrome development. Nutr. Hosp. 2019, 36, 43–50. [Google Scholar]
- Park, H.K.; Kwak, M.K.; Kim, H.J.; Ahima, R.S. Role of Murine Resistin in Insulin Resistance. Korean J. Intern. Med. 2017, 32, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Maury, E.; Noël, L.; Detry, R.; Brichard, S.M. In Vitro hyperresponsiveness to tumor necrosis factor-α contributes to adipokine dysregulation in omental adipocytes of obese subjects. J. Clin. Endocrinol. Metab. 2009, 94, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Fan, Z.; Li, L.; Lu, J.; Zhai, Y.; Zhao, J. The chemokine system and its role in obesity. J. Cell. Physiol. 2019, 234, 3336–3346. [Google Scholar] [CrossRef]
- Aass, K.R.; Kastnes, M.H.; Standal, T. Molecular interactions and functions of IL-32. J. Leukoc. Biol. 2020, 109, 143–159. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Ortega, V.A.; Hernández-Lizoain, J.L.; Baixauli, J.; Becerril, S.; Rotellar, F.; Valentí, V.; et al. IL-32α-induced inflammation constitutes a link between obesity and colon cancer. Oncoimmunology 2017, 6, e1328338. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Valentí, V.; Moncada, R.; Landecho, M.F.; Silva, C.; Salvador, J.; Frühbeck, G. Increased interleukin-32 levels in obesity promote adipose tissue inflammation and extracellular matrix remodeling: Effect of weight loss. Diabetes 2016, 65, 3636–3648. [Google Scholar] [CrossRef] [PubMed]
- Kaji, H. Adipose tissue-derived plasminogen activator inhibitor-1 function and regulation. Compr. Physiol. 2016, 6, 1873–1896. [Google Scholar] [PubMed]
- Kang, S.; Narazaki, M.; Metwally, H.; Kishimoto, T. Historical overview of the interleukin-6 family cytokine. J. Exp. Med. 2020, 217, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hoejberg, L.; Bastholt, L.; Schmidt, H. Interleukin-6 and melanoma. Melanoma Res. 2012, 22, 327–333. [Google Scholar] [CrossRef]
- Schett, G. Physiological effects of modulating the interleukin-6 axis. Rheumatology 2018, 57, 43–50. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Narazaki, M.; Kishimoto, T. Targeting Interleukin-6 Signaling in Clinic. Immunity 2019, 50, 1007–1023. [Google Scholar] [CrossRef]
- Aubert, J.; Dessolin, S.; Belmonte, N.; Li, M.; McKenzie, F.R.; Staccini, L.; Villageois, P.; Barhanin, B.; Vernallis, A.; Smith, A.G.; et al. Leukemia inhibitory factor and its receptor promote adipocyte differentiation via the mitogen-activated protein kinase cascade. J. Biol. Chem. 1999, 274, 24965–24972. [Google Scholar] [CrossRef][Green Version]
- Mathieu, M.E.; Saucourt, C.; Mournetas, V.; Gauthereau, X.; Thézé, N.; Praloran, V.; Thiébaud, P.; Bœuf, H. LIF-Dependent Signaling: New Pieces in the Lego. Stem Cell Rev. Rep. 2012, 8, 1–15. [Google Scholar] [CrossRef]
- Licursi, M.; Alberto, C.O.; Dias, A.; Hirasawa, K.; Hirasawa, M. High-fat diet-induced downregulation of anorexic leukemia inhibitory factor in the brain stem. Obesity 2016, 24, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, J.; Wang, J.; Hu, W.; Feng, Z. The emerging role of leukemia inhibitory factor in cancer and therapy. Pharmacol. Ther. 2020, 221, 107754. [Google Scholar] [CrossRef] [PubMed]
- Fain, J.N.; Bahouth, S.W.; Madan, A.K. TNFα release by the nonfat cells of human adipose tissue. Int. J. Obes. 2004, 28, 616–622. [Google Scholar] [CrossRef]
- Aggarwal, B.B. Signalling pathways of the TNF superfamily: A double-edged sword. Nat. Rev. Immunol. 2003, 3, 745–756. [Google Scholar] [CrossRef]
- Vanamee, É.S.; Faustman, D.L. Structural principles of tumor necrosis factor superfamily signaling. Sci. Signal. 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Gupta, S.C.; Kim, J.H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 2012, 119, 651–665. [Google Scholar] [CrossRef]
- Basurto, L.; Sánchez, L.; Díaz, A.; Valle, M.; Robledo, A.; Martínez-Murillo, C. Differences between metabolically healthy and unhealthy obesity in PAI-1 level: Fibrinolysis, body size phenotypes and metabolism. Thromb. Res. 2019, 180, 110–114. [Google Scholar] [CrossRef]
- Mertens, I.; Van Gaal, L.F. Obesity, haemostasis and the fibrinolytic system. Obes. Rev. 2002, 3, 85–101. [Google Scholar] [CrossRef]
- Milenkovic, J.; Milojkovic, M.; Jevtovic Stoimenov, T.; Djindjic, B.; Miljkovic, E. Mechanisms of plasminogen activator inhibitor 1 action in stromal remodeling and related diseases. Biomed. Pap. 2017, 161, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Curat, C.A.; Wegner, V.; Sengenès, C.; Miranville, A.; Tonus, C.; Busse, R.; Bouloumié, A. Macrophages in human visceral adipose tissue: Increased accumulation in obesity and a source of resistin and visfatin. Diabetologia 2006, 49, 744–747. [Google Scholar] [CrossRef]
- Fukuhara, A.; Matsuda, M.; Nishizawa, M.; Segawa, K.; Tanaka, M.; Kishimoto, K.; Matsuki, Y.; Murakami, M.; Ichisaka, T.; Murakami, H.; et al. Visfatin: A protein secreted by visceral fat that Mimics the effects of insulin. Science 2005, 307, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, G.; Li, Q.; Tang, Y.; Yang, M.; Yang, H.; Li, K. Changes and relations of circulating visfatin, apelin, and resistin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Exp. Clin. Endocrinol. Diabetes 2006, 114, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Novak, S.; Divkovic, D.; Drenjancevic, I.; Cosic, A.; Selthofer-Relatic, K. Visfatin serum level and expression in subcutaneous and visceral adipose tissue in prepubertal boys. Pediatr. Obes. 2016, 11, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Christodoulatos, G.S. Visfatin, Obesity, and Cancer. In Adipocytokines, Energy Balance, and Cancer; Springer: Berlin/Heidelberg, Germany, 2017; Volume 12, pp. 109–136. [Google Scholar]
- Candelaria, P.V.; Rampoldi, A.; Harbuzariu, A.; Gonzalez-Perez, R.R. Leptin signaling and cancer chemoresistance: Perspectives. World J. Clin. Oncol. 2017, 8, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Malvi, P.; Chaube, B.; Singh, S.V.; Mohammad, N.; Vijayakumar, M.V.; Singh, S.; Chouhan, S.; Bhat, M.K. Elevated circulatory levels of leptin and resistin impair therapeutic efficacy of dacarbazine in melanoma under obese state. Cancer Metab. 2018, 6, 1–14. [Google Scholar] [CrossRef]
- Ellerhorst, J.A.; Diwan, A.H.; Dang, S.M.; Uffort, D.G.; Johnson, M.K.; Cooke, C.P.; Grimm, E.A. Promotion of Melanoma Growth by the Metabolic Hormone Leptin. Oncol. Rep. 2010, 23, 901–907. [Google Scholar] [CrossRef]
- Amjadi, F.; Javanmard, S.H.; Zarkesh-esfahani, H.; Khazaei, M. Leptin promotes melanoma tumor growth in mice related to increasing circulating endothelial progenitor cells numbers and plasma NO production. J. Exp. Clin. Cancer Res. 2011, 30, 1–6. [Google Scholar] [CrossRef]
- Gogas, H.; Trakatelli, M.; Dessypris, N.; Terzidis, A.; Katsambas, A.; Chrousos, G.P.; Petridou, E.T. Melanoma risk in association with serum leptin levels and lifestyle parameters: A case-control study. Ann. Oncol. 2008, 19, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Torisu-Itakura, H.; Lee, J.H.; Scheri, R.P.; Huynh, Y.; Ye, X.; Essner, R.; Morton, D.L. Human Cancer Biology Molecular Characterization of Inflammatory Genes in Sentinel and Nonsentinel Nodes in Melanoma. Hum. Cancer Biol. 2007, 13, 3125–3133. [Google Scholar]
- Oba, J.; Wei, W.; Gershenwald, J.E.; Johnson, M.M.; Wyatt, C.M.; Ellerhorst, J.A.; Grimm, E.A. Elevated Serum Leptin Levels are Associated With an Increased Risk of Sentinel Lymph Node Metastasis in Cutaneous Melanoma. Medicine 2016, 95, e3073. [Google Scholar] [CrossRef] [PubMed]
- Maldi, E.; Travelli, C.; Caldarelli, A.; Agazzone, N.; Cintura, S.; Galli, U.; Scatolini, M.; Ostano, P.; Miglino, B.; Chiorino, G.; et al. Nicotinamide phosphoribosyltransferase (NAMPT) is over-expressed in melanoma lesions. Pigment Cell Melanoma Res. 2012, 26, 144–146. [Google Scholar] [CrossRef]
- Zhao, H.; Tang, W.; Chen, X.; Wang, S.; Wang, X.; Hu, H.; Li, L. The NAMPT/E2F2/SIRT1 axis promotes proliferation and inhibits p53-dependent apoptosis in human melanoma cells. Biochem. Biophys. Res. Commun. 2017, 493, 77–84. [Google Scholar] [CrossRef]
- Wachsman, W.; Morhenn, V.; Palmer, T.; Walls, L.; Hata, T.; Zalla, J.; Scheinberg, R.; Sofen, H.; Mraz, S.; Gross, K.; et al. Noninvasive genomic detection of melanoma. Br. J. Dermatol. 2011, 164, 797–806. [Google Scholar] [CrossRef]
- La Vecchia, S.; Zamporlini, F.; Audrito, V.; Manag, A.; Vitale, N.; Baroni, G.; Cignetto, S.; Serra, S.; Bologna, C.; Stingi, A.; et al. Nicotinamide Phosphoribosyltransferase (NAMPT) as a Therapeutic Target in BRAF-Mutated Metastatic Melanoma. JNCI J. Natl. Cancer Inst. 2018, 110, 290–303. [Google Scholar]
- Audrito, V.; Managò, A.; Zamporlini, F.; Rulli, E.; Gaudino, F.; Madonna, G.; Atri, S.D.; Antonini, G.C.; Ascierto, P.A.; Massi, D.; et al. Extracellular nicotinamide phosphoribosyltransferase (eNAMPT) is a novel marker for patients with BRAF-mutated metastatic melanoma. Oncotarget 2018, 9, 18997–19005. [Google Scholar] [CrossRef]
- Elias, E.G.; Hasskamp, J.H.; Sharma, B.K. Cytokines and growth factors expressed by human cutaneous melanoma. Cancers 2010, 2, 794–808. [Google Scholar] [CrossRef]
- Zhou, Y.; Dai, D.L.; Martinka, M.; Su, M.; Zhang, Y.; Campos, E.I.; Dorocicz, I.; Tang, L.; Huntsman, D.; Nelson, C.; et al. Osteopontin expression correlates with melanoma invasion. J. Investig. Dermatol. 2005, 124, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Riker, A.I.; Enkemann, S.A.; Fodstad, O.; Liu, S.; Ren, S.; Morris, C.; Xi, Y.; Howell, P.; Metge, B.; Samant, R.S.; et al. The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Med. Genom. 2008, 1, 1–16. [Google Scholar] [CrossRef]
- Kiss, T.; Ecsedi, S.; Vizkeleti, L.; Koroknai, V.; Emri, G.; Kovács, N.; Adany, R.; Balazs, M. The role of osteopontin expression in melanoma progression. Tumor Biol. 2015, 36, 7841–7847. [Google Scholar] [CrossRef] [PubMed]
- Rangel, J.; Nosrati, M.; Torabian, S.; Shaikh, L.; Leong, S.P.L.; Haqq, C.; Miller, J.R.; Sagebiel, R.W.; Kashani-Sabet, M. Osteopontin as a molecular prognostic marker for melanoma. Cancer 2008, 112, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Soikkeli, J.; Jahkola, T.; Virolainen, S.; Saksela, O.; Hölttä, E. Osteopontin promotes the invasive growth of melanoma cells by activating integrin αvβ3 and down-regulating tetraspanin CD9. Am. J. Pathol. 2014, 184, 842–858. [Google Scholar] [CrossRef] [PubMed]
- Rangaswami, H.; Kundu, G.C. Osteopontin stimulates melanoma growth and lung metastasis through NIK/MEKK1-dependent MMP-9 activation pathways. Oncol. Rep. 2007, 18, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Conway, C.; Mitra, A.; Jewell, R.; Randerson-Moor, J.; Lobo, S.; Nsengimana, J.; Edward, S.; Sanders, D.S.; Cook, M.; Powell, B.; et al. Gene expression profiling of paraffin-embedded primary melanoma using the DASL assay identifies increased osteopontin expression as predictive of reduced relapse-free survival. Clin. Cancer Res. 2009, 15, 6939–6946. [Google Scholar] [CrossRef] [PubMed]
- Philip, S.; Kundu, G.C. Osteopontin induces nuclear factor κB-mediated promatrix metalloproteinase-2 activation through IκBα/IKK signaling pathways, and curcumin (diferulolylmethane) down-regulates these pathways. J. Biol. Chem. 2003, 278, 14487–14497. [Google Scholar] [CrossRef] [PubMed]
- Kadkol, S.S.; Lin, A.Y.; Barak, V.; Kalickman, I.; Lu, L.; Valyi-Nagy, K.; Majumdar, D.; Setty, S.; Maniotis, A.J.; Folberg, R.; et al. Osteopontin Expression and Serum Levels in Metastatic Uveal Melanoma—A Pilot Study. Anat. Pathol. 2006, 47, 802–806. [Google Scholar] [CrossRef]
- Sevim, D.G.; Kiratli, H. Serum adiponectin, insulin resistance, and uveal melanoma: Clinicopathological correlations. Melanoma Res. 2016, 26, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Tura, A.; Thieme, C.; Brosig, A.; Merz, H.; Ranjbar, M.; Vardanyan, S.; Zuo, H.; Maassen, T.; Kakkassery, V.; Grisanti, S. Lower levels of adiponectin and its receptor adipor1 in the uveal melanomas with monosomy-3. Investig. Ophthalmol. Vis. Sci. 2020, 61, 12. [Google Scholar] [CrossRef] [PubMed]
- Pachynski, R.K.; Zabel, B.A.; Kohrt, H.E.; Tejeda, N.M.; Monnier, J.; Swanson, C.D.; Holzer, A.K.; Gentles, A.J.; Sperinde, G.V.; Edalati, A.; et al. The chemoattractant chemerin suppresses melanoma by recruiting natural killer cell antitumor defenses. J. Exp. Med. 2012, 209, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Berta, J.; Török, S.; Tárnoki-Zách, J.; Drozdovszky, O.; Tóvári, J.; Paku, S.; Kovács, I.; Czirók, A.; Masri, B.; Megyesfalvi, Z.; et al. Apelin promotes blood and lymph vessel formation and the growth of melanoma lung metastasis. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Rossi, S.; Cordella, M.; Tabolacci, C.; Nassa, G.; D’Arcangelo, D.; Senatore, C.; Pagnotto, P.; Magliozzi, R.; Salvati, A.; Weisz, A.; et al. TNF-alpha and metalloproteases as key players in melanoma cells aggressiveness. J. Exp. Clin. Cancer Res. 2018, 37, 1–17. [Google Scholar] [CrossRef]
- Paz, H.; Tsoi, J.; Kalbasi, A.; Grasso, C.S.; McBride, W.H.; Schaue, D.; Butterfield, L.H.; Maurer, D.M.; Ribas, A.; Graeber, T.G.; et al. Interleukin 32 expression in human melanoma. J. Transl. Med. 2019, 17, 1–13. [Google Scholar] [CrossRef]
- Castelli, C.; Sensi, M.; Lupetti, R.; Mortarini, R.; Panceri, P.; Anichini, A.; Parmiani, G. Expression of Interleukin la, Interleukin 6, and Tumor Necrosis Factor a Genes in Human Melanoma Clones Is Associated with That of Mutated N-RAS Oncogene. Cancer Res. 1994, 54, 4785–4790. [Google Scholar]
- Hoejberg, L.; Bastholt, L.; Johansen, J.S.; Christensen, I.J.; Gehl, J.; Schmidt, H. Serum interleukin-6 as a prognostic biomarker in patients with metastatic melanoma. Melanoma Res. 2012, 22, 287–293. [Google Scholar] [CrossRef]
- Maruta, S.; Takiguchi, S.; Ueyama, M.; Kataoka, Y.; Oda, Y.; Tsuneyoshi, M.; Iguchi, H. A role for leukemia inhibitory factor in melanoma-induced bone metastasis. Clin. Exp. Metastasis 2009, 26, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.M.; Bernstein, D.; Higgins, S.P.; Higgins, C.E.; Higgins, P.J. SERPINE1 expression discriminates site-specific metastasis in human melanoma. Exp. Dermatol. 2012, 21, 551–554. [Google Scholar] [CrossRef]
- Amjadi, F.; Mehdipoor, R.; Zarkesh-Esfahani, H.; Javanmard, S. Leptin serves as angiogenic/mitogenic factor in melanoma tumor growth. Adv. Biomed. Res. 2016, 5, 127. [Google Scholar]
- Mcmurphy, T.; Xiao, R.; Magee, D.; Slater, A.; Zabeau, L.; Tavernier, J. The Anti-Tumor Activity of a Neutralizing Nanobody Targeting Leptin Receptor in a Mouse Model of Melanoma. PLoS ONE 2014, 9, e89895. [Google Scholar] [CrossRef] [PubMed]
- Daquinag, A.C.; Zhang, Y.; Amaya-Manzanares, F.; Simmons, P.J.; Kolonin, M.G. An Isoform of Decorin Is a Resistin Receptor on the Surface of Adipose Progenitor Cells. Cell Stem Cell 2011, 9, 74–86. [Google Scholar] [CrossRef]
- Benomar, Y.; Taouis, M. Molecular mechanisms underlying obesity-induced hypothalamic inflammation and insulin resistance: Pivotal role of resistin/tlr4 pathways. Front. Endocrinol. 2019, 10, 140. [Google Scholar] [CrossRef]
- Zieba, D.A.; Biernat, W.; Barć, J. Roles of leptin and resistin in metabolism, reproduction and leptin resistance. Domest. Anim. Endocrinol. 2020, 73, 106472. [Google Scholar] [CrossRef]
- Tripathi, D.; Kant, S.; Pandey, S. Resistin in metabolism, inflammation, and disease. FEBS J. 2020, 287, 3141–3149. [Google Scholar] [CrossRef] [PubMed]
- Diakowska, D.; Markocka-Maczka, K.; Nienartowicz, M.; Rosińczuk, J.; Krzystek-Korpacka, M. Assessment of apelin, apelin receptor, resistin, and adiponectin levels in the primary tumor and serum of patients with esophageal squamous cell carcinoma. Adv. Clin. Exp. Med. 2019, 28, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Malvi, P.; Chaube, B.; Pandey, V.; Vijayakumar, V.; Boreddy, P.R.; Mohammad, N.; Singh, S.V.; Bhat, M.K. Obesity induced rapid melanoma progression is reversed by orlistat treatment and dietary intervention: Role of adipokines. Mol. Oncol. 2014, 9, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Kushiro, K.; Núñez, N.P. Ob/ob serum promotes a mesenchymal cell phenotype in B16BL6 melanoma cells. Clin. Exp. Metastasis 2011, 28, 877–886. [Google Scholar] [CrossRef]
- Heo, Y.J.; Choi, S.E.; Jeon, J.Y.; Han, S.J.; Kim, D.J.; Kang, Y.; Lee, K.W.; Kim, H.J. Visfatin Induces Inflammation and Insulin Resistance via the NF-κ B and STAT3 Signaling Pathways in Hepatocytes. J. Diabetes Res. 2019, 2019, 4021623. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, S.; Gao, H.; Ren, L.; Song, G. Visfatin induces the apoptosis of endothelial progenitor cells via the induction of pro-inflammatory mediators through the NF-κ B pathway. Int. J. Mol. Med. 2017, 40, 637–646. [Google Scholar] [CrossRef]
- Grolla, A.A.; Torretta, S.; Gnemmi, I.; Amoruso, A.; Orsomando, G.; Gatti, M.; Caldarelli, A.; Lim, D.; Penengo, L.; Brunelleschi, S.; et al. Nicotinamide phosphoribosyltransferase (NAMPT/PBEF/visfatin) is a tumoural cytokine released from melanoma. Pigment Cell Melanoma Res. 2015, 28, 718–729. [Google Scholar] [CrossRef]
- Lee, W.; Wu, C.; Lin, H.; Lee, I.; Wu, C.; Tseng, J.; Chou, M.; Sheu, W. Visfatin-induced expression of inflammatory mediators in human endothelial cells through the NF-j B pathway. Int. J. Obes. 2009, 33, 465–472. [Google Scholar] [CrossRef]
- Adya, R.; Tan, B.K.; Punn, A.; Chen, J.; Randeva, H.S. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: Novel insights into visfatin-induced angiogenesis. Cardiovasc. Res. 2008, 78, 356–365. [Google Scholar] [CrossRef]
- Kamińska, A.; Kopczyńska, E.; Bronisz, A.; Zmudzińska, M.; Bieliński, M.; Borkowska, A.; Tyrakowski, T.; Junik, R. An evaluation of visfatin levels in obese subjects. Endokrynol. Pol. 2010, 61, 169–173. [Google Scholar]
- Berndt, J.; Klöting, N.; Kralisch, S.; Kovacs, P.; Fasshauer, M.; Schön, M.R.; Stumvoll, M.; Blüher, M. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes 2005, 54, 2911–2916. [Google Scholar] [CrossRef] [PubMed]
- Zahorska-Markiewicz, B.; Olszanecka-Glinianowicz, M.; Janowska, J.; Kocełak, P.; Semik-Grabarczyk, E.; Holecki, M.; Dabrowski, P.; Skorupa, A. Serum concentration of visfatin in obese women. Metabolism 2007, 56, 1131–1134. [Google Scholar] [CrossRef]
- Haider, D.G.; Holzer, G.; Schaller, G.; Weghuber, D.; Widhalm, K.; Wagner, O.; Kapiotis, S.; Wolzt, M. The adipokine visfatin is markedly elevated in obese children. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 548–549. [Google Scholar] [CrossRef]
- Lin, T.C. The role of visfatin in cancer proliferation, angiogenesis, metastasis, drug resistance and clinical prognosis. Cancer Manag. Res. 2019, 11, 3481–3491. [Google Scholar] [CrossRef]
- Ohanna, M.; Cerezo, M.; Nottet, N.; Bille, K.; Didier, R.; Beranger, G.; Mograbi, B.; Rocchi, S.; Yvan-charvet, L.; Ballotti, R.; et al. Pivotal role of NAMPT in the switch of melanoma cells toward an invasive and drug-resistant phenotype. Genes Dev. 2018, 32, 448–461. [Google Scholar] [CrossRef]
- Bułdak, R.J.; Bułdak, Ł.; Polaniak, R.; Kukla, M.; Birkner, E.; Kubina, R.; Kabała-Dzik, A.; Duława-Bułdak, A.; Zwirska-Korczala, K. Visfatin affects redox adaptative responses and proliferation in Me45 human malignant melanoma cells: An in vitro study. Oncol. Rep. 2013, 29, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Buldak, R.J.; Polaniak, R.; Buldak, L.; Mielanczyk, L.; Kukla, M.; Skonieczna, M.; Matysiak, N.; Pharmacology, C. Exogenous administration of visfatin affects cytokine secretion and increases oxidative stress in human melignant melanoma Me45 cells. J. Physiol. Pharmacol. 2013, 64, 377–385. [Google Scholar]
- Audrito, V.; Managò, A.; Gaudino, F.; Deaglio, S. Targeting metabolic reprogramming in metastatic melanoma: The key role of nicotinamide phosphoribosyltransferase (NAMPT). Semin. Cell Dev. Biol. 2020, 98, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Lok, Z.S.Y.; Lyle, A.N. Osteopontin in Vascular Disease. Arter. Thromb. Vasc. Biol. 2019, 39, 613–622. [Google Scholar] [CrossRef]
- Nazneen, F.; Bai, F. The Roles of Osteopontin in the Pathogenesis of West Nile Encephalitis. Vaccines 2020, 8, 748. [Google Scholar] [CrossRef] [PubMed]
- Rizk, E.M.; Seffens, A.M.; Trager, M.H.; Moore, M.R.; Geskin, L.J.; Gartrell-Corrado, R.D.; Wong, W.; Saenger, Y.M. Biomarkers Predictive of Survival and Response to Immune Checkpoint Inhibitors in Melanoma. Am. J. Clin. Dermatol. 2020, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, H.; Rittling, S.R.; Yoshitake, H.; Furuya, K.; Amagasa, T.; Tsuji, K.; Nifuji, A.; Denhardt, D.T.; Noda, M. Osteopontin Deficiency Reduces Experimental Tumor Cell Metastasis to Bone and Soft Tissues. J. Bone Miner. Res. 2001, 16, 652–659. [Google Scholar] [CrossRef]
- Hayashi, C.; Rittling, S.; Hayata, T.; Amagasa, T.; Denhardt, D.; Ezura, Y.; Nakashima, K.; Noda, M. Serum Osteopontin, an Enhancer of Tumor Metastasis to Bone, Promotes B16 Melanoma Cell Migration. J. Cell Biochem. 2007, 101, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Alonso, S.R.; Tracey, L.; Ortiz, P.; Pérez-Gómez, B.; Palacios, J.; Pollán, M.; Linares, J.; Serrano, S.; Sáez-Castillo, A.I.; Sánchez, L.; et al. A high-throughput study in melanoma identifies epithelial-mesenchymal transition as a major determinant of metastasis. Cancer Res. 2007, 67, 3450–3460. [Google Scholar] [CrossRef]
- Kale, S.; Raja, R.; Thorat, D.; Soundararajan, G.; Patil, T.V.; Kundu, G.C. Osteopontin signaling upregulates cyclooxygenase-2 expression in tumor-associated macrophages leading to enhanced angiogenesis and melanoma growth via α9β1 integrin. Oncogene 2014, 33, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.D. Adiponectin: Role in Physiology and Pathophysiology. Int. J. Prev. Med. 2020, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Lord, J.M.; Ampk, M.-Á. Adiponectin inhibits neutrophil apoptosis via activation of AMP kinase, PKB and ERK 1/2 MAP kinase. Apoptosis 2013, 18, 1469–1480. [Google Scholar] [CrossRef]
- Ouchi, N.; Kihara, S.; Funahashi, T.; Matsuzawa, Y.; Walsh, K. Obesity, adiponectin and vascular inflammatory disease. Curr. Opin. Lipidol. 2003, 14, 561–566. [Google Scholar] [CrossRef]
- Dumas, J.; Brisson, L. Interaction between adipose tissue and cancer cells: Role for cancer progression. Cancer Metastasis Rev. 2020, 40, 31–46. [Google Scholar] [CrossRef]
- Bang, S.; Won, K.H.; Moon, H.R.; Yoo, H.; Hong, A.; Song, Y.; Chang, S.E. Novel regulation of melanogenesis by adiponectin via the AMPK/CRTC pathway. Pigment Cell Melanoma Res. 2017, 30, 553–557. [Google Scholar] [CrossRef]
- Vachtenheim, J. The Many Roles of MITF in Melanoma. Single Cell Biol. 2017, 6, 10–13. [Google Scholar] [CrossRef]
- Chou, S.H.; Tseleni-Balafouta, S.; Moon, H.S.; Chamberland, J.P.; Liu, X.; Kavantzas, N.; Mantzoros, C.S. Adiponectin receptor expression in human malignant tissues. Horm. Cancer 2010, 1, 136–145. [Google Scholar] [CrossRef]
- Sun, Y.; Lodish, H.F. Adiponectin deficiency promotes tumor growth in mice by reducing macrophage infiltration. PLoS ONE 2010, 5, e11987. [Google Scholar] [CrossRef] [PubMed]
- Zegers, D.; Beckers, S.; Mertens, I.L.; Van Gaal, L.F.; Van Hul, W. Association between polymorphisms of the Nesfatin gene, NUCB2, and obesity in men. Mol. Genet. Metab. 2011, 9, 39. [Google Scholar] [CrossRef]
- Zegers, D.; Beckers, S.; de Freitas, F.; Jennes, K.; Van Camp, J.K.; Mertens, I.L.; Van Hoorenbeeck, K.; Rooman, R.P.; Desager, K.N.; Massa, G.; et al. Identification of mutations in the NUCB2/nesfatin gene in children with severe obesity. Mol. Genet. Metab. 2012, 107, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.; Szarszewska, M.; Knapp, P.; Grybos, A.; Grybos, M.; Marszalek, A.; Filas, V.; Wojcik-Krowiranda, K.; Swornik, M.; Markowska, J. The role of nesfatin and selected molecular factors in various types of endometrial cancer. Ginekol. Pol. 2019, 90, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Q.; Zheng, Y.; Fang, P.-F.; Song, X.-B. Nesfatin-1 is a potential diagnostic biomarker for gastric cancer. Oncol. Lett. 2020, 19, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.M.; Xu, Z.Q.; Ma, H.S. Nesfatin-1/nucleobindin-2 is a potent prognostic marker and enhances cell proliferation, migration, and invasion in bladder cancer. Dis. Markers 2018, 2018, 4272064. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, W.; Qi, K.; Zhou, J.; Gu, M.; Wang, Z. A novel function of NUCB2 in promoting the development and invasion of renal cell carcinoma. Oncol. Lett. 2018, 15, 2425–2430. [Google Scholar] [CrossRef]
- Suzuki, S.; Takagi, K.; Miki, Y.; Onodera, Y.; Akahira, J.I.; Ebata, A.; Ishida, T.; Watanabe, M.; Sasano, H.; Suzuki, T. Nucleobindin 2 in human breast carcinoma as a potent prognostic factor. Cancer Sci. 2012, 103, 136–143. [Google Scholar] [CrossRef]
- Zhang, D.; Lin, J.; Chao, Y.; Zhang, L.; Jin, L.; Li, N.; He, R.; Ma, B.; Zhao, W.; Han, C. Regulation of the adaptation to ER stress by KLF4 facilitates melanoma cell metastasis via upregulating NUCB2 expression. J. Exp. Clin. Cancer Res. 2018, 37, 1–14. [Google Scholar] [CrossRef]
- Treeck, O.; Buechler, C.; Ortmann, O. Chemerin and Cancer. Int. J. Mol. Sci. 2019, 20, 3750. [Google Scholar] [CrossRef] [PubMed]
- Buechler, C.; Feder, S.; Haberl, E.M.; Aslanidis, C. Chemerin isoforms and activity in obesity. Int. J. Mol. Sci. 2019, 20, 1128. [Google Scholar] [CrossRef]
- Rourke, J.L.; Dranse, H.J.; Sinal, C.J. Towards an integrative approach to understanding the role of chemerin in human health and disease. Obes. Rev. 2013, 14, 245–262. [Google Scholar] [CrossRef]
- Song, Y.; Yin, W.; Dan, Y.; Sheng, J.; Zeng, Y.; He, R. Chemerin partly mediates tumor-inhibitory effect of all-trans retinoic acid via CMKLR1-dependent natural killer cell recruitment. Immunology 2019, 157, 248–256. [Google Scholar] [CrossRef]
- Habata, Y.; Fujii, R.; Hosoya, M.; Fukusumi, S.; Kawamata, Y.; Hinuma, S.; Kitada, C.; Nishizawa, N.; Murosaki, S.; Kurokawa, T.; et al. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim. Biophys. Acta Mol. Cell Res. 1999, 1452, 25–35. [Google Scholar] [CrossRef]
- Yan, J.; Wang, A.; Cao, J.; Chen, L. Apelin/APJ system: An emerging therapeutic target for respiratory diseases. Cell. Mol. Life Sci. 2020, 77, 2919–2930. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Yao, G.; Yu, H.; Qing, Y.; Wang, K. Tumor apelin, not serum apelin, is associated with the clinical features and prognosis of gastric cancer. BMC Cancer 2016, 16, 794. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Fiedor, E.; Ptak, A. Bisphenol A and its derivatives tetrabromobisphenol A and tetrachlorobisphenol A induce apelin expression and secretion in ovarian cancer cells through a peroxisome proliferator-activated receptor gamma-dependent mechanism. Toxicol. Lett. 2017, 269, 15–22. [Google Scholar] [CrossRef]
- Podgórska, M.; Diakowska, D.; Pietraszek-Gremplewicz, K.; Nienartowicz, M.; Nowak, D. Evaluation of Apelin and Apelin Receptor Level in the Primary Tumor and Serum of Colorectal Cancer Patients. J. Clin. Med. 2019, 8, 1513. [Google Scholar] [CrossRef]
- Podgórska, M.; Pietraszek-Gremplewicz, K.; Nowak, D. Apelin Effects Migration and Invasion Abilities of Colon Cancer Cells. Cells 2018, 7, 113. [Google Scholar] [CrossRef]
- Cabiati, M.; Gaggini, M.; De Simone, P.; Del Ry, S. Evaluation of Apelin/APJ system expression in hepatocellular carcinoma as a function of clinical severity. Clin. Exp. Med. 2020, 21, 3–9. [Google Scholar] [CrossRef]
- Chen, H.; Wong, C.-C.; Liu, D.; Go, M.Y.Y.; Wu, B.; Peng, S.; Kuang, M.; Wong, N.; Yu, J. APLN promotes hepatocellular carcinoma through activating PI3K/Akt pathway and is a druggable target. Theranostics 2019, 9, 5246–5260. [Google Scholar] [CrossRef]
- Heo, K.; Kim, Y.H.; Sung, H.J.; Li, H.Y.; Yoo, C.W.; Kim, J.Y.; Park, J.Y.; Lee, U.L.; Nam, B.H.; Kim, E.O.; et al. Hypoxia-induced up-regulation of apelin is associated with a poor prognosis in oral squamous cell carcinoma patients. Oral Oncol. 2012, 48, 500–506. [Google Scholar] [CrossRef]
- Yang, Y.; Lv, S.Y.; Ye, W.; Zhang, L. Apelin/APJ system and cancer. Clin. Chim. Acta 2016, 457, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Berta, J.; Hoda, M.A.; Laszlo, V.; Rozsas, A.; Garay, T.; Torok, S.; Grusch, M.; Berger, W.; Paku, S.; Renyi-Vamos, F.; et al. Apelin promotes lymphangiogenesis and lymph node metastasis. Oncotarget 2014, 5, 4426–4437. [Google Scholar] [CrossRef]
- Komina, A.; Palkina, N.; Aksenenko, M.; Tsyrenzhapova, S.; Ruksha, T. Antiproliferative and pro-apoptotic effects of MiR-4286 inhibition in melanoma cells. PLoS ONE 2016, 11, e0168229. [Google Scholar] [CrossRef] [PubMed]
- Milenkovic, V.M.; Stanton, E.H.; Nothdurfter, C.; Rupprecht, R.; Wetzel, C.H. The role of chemokines in the pathophysiology of major depressive disorder. Int. J. Mol. Sci. 2019, 20, 2283. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, W.; Ma, J.; Dai, W.; Liu, L.; Guo, S.; Chen, J.; Wang, H.; Yang, Y.; Yi, X.; et al. Aberrant SIRT6 expression contributes to melanoma growth: Role of the autophagy paradox and IGF-AKT signaling. Autophagy 2018, 14, 518–533. [Google Scholar] [CrossRef]
- Chen, K.; Bao, Z.; Tang, P.; Gong, W.; Yoshimura, T.; Wang, J.M. Chemokines in homeostasis and diseases. Cell. Mol. Immunol. 2018, 15, 324–334. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, Y.K.; Kim, S.; Kim, J.E.; Tian, Y.D.; Doh, E.J.; Lee, D.H. Adipochemokines induced by ultraviolet irradiation contribute to impaired. Br. J. Dermatol. 2017, 178, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Duffaut, C.; Zakaroff-girard, A.; Bourlier, V.; Decaunes, P.; Maumus, M.; Chiotasso, P.; Sengene, C.; Lafontan, M.; Galitzky, J.; Bouloumie, A. Interplay Between Human Adipocytes and T Lymphocytes in Obesity CCL20 as an Adipochemokine and T Lymphocytes as Lipogenic Modulators. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1608–1614. [Google Scholar] [CrossRef]
- Gerhardt, C.C.; Romero, I.A.; Cancello, R.; Camoin, L.; Strosberg, A.D. Chemokines control fat accumulation and leptin secretion by cultured human adipocytes. Mol. Cell. Endocrinol. 2001, 175, 81–92. [Google Scholar] [CrossRef]
- Kochumon, S.; Al-Rashed, F.; Abu-Farha, M.; Devarajan, S.; Tuomilehto, J.; Ahmad, R. Adipose tissue expression of CCL19 chemokine is positively associated with insulin resistance. Diabetes Metab. Res. Rev. 2019, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Laurent, V.; Guérard, A.; Mazerolles, C.; Le Gonidec, S.; Toulet, A.; Nieto, L.; Zaidi, F.; Majed, B.; Garandeau, D.; Socrier, Y.; et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat. Commun. 2016, 7, 1–15. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, D.H.; Lee, S.H.; Nam, H.S.; Roh, M.R.; Cho, M.K. Chemokine receptor CCR3 expression in malignant cutaneous tumors. Ann. Dermatol. 2010, 22, 412–417. [Google Scholar] [CrossRef]
- Hong, J.T.; Son, D.J.; Lee, C.K.; Yoon, D.Y.; Lee, D.H.; Park, M.H. Interleukin 32, inflammation and cancer. Pharmacol. Ther. 2017, 174, 127–137. [Google Scholar] [CrossRef]
- Choi, J.D.; Bae, S.Y.; Hong, J.W.; Azam, T.; Dinarello, C.A.; Her, E.; Choi, W.S.; Kim, B.K.; Lee, C.K.; Yoon, D.Y.; et al. Identification of the most active interleukin-32 isoform. Immunology 2009, 126, 535–542. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.E.; Cheon, S.; Song, J.H.; Houh, Y.; Kim, T.S.; Gil, M.; Lee, K.J.; Kim, S.; Kim, D.; et al. Interleukin-32α induces migration of human melanoma cells through downregulation of E-cadherin. Oncotarget 2016, 7, 65825–65836. [Google Scholar] [CrossRef][Green Version]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumor Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef] [PubMed]
- Golan, T.; Parikh, R.; Jacob, E.; Vaknine, H.; Zemser-Werner, V.; Hershkovitz, D.; Malcov, H.; Leibou, S.; Reichman, H.; Sheinboim, D.; et al. Adipocytes sensitize melanoma cells to environmental TGF-β cues by repressing the expression of miR-211. Sci. Signal. 2019, 12, eaav6847. [Google Scholar] [CrossRef]
- Chen, G.L.; Luo, Y.; Eriksson, D.; Meng, X.; Qian, C.; Bäuerle, T.; Chen, X.X.; Schett, G.; Bozec, A. High fat diet increases melanoma cell growth in the bone marrow by inducing osteopontin and interleukin 6. Oncotarget 2016, 7, 26653–26669. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, X. IRE1α-XBP1 pathway promotes melanoma progression by regulating IL-6/STAT3 signaling. J. Transl. Med. 2017, 15, 1–9. [Google Scholar] [CrossRef]
- Laino, A.S.; Woods, D.; Vassallo, M.; Qian, X.; Tang, H.; Wind-Rotolo, M.; Weber, J. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J. Immunother. Cancer 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Wang, T.; Yan, R.; Xu, X.; Yu, H.; Wu, J.; Yang, Y.; Li, W. Effects of leukemia inhibitory factor receptor on the adipogenic differentiation of human bone marrow mesenchymal stem cells. Mol. Med. Rep. 2019, 19, 4719–4726. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 family cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fioravante, M.; Bombassaro, B.; Ramalho, A.F.; Dragano, N.R.; Morari, J.; Solon, C.; Tobar, N.; Ramos, C.D.; Velloso, L.A. Inhibition of hypothalamic leukemia inhibitory factor exacerbates diet-induced obesity phenotype. J. Neuroinflamm. 2017, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pinho, V.; Fernandes, M.; da Costa, A.; Machado, R.; Gomes, A.C. Leukemia inhibitory factor: Recent advances and implications in biotechnology. Cytokine Growth Factor Rev. 2020, 52, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Kuphal, S.; Wallner, S.; Bosserhoff, A.K. Impact of LIF (leukemia inhibitory factor) expression in malignant melanoma. Exp. Mol. Pathol. 2013, 95, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Humbert, L.; Ghozlan, M.; Canaff, L.; Tian, J.; Lebrun, J.J. The leukemia inhibitory factor (LIF) and p21 mediate the TGFβ tumor suppressive effects in human cutaneous melanoma. BMC Cancer 2015, 15, 1–16. [Google Scholar] [CrossRef]
- Paglia, D.; Oran, A.; Lu, C.; Kerbel, R.S.; Sauder, D.N.; McKenzie, R.C. Expression of Leukemia Inhibitory Factor and Interleukin-11 by human melanoma cell lines: LIF, IL-6, and IL-11 are not coregulated. J. Interferon Cytokine Res. 1995, 160, 455–460. [Google Scholar] [CrossRef]
- Guo, H.; Cheng, Y.; Martinka, M.; Elwee, K.M. High LIFr expression stimulates melanoma cell migration and is associated with unfavorable prognosis in melanoma. Oncotarget 2015, 6, 25484–25498. [Google Scholar] [CrossRef]
- Mori, M.; Yamaguchi, K.; Honda, S.; Nagasaki, K.; Ueda, M.; Abe, O.; Abe, K. Cancer Cachexia Syndrome Developed in Nude Mice Bearing Melanoma Cells Producing Leukemia-inhibitory Factor. Cancer Res. 1991, 51, 6656–6659. [Google Scholar] [PubMed]
- Mehaffey, E.; Majid, D.S.A. Tumor necrosis factor-α, kidney function, and hypertension. Am. J. Physiol. Ren. Physiol. 2017, 313, F1005–F1008. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.H.; Rehman, K.; Liaqat, A. Tumor Necrosis Factor-Alpha: Role in Development of Insulin Resistance and Pathogenesis of Type 2 Diabetes Mellitus. J. Cell. Biochem. 2018, 119, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Vincent, A.; Cates, J.; Brantley-Sieders, D.M.; Polk, D.B.; Young, P.P. Low levels of tumor necrosis factor α increase tumor growth by inducing an endothelial phenotype of monocytes recruited to the tumor site. Cancer Res. 2009, 69, 338–348. [Google Scholar] [CrossRef]
- Donia, M.; Andersen, R.; Kjeldsen, J.W.; Fagone, P.; Munir, S.; Nicoletti, F.; Andersen, M.H.; Straten, P.T.; Svane, I.M. Aberrant expression of MHC class II in melanoma attracts inflammatory tumor-specific CD4+T-cells, which dampen CD8+T-cell antitumor reactivity. Cancer Res. 2015, 75, 3747–3759. [Google Scholar] [CrossRef]
- Reinhardt, J.; Landsberg, J.; Schmid-Burgk, J.L.; Ramis, B.B.; Bald, T.; Glodde, N.; Lopez-Ramos, D.; Young, A.; Ngiow, S.F.; Nettersheim, D.; et al. MAPK signaling and inflammation link melanoma phenotype switching to induction of CD73 during immunotherapy. Cancer Res. 2017, 77, 4697–4709. [Google Scholar] [CrossRef]
- Bertrand, F.; Rochotte, J.; Colacios, C.; Montfort, A.; Tilkin-Mariamé, A.F.; Touriol, C.; Rochaix, P.; Lajoie-Mazenc, I.; Andrieu-Abadie, N.; Levade, T.; et al. Blocking tumor necrosis factor α enhances CD8 T-cell-dependent immunity in experimental melanoma. Cancer Res. 2015, 75, 2619–2628. [Google Scholar] [CrossRef] [PubMed]
- Humbert, L.; Lebrun, J.J. TGF-beta inhibits human cutaneous melanoma cell migration and invasion through regulation of the plasminogen activator system. Cell. Signal. 2013, 25, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.A.; Krause, M.P. PAI-1, the plasminogen system, and skeletal muscle. Int. J. Mol. Sci. 2020, 21, 7066. [Google Scholar] [CrossRef] [PubMed]
- Tokuo, H.; Bhawan, J.; Coluccio, L.M. Myosin X is required for efficient melanoblast migration and melanoma initiation and metastasis. Sci. Rep. 2018, 8, 1–19. [Google Scholar]
- Brooks, T.D.; Slomp, J.; Quax, P.H.A.; De Bart, A.C.W.; Spencer, M.T.; Verheijen, J.H.; Charlton, P.A. Antibodies to PAI-1 alter the invasive and migratory properties of human tumour cells in vitro. Clin. Exp. Metastasis 2000, 18, 445–453. [Google Scholar] [CrossRef]
- Ramont, L.; Pasco, S.; Hornebeck, W.; Maquart, F.X.; Monboisse, J.C. Transforming growth factor-β1 inhibits tumor growth in a mouse melanoma model by down-regulating the plasminogen activation system. Exp. Cell Res. 2003, 291, 1–10. [Google Scholar] [CrossRef]
- Thapa, B.; Koo, B.H.; Kim, Y.H.; Kwon, H.J.; Kim, D.S. Plasminogen activator inhibitor-1 regulates infiltration of macrophages into melanoma via phosphorylation of FAK-Tyr925. Biochem. Biophys. Res. Commun. 2014, 450, 1696–1701. [Google Scholar] [CrossRef]
- Masuda, T.; Hattori, N.; Senoo, T.; Akita, S.; Ishikawa, N.; Fujitaka, K.; Haruta, Y.; Murai, H.; Kohno, N. SK-216, an inhibitor of plasminogen activator inhibitor-1, limits tumor progression and angiogenesis. Mol. Cancer Ther. 2013, 12, 2378–2388. [Google Scholar] [CrossRef]
- McMahon, G.A.; Petitclerc, E.; Stefansson, S.; Smith, E.; Wong, M.K.K.; Westrick, R.J.; Ginsburg, D.; Brooks, P.C.; Lawrence, D.A. Plasminogen Activator Inhibitor-1 Regulates Tumor Growth and Angiogenesis. J. Biol. Chem. 2001, 276, 33964–33968. [Google Scholar] [CrossRef]
- Ma, D.; Gerard, R.D.; Li, X.Y.; Alizadeh, H.; Niederkorn, J.Y. Inhibition of metastasis of intraocular melanomas by adenovirus-mediated gene transfer of plasminogen activator inhibitor type I (PAI-1) in an athymic mouse model. Blood 1997, 90, 2738–2746. [Google Scholar] [CrossRef]
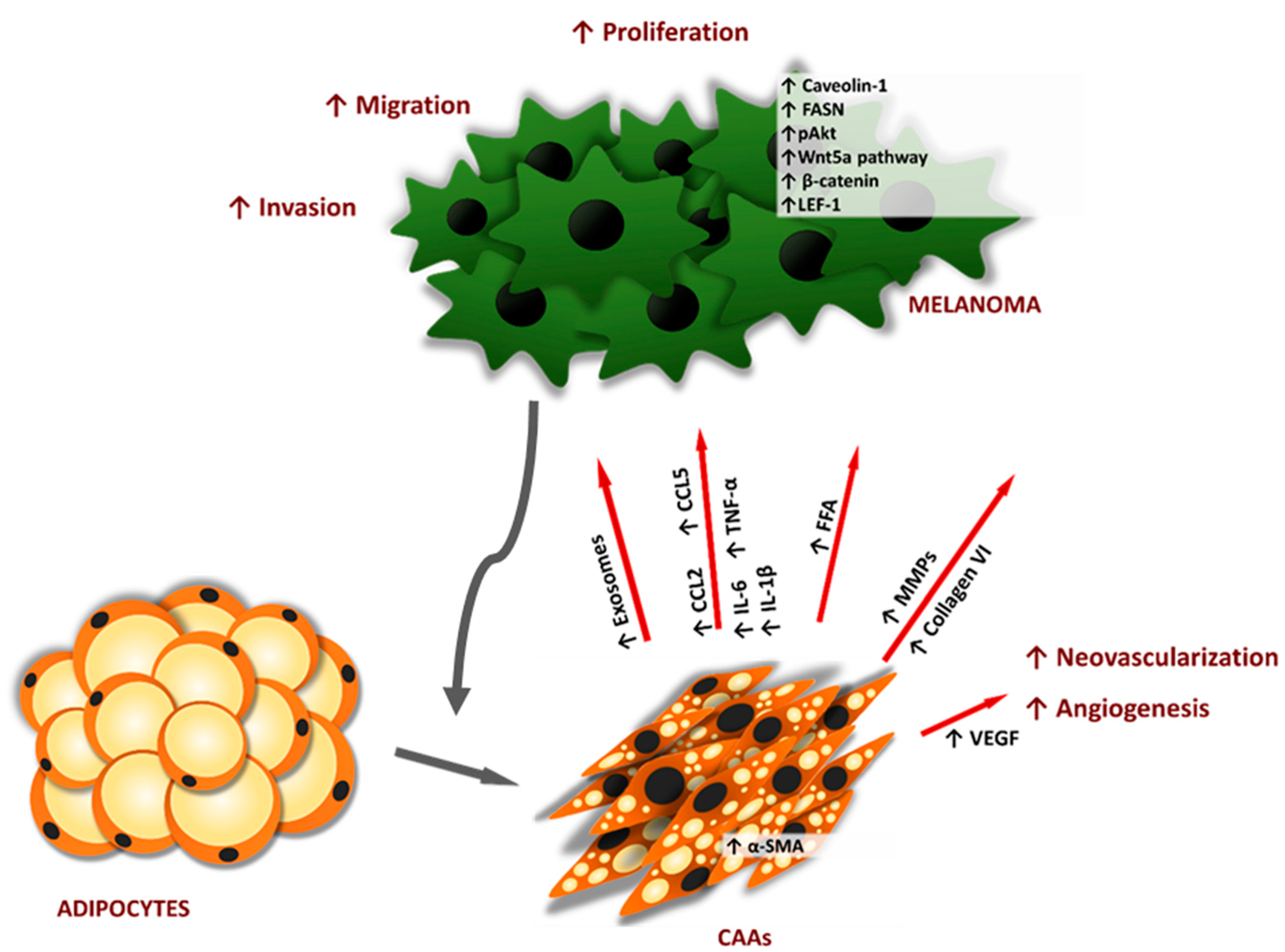
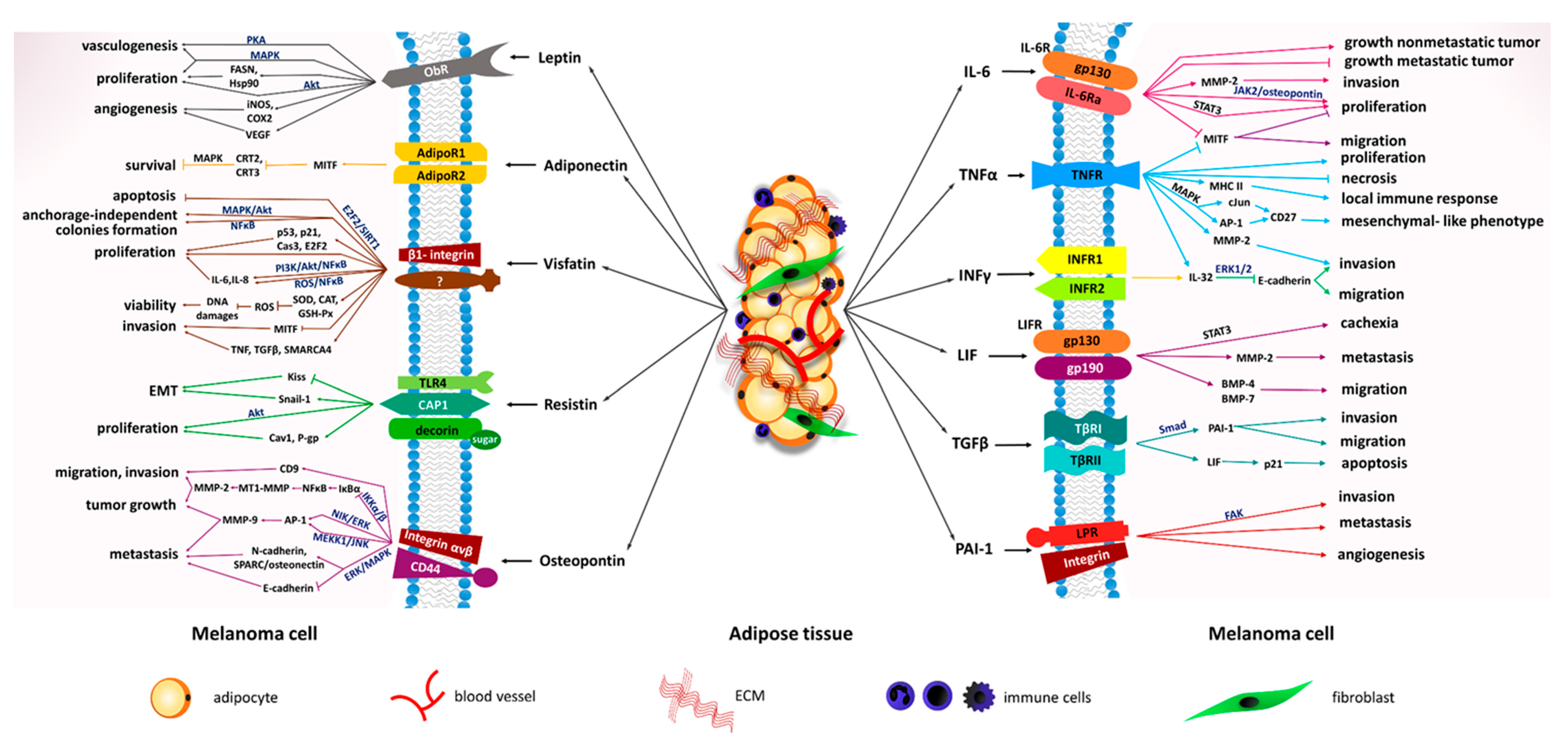
| Molecule | Expression in Adipose Tissue Cell Type | Type of Adipose Tissue | Level under Obesity | Main Biological Functions | |
|---|---|---|---|---|---|
| Leptin | preadipocytes, adipocytes [40] | SAT [39], VAT [6] | WAT [40], BAT [38] | ↑ in serum [39] | control food intake, regulation of energy expenditure, thermogenesis, inflammation, immune responses [44], regulation of bone metabolism and vascular functions [37] |
| Osteopontin | adipocytes [45] | VAT [46], SAT [47] | no data | ↑ in serum [47,48,49] in VAT [48] ↑in SAT [47] | functions in immunity inflammation [50], control of biomineralization, calcification and bone destruction, insulin resistance [49], promotion of angiogenesis [51], induction of neovascularization [52] |
| Adiponectin | adipocytes [53] | VAT, SAT [53] | WAT [6,53] | ↓ in serum [54,55] ↓ in SAT [56] | enhancement of glucose uptake and fatty acid oxidation, act as proangiogenic and anti-apoptotic factor in vascular endothelium [57], insulin sensitivity, act as an anti-inflammatory factor [58] |
| Nesfatin-1 | adipocytes [59] | SAT, VAT [59] | BAT [60] | ↑ in serum [59,61] | regulation of food uptake [62], control body core temperature and energy homeostasis [63], regulation of, reproduction, depressive behavior, cardiovascular and digestive systems [64], promotion of differentiation of primary brown from white adipocytes [60] |
| Chemerin | adipocytes [62,65] | SAT, VAT [66] | WAT [62,65], BAT [67] | ↑ in serum [66] | implication in osteoclastogenesis and insulin-stimulated glucose homeostasis [68], regulation of adipogenesis, angiogenesis and inflammation [69], adipocyte differentiation [62] |
| CCL5 | adipocytes [70] | SAT, VAT [71] | WAT [70] | ↑ in serum [72,73] ↑ in SAT ↑ in VAT [71,73] | act as proinflammatory and potent anti-microbial factor [74] |
| Apelin | adipocytes [70,75] | VAT [76] | WAT [70,75], BAT [77] | ↑ in serum [62,75] | regulation of body fluid homeostasis, angiogenesis, and energy metabolism [75], remodeling of cardiac tissue, regulation of food and fluid intake, control of the release of insulin and histamine [62] |
| Resistin | immune cells [55,78], preadipocytes, adipocytes [3] | SAT [56], VAT [79] | WAT [3,55,78] | ↑ in SAT [56] ↑ in VAT ↑ in serum [79] | energy homeostasis [62], stimulation of inflammation, insulin resistance, enhancement a proliferation and migration of human endothelial cells and vascular smooth muscle cells [80] |
| CCL2 | immune cells [6,72], adipocytes [72] | SAT, VAT [6,72,81] | WAT [81] | ↑ in serum [72,73] ↑ in SAT ↑ in VAT [71,73] | act as proinflammatory factor [74], major chemoattractant for monocytes, NK cells, memory T cells, eosinophils and DCs [82] |
| IL-32 | immune cells, adipocytes [83] | SAT [83], VAT [84] | no data | ↑ in serum ↑ in AT [83,85] | defense against pathogens in viral infections, support chronic infection, regulation of lipid transport and metabolism, control adhesion, migration, and angiogenesis [83] |
| IL-6 | immune cells [86], adipocytes [87] | SAT, VAT [6,72] | no data | ↑ in serum [71,72] ↑ in AT [72] | differentiation of B lymphocytes into plasma cells [88], control Th17/regulatory T cells balance [89], regulate of insulin sensitivity [72], enhancement angiogenesis [90] |
| LIF | immune cells [91], preadipocytes [92] | SAT, VAT [6] | no data | no data | suppress food intake and body weight [93], enhancement the proliferation of hematopoietic stem cells, development and regeneration of tissues and organs, regulation immune response and inflammation [94] |
| TNFα | immune cells [86,95], adipocytes [81,95] | SAT [95], VAT [81,86,95] | no data | ↑ in AT [72] | necessary for proliferation of cells during hematopoiesis and protection against infections [96], essential for immune regulation and morphogenesis [97], role in inflammation and angiogenesis [98], involvement in insulin resistance [72], promotion of tissue repair and of B cells differentiation [98] |
| PAI-1 | immune cells, adipocytes [72,86] | SAT, VAT [72,86] | WAT [70] | ↑ in serum ↑ In VAT [99] ↑ SAT [99,100] | main physiologic inhibitor of fibrinolysis (specifically t-PA and u-PA), enhancement of inflammation, coagulation, fibrosis, and adhesion [101], control angiogenesis and wound healing [86] |
| Visfatin | immune cells [102] | VAT [103,104], SAT [105] | WAT [102] | ↑ in plasma [106] | regulation of cellular energetics via rate-limiting of biosynthesis of NAD, insulin-like functions, immune cell signaling [3], role in the maturation of B cells and vascular smooth muscle cells [62], promotion of migration and formation of blood vessels [55] |
| Molecule | Expression in Melanoma | Level under Obesity | Importance in Melanoma |
|---|---|---|---|
| Leptin | ↑ [109] | ↑ [39] | enhanced level increases the melanoma risk [111], positive correlation between serum leptin level and melanoma metastases to sentinel lymph nodes [113], |
| Resistin | no data | ↑ [47,48,49] | no data |
| Visfatin | ↑ [114,115] | ↓ [54,55,56] | enable a distinction of melanoma from nevi or normal skin [116,117], patients with higher levels live shorter lives [115,118], positive correlation with markers of tumor mass [118] elevated expression in vertical growth phase melanoma and in metastases [114] |
| Osteopontin | ↑ [119,120] | ↑ [59,61] | higher expression in malignant than primary melanoma [121] negative correlation with patient survival and clinical outcomes in primary melanoma patients [122], prognostic marker of survival, the risk of recurrence and lymph node metastases [123], positive correlation with melanoma stage (tumor thickness, Clark’s level and mitotic index) [122,123,124,125], prognostic marker of metastatic-free and overall survival [126], positive correlation with metastases [127] into the liver [128], higher levels in invasive and metastatic melanoma compared to benign and dysplastic moles [120], |
| Adiponectin | no data | ↑ [66] | low serum level may promote growth and more aggressive clinical course of uveal melanoma [129], low serum level improves the metastatic potential of the uveal melanoma with monosomy-3 [130] |
| Nesfatin-1 | no data | ↑ [71,72,73] | no data |
| Chemerin | ↓ [131] | ↑ [62,75] | high expression in melanoma correlates with enhanced outcome [131] |
| Apelin | + [132] | ↑ [56,79] | no data |
| CCL2 | no data | ↑ [71,72,73] | no data |
| CCL5 | + [133] | ↑ [83,85] | no data |
| IL-32 | + [134] | ↑ [71,72] | no data |
| IL-6 | + [119,135] | no data | higher serum level is connected with shorter overall patients’ survival [136] |
| LIF | ↑ [137] | ↑ [72] | elevated expression in melanoma with lymph node metastasis [138] |
| TNFα | + [119,133,135] | ↑ [99,100] | no data |
| PAI-1 | ↑ [138] | ↑ [106] | no data |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olszańska, J.; Pietraszek-Gremplewicz, K.; Nowak, D. Melanoma Progression under Obesity: Focus on Adipokines. Cancers 2021, 13, 2281. https://doi.org/10.3390/cancers13092281
Olszańska J, Pietraszek-Gremplewicz K, Nowak D. Melanoma Progression under Obesity: Focus on Adipokines. Cancers. 2021; 13(9):2281. https://doi.org/10.3390/cancers13092281
Chicago/Turabian StyleOlszańska, Joanna, Katarzyna Pietraszek-Gremplewicz, and Dorota Nowak. 2021. "Melanoma Progression under Obesity: Focus on Adipokines" Cancers 13, no. 9: 2281. https://doi.org/10.3390/cancers13092281
APA StyleOlszańska, J., Pietraszek-Gremplewicz, K., & Nowak, D. (2021). Melanoma Progression under Obesity: Focus on Adipokines. Cancers, 13(9), 2281. https://doi.org/10.3390/cancers13092281






