The PI3K/mTOR Pathway Is Targeted by Rare Germline Variants in Patients with Both Melanoma and Renal Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
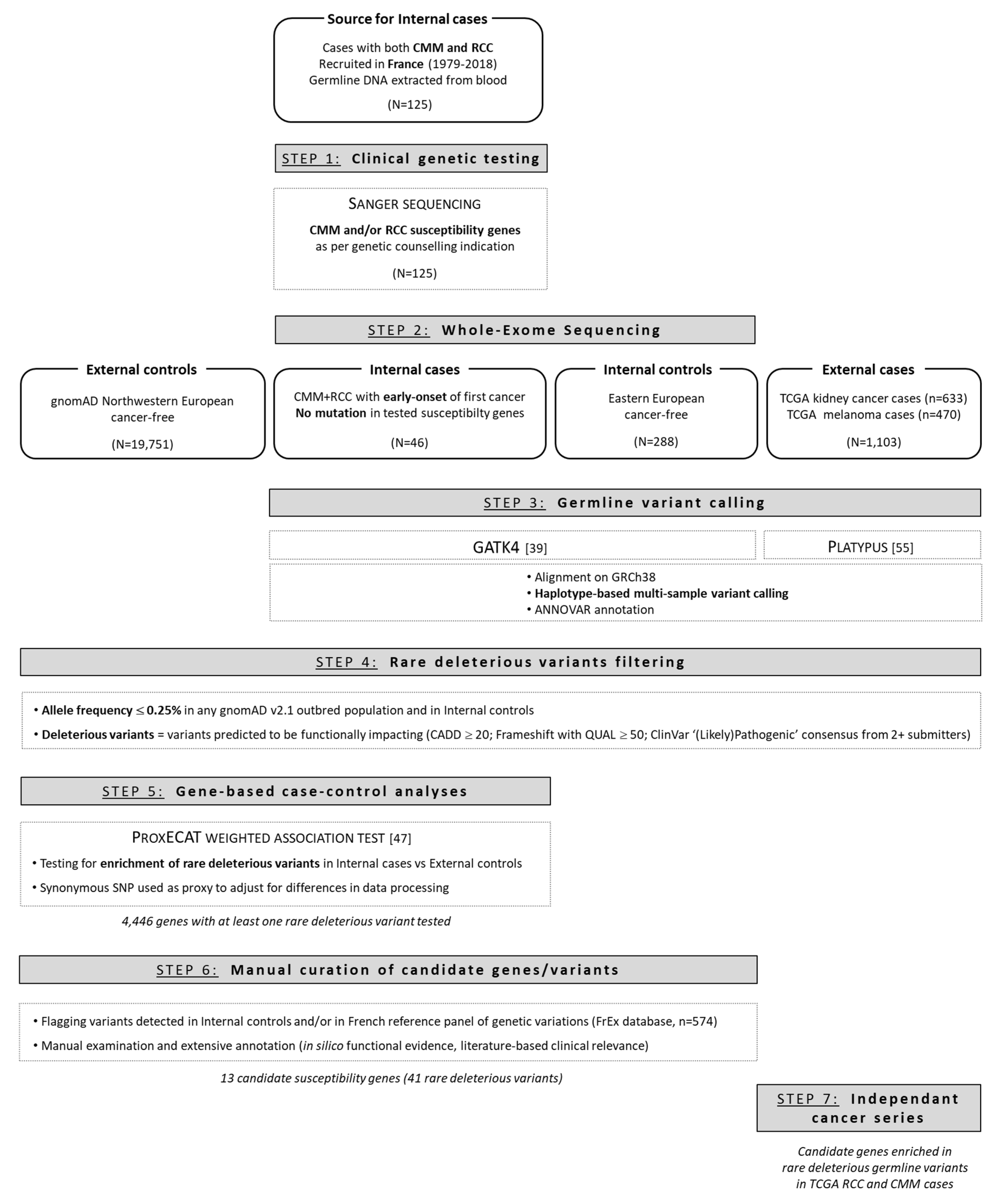
2. Materials and Methods
2.1. Recruitment of Patients and Data Collection
2.2. Ethic and Consent
2.3. Clinical Genetic Testing
2.4. Exome Sequencing, Variant Calling, and Filtering
2.5. Gene-Based Case-Control Analyses
2.6. Validation of Candidate Genes and Variants
3. Results
3.1. Overview of Clinical Sequencing Results
3.2. WES Confirmed Infrequent Pathogenic Variants in Melanoma and/or RCC Risk Genes
3.3. Gene-Based Case-Control Analysis Identified 13 Candidate Susceptibility Genes
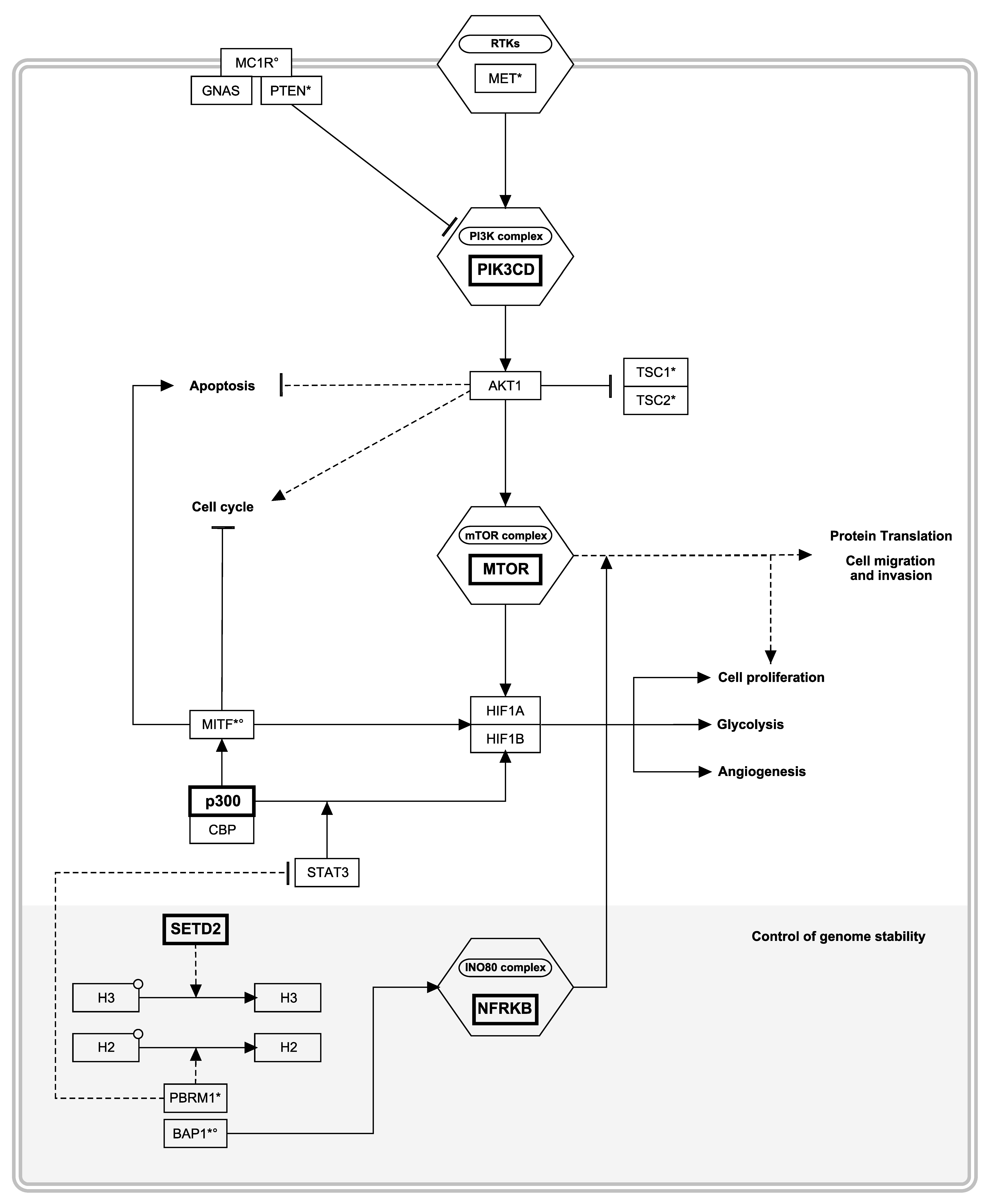
3.4. Pathway Level Analyses Highlighted the Central Role of PI3K/Akt and Its Downstream mTOR/HIF Axis
3.5. Relevance of Our Candidate Susceptibility Genes in Malignant Melanoma and RCC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Chang, A.E.; Karnell, L.H.; Menck, H.R. The National Cancer Data Base Report on Cutaneous and Noncutaneous Melanoma: A Summary of 84,836 Cases from the Past Decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer 1998, 83, 1664–1678. [Google Scholar] [CrossRef]
- Maubec, E.; Chaudru, V.; Mohamdi, H.; Grange, F.; Patard, J.-J.; Dalle, S.; Crickx, B.; Paillerets, B.B.; Demenais, F.; Avril, M.-F. Characteristics of the Coexistence of Melanoma and Renal Cell Carcinoma. Cancer 2010, 116, 5716–5724. [Google Scholar] [CrossRef]
- Abern, M.R.; Tsivian, M.; Coogan, C.L.; Kaufman, H.L.; Polascik, T.J. Characteristics of Patients Diagnosed with Both Melanoma and Renal Cell Cancer. Cancer Causes Control 2013, 24, 1925–1933. [Google Scholar] [CrossRef]
- Flynn, M.; Pickering, L.; Larkin, J.; Turajlic, S. Immune-Checkpoint Inhibitors in Melanoma and Kidney Cancer: From Sequencing to Rational Selection. Ther. Adv. Med. Oncol. 2018, 10, 1758835918777427. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, G.C.; Falzone, L.; Salemi, R.; Zanghì, A.; Spandidos, D.A.; Mccubrey, J.A.; Candido, S.; Libra, M. Cutaneous Melanoma: From Pathogenesis to Therapy (Review). Int. J. Oncol. 2018, 52, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Scelo, G.; Larose, T.L. Epidemiology and Risk Factors for Kidney Cancer. J. Clin. Oncol. 2018, 36, 3574. [Google Scholar] [CrossRef]
- Clement, E.; Lazar, I.; Muller, C.; Nieto, L. Obesity and Melanoma: Could Fat Be Fueling Malignancy? Pigment Cell Melanoma Res. 2017, 30, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Dusingize, J.C.; Olsen, C.M.; An, J.; Pandeya, N.; Law, M.H.; Thompson, B.S.; Goldstein, A.M.; Iles, M.M.; Webb, P.M.; Neale, R.E.; et al. Body Mass Index and Height and Risk of Cutaneous Melanoma: Mendelian Randomization Analyses. Int. J. Epidemiol. 2020, 49, 1236–1245. [Google Scholar] [CrossRef]
- Scelo, G.; Purdue, M.P.; Brown, K.M.; Johansson, M.; Wang, Z.; Eckel-Passow, J.E.; Ye, Y.; Hofmann, J.N.; Choi, J.; Foll, M.; et al. Genome-Wide Association Study Identifies Multiple Risk Loci for Renal Cell Carcinoma. Nat. Commun. 2017, 8, 15724. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ek, W.E.; Whiteman, D.; Vaughan, T.L.; Spurdle, A.B.; Easton, D.F.; Pharoah, P.D.; Thompson, D.J.; Dunning, A.M.; Hayward, N.K.; et al. Most Common “sporadic” Cancers Have a Significant Germline Genetic Component. Hum. Mol. Genet. 2014, 23, 6112–6118. [Google Scholar] [CrossRef] [PubMed]
- Nasti, T.H.; Timares, L. MC1R, Eumelanin and Pheomelanin: Their Role in Determining the Susceptibility to Skin Cancer. Photochem. Photobiol. 2015, 91, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, C.; Garcia-Borron, J.C.; Jiménez-Cervantes, C.; Olivares, C. MC1R Signaling. Intracellular Partners and Pathophysiological Implications. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2448–2461. [Google Scholar] [CrossRef] [PubMed]
- Robles-Espinoza, C.D.; Roberts, N.D.; Chen, S.; Leacy, F.P.; Alexandrov, L.B.; Pornputtapong, N.; Halaban, R.; Krauthammer, M.; Cui, R.; Timothy Bishop, D.; et al. Germline MC1R Status Influences Somatic Mutation Burden in Melanoma. Nat. Commun. 2016, 7, 12064. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.F.; Olsen, C.M.; Hayward, N.K.; Whiteman, D.C. Melanocortin 1 Receptor and Risk of Cutaneous Melanoma: A Meta-Analysis and Estimates of Population Burden. Int. J. Cancer 2011, 129, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Kiezun, A.; Garimella, K.; Do, R.; Stitziel, N.O.; Neale, B.M.; McLaren, P.J.; Gupta, N.; Sklar, P.; Sullivan, P.F.; Moran, J.L.; et al. Exome Sequencing and the Genetic Basis of Complex Traits. Nat. Genet. 2012, 44, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Read, J.; Wadt, K.A.W.; Hayward, N.K. Melanoma Genetics. J. Med. Genet. 2016, 53, 1–14. [Google Scholar] [CrossRef]
- Goding, C.R.; Arnheiter, H. MITF-the First 25 Years. Genes Dev. 2019, 33, 983–1007. [Google Scholar] [CrossRef]
- Phelep, A.; Laouari, D.; Bharti, K.; Burtin, M.; Tammaccaro, S.; Garbay, S.; Nguyen, C.; Vasseur, F.; Blanc, T.; Berissi, S.; et al. MITF—A Controls Branching Morphogenesis and Nephron Endowment. PLoS Genet. 2017, 13, e1007093. [Google Scholar] [CrossRef]
- Bertolotto, C.; Lesueur, F.; Giuliano, S.; Strub, T.; de Lichy, M.; Bille, K.; Dessen, P.; d’Hayer, B.; Mohamdi, H.; Remenieras, A.; et al. A SUMOylation-Defective MITF Germline Mutation Predisposes to Melanoma and Renal Carcinoma. Nature 2011, 480, 94–98. [Google Scholar] [CrossRef]
- Walpole, S.; Pritchard, A.L.; Cebulla, C.M.; Pilarski, R.; Stautberg, M.; Davidorf, F.H.; de la Fouchardière, A.; Cabaret, O.; Golmard, L.; Stoppa-Lyonnet, D.; et al. Comprehensive Study of the Clinical Phenotype of Germline BAP1 Variant-Carrying Families Worldwide. J. Natl. Cancer Inst. 2018, 110, 1328–1341. [Google Scholar] [CrossRef]
- Louie, B.H.; Kurzrock, R. BAP1: Not Just a BRCA1-Associated Protein. Cancer Treat. Rev. 2020, 90, 102091. [Google Scholar] [CrossRef]
- Bedogni, B.; Powell, M.B. Skin Hypoxia: A Promoting Environmental Factor in Melanomagenesis. Cell Cycle 2006, 5, 1258–1261. [Google Scholar] [CrossRef]
- Zou, A.-P.; Cowley, A.W. Reactive Oxygen Species and Molecular Regulation of Renal Oxygenation. Acta Physiol. Scand. 2003, 179, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Tempé, D.; Piechaczyk, M.; Bossis, G. SUMO under Stress. Biochem. Soc. Trans. 2008, 36, 874–878. [Google Scholar] [CrossRef] [PubMed]
- Bonet, C.; Luciani, F.; Ottavi, J.-F.; Leclerc, J.; Jouenne, F.-M.; Boncompagni, M.; Bille, K.; Hofman, V.; Bossis, G.; Marco de Donatis, G.; et al. Deciphering the Role of Oncogenic MITFE318K in Senescence Delay and Melanoma Progression. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef]
- Jafri, M.; Wake, N.C.; Ascher, D.B.; Pires, D.E.V.; Gentle, D.; Morris, M.R.; Rattenberry, E.; Simpson, M.A.; Trembath, R.C.; Weber, A.; et al. Germline Mutations in the CDKN2B Tumor Suppressor Gene Predispose to Renal Cell Carcinoma. Cancer Discov. 2015, 5, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Hebert, L.; Bellanger, D.; Guillas, C.; Campagne, A.; Dingli, F.; Loew, D.; Fievet, A.; Jacquemin, V.; Popova, T.; Jean, D.; et al. Modulating BAP1 Expression Affects ROS Homeostasis, Cell Motility and Mitochondrial Function. Oncotarget 2017, 8, 72513–72527. [Google Scholar] [CrossRef] [PubMed]
- Lesueur, F.; de Lichy, M.; Barrois, M.; Durand, G.; Bombled, J.; Avril, M.-F.; Chompret, A.; Boitier, F.; Lenoir, G.M.; French Familial Melanoma Study Group; et al. The Contribution of Large Genomic Deletions at the CDKN2A Locus to the Burden of Familial Melanoma. Br. J. Cancer 2008, 99, 364–370. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, H.; Durbin, R. Fast and Accurate Short Read Alignment with Burrows-Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- DePristo, M.A.; Banks, E.; Poplin, R.; Garimella, K.V.; Maguire, J.R.; Hartl, C.; Philippakis, A.A.; del Angel, G.; Rivas, M.A.; Hanna, M.; et al. A Framework for Variation Discovery and Genotyping Using Next-Generation DNA Sequencing Data. Nat. Genet. 2011, 43, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A Program for Annotating and Predicting the Effects of Single Nucleotide Polymorphisms, SnpEff: SNPs in the Genome of Drosophila Melanogaster Strain W1118; Iso-2; Iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Wendt, J.; Mueller, C.; Rauscher, S.; Fae, I.; Fischer, G.; Okamoto, I. Contributions by MC1R Variants to Melanoma Risk in Males and Females. JAMA Derm. 2018, 154, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.D.; Pairo-Castineira, E.; Rawlik, K.; Canela-Xandri, O.; Rees, J.; Sims, D.; Tenesa, A.; Jackson, I.J. Genome-Wide Study of Hair Colour in UK Biobank Explains Most of the SNP Heritability. Nat. Commun. 2018, 9, 5271. [Google Scholar] [CrossRef]
- Di Tommaso, P.; Chatzou, M.; Floden, E.W.; Barja, P.P.; Palumbo, E.; Notredame, C. Nextflow Enables Reproducible Computational Workflows. Nat. Biotechnol. 2017, 35, 316–319. [Google Scholar] [CrossRef]
- Tarasov, A.; Vilella, A.J.; Cuppen, E.; Nijman, I.J.; Prins, P. Sambamba: Fast Processing of NGS Alignment Formats. Bioinformatics 2015, 31, 2032–2034. [Google Scholar] [CrossRef]
- Van der Auwera, G.A.; Carneiro, M.O.; Hartl, C.; Poplin, R.; Del Angel, G.; Levy-Moonshine, A.; Jordan, T.; Shakir, K.; Roazen, D.; Thibault, J.; et al. From FastQ Data to High Confidence Variant Calls: The Genome Analysis Toolkit Best Practices Pipeline. Curr. Protoc. Bioinform. 2013, 43. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-Generation PLINK: Rising to the Challenge of Larger and Richer Datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional Annotation of Genetic Variants from High-Throughput Sequencing Data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef] [PubMed]
- Wigginton, J.E.; Cutler, D.J.; Abecasis, G.R. A Note on Exact Tests of Hardy-Weinberg Equilibrium. Am. J. Hum. Genet. 2005, 76, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Lek, M.; Karczewski, K.J.; Minikel, E.V.; Samocha, K.E.; Banks, E.; Fennell, T.; O’Donnell-Luria, A.H.; Ware, J.S.; Hill, A.J.; Cummings, B.B.; et al. Analysis of Protein-Coding Genetic Variation in 60,706 Humans. Nature 2016, 536, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The Mutational Constraint Spectrum Quantified from Variation in 141,456 Humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Rentzsch, P.; Witten, D.; Cooper, G.M.; Shendure, J.; Kircher, M. CADD: Predicting the Deleteriousness of Variants throughout the Human Genome. Nucleic Acids Res. 2019, 47, D886–D894. [Google Scholar] [CrossRef]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving Access to Variant Interpretations and Supporting Evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef]
- Hendricks, A.E.; Billups, S.C.; Pike, H.N.C.; Farooqi, I.S.; Zeggini, E.; Santorico, S.A.; Barroso, I.; Dupuis, J. ProxECAT: Proxy External Controls Association Test. A New Case-Control Gene Region Association Test Using Allele Frequencies from Public Controls. PLoS Genet. 2018, 14, e1007591. [Google Scholar] [CrossRef]
- Jiang, Y.; Epstein, M.P.; Conneely, K.N. Assessing the Impact of Population Stratification on Association Studies of Rare Variation. Hum. Hered. 2013, 76, 28–35. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, Y.; Zhang, R.; Su, L.; Gogarten, S.M.; Liu, G.; Brennan, P.; Field, J.K.; McKay, J.D.; Lissowska, J.; et al. Multi-Omics Analysis Reveals a HIF Network and Hub Gene EPAS1 Associated with Lung Adenocarcinoma. EBioMedicine 2018, 32, 93–101. [Google Scholar] [CrossRef]
- Husson, T.; Duboc, J.-B.; Quenez, O.; Charbonnier, C.; Rotharmel, M.; Cuenca, M.; Jegouzo, X.; Richard, A.-C.; Frebourg, T.; Deleuze, J.-F.; et al. Identification of Potential Genetic Risk Factors for Bipolar Disorder by Whole-Exome Sequencing. Transl. Psychiatry 2018, 8, 268. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdóttir, H.; Wenger, A.M.; Zehir, A.; Mesirov, J.P. Variant Review with the Integrative Genomics Viewer. Cancer Res. 2017, 77, e31–e34. [Google Scholar] [CrossRef] [PubMed]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g: Profiler: A Web Server for Functional Enrichment Analysis and Conversions of Gene Lists (2019 Update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-L.; Mashl, R.J.; Wu, Y.; Ritter, D.I.; Wang, J.; Oh, C.; Paczkowska, M.; Reynolds, S.; Wyczalkowski, M.A.; Oak, N.; et al. Pathogenic Germline Variants in 10,389 Adult Cancers. Cell 2018, 173, 355–370.e14. [Google Scholar] [CrossRef]
- Rimmer, A.; Phan, H.; Mathieson, I.; Iqbal, Z.; Twigg, S.R.F.; Wilkie, A.O.M.; McVean, G.; Lunter, G.; WGS500 Consortium. Integrating Mapping-, Assembly- and Haplotype-Based Approaches for Calling Variants in Clinical Sequencing Applications. Nat. Genet. 2014, 46, 912–918. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, W.; Comiskey, D.F.; Liyanarachchi, S.; Nieminen, T.T.; Wang, Y.; DeLap, K.E.; Brock, P.; de la Chapelle, A. A Truncating Germline Mutation of TINF2 in Individuals with Thyroid Cancer or Melanoma Results in Longer Telomeres. Thyroid 2020, 30, 204–213. [Google Scholar] [CrossRef]
- Maher, E.R. Hereditary Renal Cell Carcinoma Syndromes: Diagnosis, Surveillance and Management. World J. Urol. 2018, 36, 1891–1898. [Google Scholar] [CrossRef]
- Schmidt, L.S.; Linehan, W.M. FLCN: The Causative Gene for Birt-Hogg-Dubé Syndrome. Gene 2018, 640, 28–42. [Google Scholar] [CrossRef]
- Aoude, L.G.; Pritchard, A.L.; Robles-Espinoza, C.D.; Wadt, K.; Harland, M.; Choi, J.; Gartside, M.; Quesada, V.; Johansson, P.; Palmer, J.M.; et al. Nonsense Mutations in the Shelterin Complex Genes ACD and TERF2IP in Familial Melanoma. J. Natl. Cancer Inst. 2015, 107, dju408. [Google Scholar] [CrossRef]
- Pastorino, L.; Andreotti, V.; Dalmasso, B.; Vanni, I.; Ciccarese, G.; Mandalà, M.; Spadola, G.; Pizzichetta, M.A.; Ponti, G.; Tibiletti, M.G.; et al. Insights into Genetic Susceptibility to Melanoma by Gene Panel Testing: Potential Pathogenic Variants in ACD, ATM, BAP1, and POT1. Cancers 2020, 12, 1007. [Google Scholar] [CrossRef]
- Malińska, K.; Deptuła, J.; Rogoża-Janiszewska, E.; Górski, B.; Scott, R.; Rudnicka, H.; Kashyap, A.; Domagała, P.; Hybiak, J.; Masojć, B.; et al. Constitutional Variants in POT1, TERF2IP, and ACD Genes in Patients with Melanoma in the Polish Population. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. 2020, 29, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Machiela, M.J.; Hofmann, J.N.; Carreras-Torres, R.; Brown, K.M.; Johansson, M.; Wang, Z.; Foll, M.; Li, P.; Rothman, N.; Savage, S.A.; et al. Genetic Variants Related to Longer Telomere Length Are Associated with Increased Risk of Renal Cell Carcinoma. Eur. Urol. 2017, 72, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Corradetti, M.N.; Guan, K.-L. Dysregulation of the TSC-MTOR Pathway in Human Disease. Nat. Genet. 2005, 37, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Carlo, M.I.; Hakimi, A.A.; Stewart, G.D.; Bratslavsky, G.; Brugarolas, J.; Chen, Y.-B.; Linehan, W.M.; Maher, E.R.; Merino, M.J.; Offit, K.; et al. Familial Kidney Cancer: Implications of New Syndromes and Molecular Insights. Eur. Urol. 2019, 76, 754–764. [Google Scholar] [CrossRef]
- Rosengren, T.; Nanhoe, S.; de Almeida, L.G.D.; Schönewolf-Greulich, B.; Larsen, L.J.; Hey, C.A.B.; Dunø, M.; Ek, J.; Risom, L.; Nellist, M.; et al. Mutational Analysis of TSC1 and TSC2 in Danish Patients with Tuberous Sclerosis Complex. Sci. Rep. 2020, 10, 9909. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhao, W.-Q.; Fang, C.; Yang, X.; Ji, M. Histone Methyltransferase SETD2: A Potential Tumor Suppressor in Solid Cancers. J. Cancer 2020, 11, 3349–3356. [Google Scholar] [CrossRef]
- Wang, Y.; Liyanarachchi, S.; Miller, K.E.; Nieminen, T.T.; Comiskey, D.F.; Li, W.; Brock, P.; Symer, D.E.; Akagi, K.; DeLap, K.E.; et al. Identification of Rare Variants Predisposing to Thyroid Cancer. Thyroid 2019, 29, 946–955. [Google Scholar] [CrossRef]
- Riazalhosseini, Y.; Lathrop, M. Precision Medicine from the Renal Cancer Genome. Nat. Rev. Nephrol. 2016, 12, 655–666. [Google Scholar] [CrossRef]
- Martens, M.; Ammar, A.; Riutta, A.; Waagmeester, A.; Slenter, D.N.; Hanspers, K.; A Miller, R.; Digles, D.; Lopes, E.N.; Ehrhart, F.; et al. WikiPathways: Connecting Communities. Nucleic Acids Res. 2020. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Furumichi, M.; Morishima, K.; Tanabe, M. New Approach for Understanding Genome Variations in KEGG. Nucleic Acids Res. 2019, 47, D590–D595. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, S.D.; Alvarado, A.G.; Kornblum, H.I. Building Bonds: Cancer Stem Cells Depend on Their Progeny to Drive Tumor Progression. Cell Stem Cell 2018, 22, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.; Rose-Zerilli, M.J.J.; Larrayoz, M.; Clifford, R.; Edelmann, J.; Blakemore, S.; Gibson, J.; Wang, J.; Ljungström, V.; Wojdacz, T.K.; et al. Genomic Disruption of the Histone Methyltransferase SETD2 in Chronic Lymphocytic Leukaemia. Leukemia 2016, 30, 2179–2186. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.; Schwartz, A.G.; Lusk, C.; Wenzlaff, A.S.; de Andrade, M.; Mandal, D.; Gaba, C.; Yang, P.; You, M.; Kupert, E.Y.; et al. Genome-Wide Association Study of Familial Lung Cancer. Carcinogenesis 2018, 39, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Woods, S.L.; Boyle, G.M.; Aoude, L.G.; MacGregor, S.; Zismann, V.; Gartside, M.; Cust, A.E.; Haq, R.; Harland, M.; et al. A Novel Recurrent Mutation in MITF Predisposes to Familial and Sporadic Melanoma. Nature 2011, 480, 99–103. [Google Scholar] [CrossRef]
- Potrony, M.; Puig-Butille, J.A.; Aguilera, P.; Badenas, C.; Tell-Marti, G.; Carrera, C.; Javier Del Pozo, L.; Conejo-Mir, J.; Malvehy, J.; Puig, S. Prevalence of MITF p.E318K in Patients With Melanoma Independent of the Presence of CDKN2A Causative Mutations. JAMA Dermatol. 2016, 152, 405–412. [Google Scholar] [CrossRef]
- Potjer, T.P.; Bollen, S.; Grimbergen, A.J.E.M.; van Doorn, R.; Gruis, N.A.; van Asperen, C.J.; Hes, F.J.; van der Stoep, N. Dutch Working Group for Clinical Oncogenetics Multigene Panel Sequencing of Established and Candidate Melanoma Susceptibility Genes in a Large Cohort of Dutch Non-CDKN2A/CDK4 Melanoma Families. Int. J. Cancer 2019, 144, 2453–2464. [Google Scholar] [CrossRef] [PubMed]
- Gromowski, T.; Masojć, B.; Scott, R.J.; Cybulski, C.; Górski, B.; Kluźniak, W.; Paszkowska-Szczur, K.; Rozmiarek, A.; Dębniak, B.; Maleszka, R.; et al. Prevalence of the E318K and V320I MITF Germline Mutations in Polish Cancer Patients and Multiorgan Cancer Risk-a Population-Based Study. Cancer Genet. 2014, 207, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Stoehr, C.G.; Walter, B.; Denzinger, S.; Ghiorzo, P.; Sturm, R.A.; Hinze, R.; Moch, H.; Junker, K.; Hartmann, A.; Stoehr, R. The Microphthalmia-Associated Transcription Factor p.E318K Mutation Does Not Play a Major Role in Sporadic Renal Cell Tumors from Caucasian Patients. Pathobiology 2016, 83, 165–169. [Google Scholar] [CrossRef]
- Smith, P.S.; West, H.; Whitworth, J.; Castle, B.; Sansbury, F.H.; Warren, A.Y.; Woodward, E.R.; Tischkowitz, M.; Maher, E.R. Pathogenic Germline Variants in Patients with Features of Hereditary Renal Cell Carcinoma: Evidence for Further Locus Heterogeneity. Genes Chromosomes Cancer 2021, 60, 5–16. [Google Scholar] [CrossRef]
- Lang, M.; Vocke, C.D.; Ricketts, C.J.; Metwalli, A.R.; Ball, M.W.; Schmidt, L.S.; Linehan, W.M. Clinical and Molecular Characterization of Microphthalmia-Associated Transcription Factor (MITF)-Related Renal Cell Carcinoma. Urology 2020, 149, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Chau, C.; van Doorn, R.; van Poppelen, N.M.; van der Stoep, N.; Mensenkamp, A.R.; Sijmons, R.H.; van Paassen, B.W.; van den Ouweland, A.M.W.; Naus, N.C.; van der Hout, A.H.; et al. Families with BAP1-Tumor Predisposition Syndrome in The Netherlands: Path to Identification and a Proposal for Genetic Screening Guidelines. Cancers 2019, 11, 1114. [Google Scholar] [CrossRef] [PubMed]
- Cocciolone, R.A.; Crotty, K.A.; Andrews, L.; Haass, N.K.; Moloney, F.J. Multiple Desmoplastic Melanomas in Birt-Hogg-Dubé Syndrome and a Proposed Signaling Link between Folliculin, the MTOR Pathway, and Melanoma Susceptibility. Arch. Dermatol. 2010, 146, 1316–1318. [Google Scholar] [CrossRef] [PubMed]
- Sattler, E.C.; Ertl-Wagner, B.; Pellegrini, C.; Peris, K.; Reithmair, M.; Schädle, N.; Ruzicka, T.; Steinlein, O.K. Cutaneous Melanoma in Birt-Hogg-Dubé Syndrome: Part of the Clinical Spectrum? Br. J. Dermatol. 2018, 178, e132–e133. [Google Scholar] [CrossRef]
- Ricketts, C.J.; De Cubas, A.A.; Fan, H.; Smith, C.C.; Lang, M.; Reznik, E.; Bowlby, R.; Gibb, E.A.; Akbani, R.; Beroukhim, R.; et al. The Cancer Genome Atlas Comprehensive Molecular Characterization of Renal Cell Carcinoma. Cell Rep. 2018, 23, 313–326.e5. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.-H.; Mester, J.L.; Ngeow, J.; Rybicki, L.A.; Orloff, M.S.; Eng, C. Lifetime Cancer Risks in Individuals with Germline PTEN Mutations. Clin. Cancer Res. 2012, 18, 400–407. [Google Scholar] [CrossRef]
- Jiang, N.; Dai, Q.; Su, X.; Fu, J.; Feng, X.; Peng, J. Role of PI3K/AKT Pathway in Cancer: The Framework of Malignant Behavior. Mol. Biol. Rep. 2020, 47, 4587–4629. [Google Scholar] [CrossRef]
- Pópulo, H.; Lopes, J.M.; Soares, P. The MTOR Signalling Pathway in Human Cancer. Int. J. Mol. Sci. 2012, 13, 1886–1918. [Google Scholar] [CrossRef]
- Stahl, J.M.; Cheung, M.; Sharma, A.; Trivedi, N.R.; Shanmugam, S.; Robertson, G.P. Loss of PTEN Promotes Tumor Development in Malignant Melanoma. Cancer Res. 2003, 63, 2881–2890. [Google Scholar] [PubMed]
- Keppler-Noreuil, K.M.; Parker, V.E.R.; Darling, T.N.; Martinez-Agosto, J.A. Somatic Overgrowth Disorders of the PI3K/AKT/MTOR Pathway & Therapeutic Strategies. Am. J. Med. Genet. C Semin. Med. Genet. 2016, 172, 402–421. [Google Scholar] [CrossRef]
- Yehia, L.; Ngeow, J.; Eng, C. PTEN-Opathies: From Biological Insights to Evidence-Based Precision Medicine. J. Clin. Investig. 2019, 129, 452–464. [Google Scholar] [CrossRef]
- Zöllner, J.P.; Franz, D.N.; Hertzberg, C.; Nabbout, R.; Rosenow, F.; Sauter, M.; Schubert-Bast, S.; Wiemer-Kruel, A.; Strzelczyk, A. A Systematic Review on the Burden of Illness in Individuals with Tuberous Sclerosis Complex (TSC). Orphanet J. Rare Dis. 2020, 15, 23. [Google Scholar] [CrossRef]
- Guo, H.; German, P.; Bai, S.; Barnes, S.; Guo, W.; Qi, X.; Lou, H.; Liang, J.; Jonasch, E.; Mills, G.B.; et al. The PI3K/AKT Pathway and Renal Cell Carcinoma. J. Genet. Genom. Yi Chuan Xue Bao 2015, 42, 343–353. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Roy, T.; Uddin, M.B.; Banang-Mbeumi, S.; Chamcheu, R.-C.N.; Walker, A.L.; Liu, Y.-Y.; Huang, S. Role and Therapeutic Targeting of the PI3K/Akt/MTOR Signaling Pathway in Skin Cancer: A Review of Current Status and Future Trends on Natural and Synthetic Agents Therapy. Cells 2019, 8, 803. [Google Scholar] [CrossRef]
- Chappell, J.C.; Payne, L.B.; Rathmell, W.K. Hypoxia, Angiogenesis, and Metabolism in the Hereditary Kidney Cancers. J. Clin. Investig. 2019, 129, 442–451. [Google Scholar] [CrossRef]
- Kim, E.; Zucconi, B.E.; Wu, M.; Nocco, S.E.; Meyers, D.J.; McGee, J.S.; Venkatesh, S.; Cohen, D.L.; Gonzalez, E.C.; Ryu, B.; et al. MITF Expression Predicts Therapeutic Vulnerability to P300 Inhibition in Human Melanoma. Cancer Res. 2019, 79, 2649–2661. [Google Scholar] [CrossRef] [PubMed]
- Milani, D.; Manzoni, F.M.P.; Pezzani, L.; Ajmone, P.; Gervasini, C.; Menni, F.; Esposito, S. Rubinstein-Taybi Syndrome: Clinical Features, Genetic Basis, Diagnosis, and Management. Ital. J. Pediatr. 2015, 41, 4. [Google Scholar] [CrossRef]
- Arany, Z.; Huang, L.E.; Eckner, R.; Bhattacharya, S.; Jiang, C.; Goldberg, M.A.; Bunn, H.F.; Livingston, D.M. An Essential Role for P300/CBP in the Cellular Response to Hypoxia. Proc. Natl. Acad. Sci. USA 1996, 93, 12969–12973. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Shi, R.; Zhang, Q. Hypoxia and Oxygen-Sensing Signaling in Gene Regulation and Cancer Progression. Int. J. Mol. Sci. 2020, 21, 8162. [Google Scholar] [CrossRef]
- Martínez-García, M.Á.; Riveiro-Falkenbach, E.; Rodríguez-Peralto, J.L.; Nagore, E.; Martorell-Calatayud, A.; Campos-Rodríguez, F.; Farré, R.; Hernández Blasco, L.; Bañuls Roca, J.; Chiner Vives, E.; et al. A Prospective Multicenter Cohort Study of Cutaneous Melanoma: Clinical Staging and Potential Associations with HIF-1α and VEGF Expressions. Melanoma Res. 2017, 27, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.B.; Wadt, K.; Jensen, U.B.; Lautrup, C.K.; Bojesen, A.; Krogh, L.N.; van Overeem Hansen, T.; Gerdes, A.-M. Exploring the Hereditary Background of Renal Cancer in Denmark. PLoS ONE 2019, 14, e0215725. [Google Scholar] [CrossRef]
- Rotunno, M.; Barajas, R.; Clyne, M.; Hoover, E.; Simonds, N.I.; Lam, T.K.; Mechanic, L.E.; Goldstein, A.M.; Gillanders, E.M. A Systematic Literature Review of Whole Exome and Genome Sequencing Population Studies of Genetic Susceptibility to Cancer. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1519–1534. [Google Scholar] [CrossRef]
- Artomov, M.; Stratigos, A.J.; Kim, I.; Kumar, R.; Lauss, M.; Reddy, B.Y.; Miao, B.; Daniela Robles-Espinoza, C.; Sankar, A.; Njauw, C.-N.; et al. Rare Variant, Gene-Based Association Study of Hereditary Melanoma Using Whole-Exome Sequencing. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed]
- Galvan, A.; Ioannidis, J.P.A.; Dragani, T.A. Beyond Genome-Wide Association Studies: Genetic Heterogeneity and Individual Predisposition to Cancer. Trends Genet. TIG 2010, 26, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.C.; van Overeem Hansen, T.; Sørensen, C.S. Hereditary Breast and Ovarian Cancer: New Genes in Confined Pathways. Nat. Rev. Cancer 2016, 16, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Babu, J.R.; Jeganathan, K.B.; Baker, D.J.; Wu, X.; Kang-Decker, N.; van Deursen, J.M. Rae1 Is an Essential Mitotic Checkpoint Regulator That Cooperates with Bub3 to Prevent Chromosome Missegregation. J. Cell Biol. 2003, 160, 341–353. [Google Scholar] [CrossRef]
- Walker, C.; Burggren, W. Remodeling the Epigenome and (Epi)Cytoskeleton: A New Paradigm for Co-Regulation by Methylation. J. Exp. Biol. 2020, 223. [Google Scholar] [CrossRef]
- Royle, S.J. The Role of Clathrin in Mitotic Spindle Organisation. J. Cell Sci. 2012, 125, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Gorodetska, I.; Kozeretska, I.; Dubrovska, A. BRCA Genes: The Role in Genome Stability, Cancer Stemness and Therapy Resistance. J. Cancer 2019, 10, 2109–2127. [Google Scholar] [CrossRef]
- Chan, S.H.; Ngeow, J. Germline Mutation Contribution to Chromosomal Instability. Endocr. Relat. Cancer 2017, 24, T33–T46. [Google Scholar] [CrossRef][Green Version]
- Gerstenblith, M.R.; Goldstein, A.M.; Fargnoli, M.C.; Peris, K.; Landi, M.T. Comprehensive Evaluation of Allele Frequency Differences of MC1R Variants across Populations. Hum. Mutat. 2007, 28, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wan, L.; Hacker, E.; Dai, X.; Lenna, S.; Jimenez-Cervantes, C.; Wang, Y.; Leslie, N.R.; Xu, G.X.; Widlund, H.R.; et al. MC1R Is a Potent Regulator of PTEN after UV Exposure in Melanocytes. Mol. Cell 2013, 51, 409–422. [Google Scholar] [CrossRef]
- Gong, R. The Renaissance of Corticotropin Therapy in Proteinuric Nephropathies. Nat. Rev. Nephrol. 2011, 8, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Pośpiech, E.; Ligęza, J.; Wilk, W.; Gołas, A.; Jaszczyński, J.; Stelmach, A.; Ryś, J.; Blecharczyk, A.; Wojas-Pelc, A.; Jura, J.; et al. Variants of SCARB1 and VDR Involved in Complex Genetic Interactions May Be Implicated in the Genetic Susceptibility to Clear Cell Renal Cell Carcinoma. Biomed. Res. Int. 2015, 2015, 860405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, N.; Mu, X.; Zhi, L.; Zhai, L.; Jiang, Y.; Fu, Z.; Yao, Z. G Protein Alpha S Subunit Promotes Cell Proliferation of Renal Cell Carcinoma with Involvement of Protein Kinase A Signaling. DNA Cell Biol. 2017, 36, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.; Locke, A.E.; Flickinger, M.; Zawistowski, M.; Levy, S.; Myers, R.M.; Boehnke, M.; Kang, H.M.; Scott, L.J.; Li, J.Z.; et al. Extremely Rare Variants Reveal Patterns of Germline Mutation Rate Heterogeneity in Humans. Nat. Commun. 2018, 9, 3753. [Google Scholar] [CrossRef]
| Demographics | ||
| No. of patients | 125 | |
| No. of male | 80 | 64.0% |
| No. of female | 45 | 36.0% |
| Age at 1st melanoma diagnosis | 57.3 | |
| Age at 1st RCC diagnosis | 58.8 | |
| Melanoma features | ||
| Melanoma site | ||
| Cutaneous | 158 | 97.5% |
| Ocular | 1 | 0.6% |
| Mucosal | 1 | 0.6% |
| Unknown | 2 | 1.2% |
| Histologic subtype for cutaneous melanoma | ||
| Superficial Spreading Melanoma | 87 | 55.1% |
| Nodular Melanoma | 21 | 13.3% |
| Lentigo Malignant Melanoma | 4 | 2.5% |
| Acral Lentiginous Melanoma | 2 | 1.3% |
| Unclassified | 7 | 4.4% |
| Unknown | 37 | 23.4% |
| Year of melanoma diagnosis | from 1984 to 2018 | |
| RCC features | ||
| RCC type | ||
| Clear cell | 93 | 72.7% |
| Papillary | 16 | 12.5% |
| Chromophobe | 8 | 6.3% |
| Other | 5 | 3.9% |
| Unknown | 6 | 4.7% |
| Year of RCC diagnosis | from 1979 to 2018 | |
| Predisposing Gene | Reference Transcript | Nucleotide Change | Amino Acid Change | MC1R Status (Class) | Sex | Age at First Melanoma | No. Melanoma | Melanoma Histological Subtype | Age at First RCC | RCC Histological Subtype | Other Cancers in Proband | Cancers in Family |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MITF | NM_000248.3 | c.952G>A | p.E318K | p.R163Q (r) | Male | 33 | 2 | SSM | 27 | chRCC | Uncle: skin cancer | |
| MITF * | NM_000248.3 | c.952G>A | p.E318K | p.V92M (r) | Male | 37 | 1 | SSM | 55 | ccRCC | ||
| MITF * | NM_000248.3 | c.952G>A | p.E318K | p.V60L (r) p.R160W (R) | Male | 62 | 1 | NM | 51 | ccRCC | ||
| MITF * | NM_000248.3 | c.952G>A | p.E318K | WT | Male | 52 | 1 | SSM | 52 | ccRCC | Mother: breast cancer Maternal uncle: colo-rectal cancer Paternal uncle: leukemia | |
| MITF | NM_000248.3 | c.952G>A | p.E318K | p.R160W (R) p.D294H (R) | Female | 56 | 2 | SSM | 59 | ccRCC | Basal cell carcinoma | Mother: RCC + lung cancer Sister: basal cell carcinoma |
| MITF | NM_000248.3 | c.952G>A | p.E318K | p.R163Q (r) | Male | 60 | 1 | SSM | 60 | chRCC | Thyroid adenocarcinoma (60) | Father: RCC? |
| MITF * | NM_000248.3 | c.952G>A | p.E318K | p.V60L (r) | Male | 69 | 1 | NM | 69 | ccRCC | ||
| MITF * | NM_000248.3 | c.952G>A | p.E318K | p.R160W (R) | Male | 75 | 2 | NM | 70 | ccRCC | ||
| MITF | NM_000248.3 | c.952G>A | p.E318K | p.V92M (r) p.R151C (R) | Male | 74 | 3 | SSM | 74 | pRCC | Basal cell carcinoma | Mother: 2 CMM? Sister: CMM |
| BAP1 | NM_004656.3 | c.37+1delG | p.? | p.V60L (r) p.R160W (R) | Female | 29 | 6 | SSM | 49 | ccRCC | Father: mesothelioma Sister: OMM Brother: CMM + lung cancer (no tobacco) | |
| BAP1 | NM_004656.3 | c.78-79del | p.V27fs | WT | Male | 45 | 1 | NM | 53 | ccRCC with a sarcomatoid feature | Sister: OMM (53) + lung cancer (53) Nephew: OMM (18) Mother: liver cancer (43) Maternal cousin 1: skin (55) + duodenal cancers (56) Maternal cousin 2: lung cancer (53) | |
| BAP1 | NM_004656.3 | c.1938T>A | p.Y646 * | p.V60L (r) | Female | 48 | 1 | SSM | 59 | ccRCC | Urothelial cancer (59) | Mother and sister 1: CMM Sister 2: meningioma |
| CDKN2A | NM_000077.4 | c.146T>G | p.I49S | p.V92M (r) p.R151C (R) | Female | 31 | 1 | SMM | 36 | ccRCC | Mother and sister: CMM | |
| CDKN2A | NM_000077.4 | c.159G>C | p.M53I | p.V60L (r) p.R151C (R) | Male | 46 | 1 | NM | 61 | ccRCC | Mother and brother: CMM | |
| FLCN | NM_144997.6 | c.663dupG | p.M222fs | WT | Female | 48 | 1 | NM | 43 | chRCC with oncocytoma components | Leiomyosarcoma | Father: lung cancer Paternal uncle: RCC |
| FLCN | NM_144997.6 | c.755dupC | p.C253fs | WT | Male | 64 | 1 | SSM | 62 | ccRCC | Cutaneous fibrofolliculoma | |
| PTEN | NM_000314.6 | c.959T>G | p.L320* | WT | Female | 55 | 1 | SSM | 55 | ccRCC | Daughter: ALM (25) with PTEN+ |
| HGNC Gene Symbol | Gene Description | Gene Length (pb) | LOEUF Mutational Constraint a | Rare b Deleterious Allele Counts | p-Value c | q-Value d | |
|---|---|---|---|---|---|---|---|
| Internal Cases (N = 46) | External Controls (N = 19,751) | ||||||
| PIK3CD | Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Delta | 6333 | 0.20 | 2 | 68 | 2 × 10−5 | 0.04 |
| MTOR | Mechanistic Target of Rapamycin Kinase | 12,163 | 0.18 | 4 | 252 | 4 × 10−5 | 0.05 |
| RAE1 | Ribonucleic Acid Export 1 | 5642 | 0.19 | 2 | 18 | 8 × 10−5 | 0.08 |
| ZBTB21 | Zinc Finger and BTB Domain Containing 21 | 8062 | 0.25 | 3 | 120 | 2 × 10−4 | 0.12 |
| ESAM | Endothelial Cell Adhesion Molecule | 2920 | 0.59 | 2 | 37 | 2 × 10−4 | 0.12 |
| TMEM192 | Transmembrane Protein 192 | 10,182 | 1.29 | 2 | 30 | 3 × 10−4 | 0.13 |
| CLTCL1 | Clathrin Heavy Chain Like 1 | 10,052 | 0.80 | 6 | 438 | 3 × 10−4 | 0.13 |
| NFRKB | Nuclear Factor Related to KappaB Binding Protein | 6335 | 0.37 | 3 | 233 | 3 × 10−4 | 0.13 |
| EP300 | E1A Binding Protein P300 | 11,692 | 0.10 | 3 | 266 | 4 × 10−4 | 0.15 |
| MTSS2 | MTSS I-BAR Domain Containing 2 | 4986 | 0.31 | 4 | 206 | 4 × 10−4 | 0.15 |
| SETD2 | SET Domain Containing 2, Histone Lysine Methyltransferase | 10,245 | 0.21 | 5 | 505 | 6 × 10−4 | 0.16 |
| SMC2 | Structural Maintenance of Chromosomes 2 | 6470 | 0.23 | 4 | 131 | 6 × 10−4 | 0.17 |
| EBF4 | EBF Family Member 4 | 3541 | 0.70 | 3 | 72 | 8 × 10−4 | 0.18 |
| Chr | Start | End | Ref | Alt | HGNC Gene Symbol | Accession Number | Reference Transcript | Nucleotide Change | Amino acid Change | CADD | AF_Cases | AF_ FrEx | AF_nc_nwe | AF_Popmax | Independent Cancer Series * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 9715647 | 9715647 | A | G | PIK3CD | . | NM_005026 | c.A248G | p.E83G | 25 | 0.01 | . | . | . | |
| 1 | 9715709 | 9715709 | C | T | PIK3CD | . | NM_005026 | c.C310T | p.R104C | 35 | 0.01 | . | 3 × 10−5 | 9 × 10−6 | |
| 1 | 11130641 | 11130641 | G | A | MTOR | rs142403193 | NM_004958 | c.C5501T | p.T1834M | 22.8 | 0.02 | . | 9 × 10−4 | 7 × 10−4 | SKCM (2) |
| 1 | 11238522 | 11238522 | G | A | MTOR | rs751242124 | NM_004958 | c.C1882T | p.R628C | 28.9 | 0.01 | . | . | 5 × 10−5 | |
| 1 | 11248030 | 11248030 | T | A | MTOR | rs761323069 | NM_004958 | c.A905T | p.D302V | 23.1 | 0.01 | . | . | 3 × 10−4 | |
| 20 | 57365381 | 57365381 | C | T | RAE1 | rs755561880 | NM_003610 | c.C314T | p.S105L | 31 | 0.01 | . | . | 6 × 10−5 | |
| 20 | 57365432 | 57365432 | A | G | RAE1 | . | NM_003610 | c.A365G | p.Q122R | 20.6 | 0.01 | . | . | . | |
| 21 | 41991340 | 41991340 | G | A | ZBTB21 | rs368359632 | NM_001098402 | c.C2756T | p.T919M | 25.3 | 0.01 | . | . | 7 × 10−4 | |
| 21 | 41992058 | 41992058 | G | A | ZBTB21 | rs371004245 | NM_001098402 | c.C2038T | p.R680C | 26.5 | 0.01 | . | 1 × 10−4 | 1 × 10−4 | |
| 21 | 41992762 | 41992762 | C | T | ZBTB21 | . | NM_001098402 | c.G1334A | p.R445H | 30 | 0.01 | . | 5 × 10−5 | 4 × 10−5 | |
| 11 | 124753942 | 124753942 | G | A | ESAM | rs760488150 | NM_138961 | c.C877T | p.R293W | 34 | 0.01 | . | 5 × 10−5 | 3 × 10−5 | |
| 11 | 124754658 | 124754658 | G | A | ESAM | rs200924772 | NM_138961 | c.C713T | p.T238M | 33 | 0.01 | 9 × 10−4 | 8 × 10−5 | 2 × 10−4 | |
| 4 | 165103020 | 165103020 | T | G | TMEM192 | . | NM_001100389 | c.A104C | p.Q35P | 22.9 | 0.01 | . | . | . | |
| 4 | 165103021 | 165103021 | G | A | TMEM192 | . | NM_001100389 | c.C103T | p.Q35X | 35 | 0.01 | . | . | . | |
| 22 | 19210459 | 19210459 | C | T | CLTCL1 | rs781878409 | NM_007098 | c.G3116A | p.R1039Q | 32 | 0.01 | . | . | 1 × 10−3 | |
| 22 | 19219929 | 19219929 | C | T | CLTCL1 | rs188611399 | NM_007098 | c.G2875A | p.V959I | 25.5 | 0.01 | . | 2 × 10−4 | 1 × 10−3 | KIRP (2) |
| 22 | 19224006 | 19224006 | T | G | CLTCL1 | rs782728804 | NM_007098 | c.A2177C | p.D726A | 29.5 | 0.01 | . | . | 9 × 10−6 | |
| 22 | 19226346 | 19226346 | T | C | CLTCL1 | rs201280856 | NM_007098 | c.A1820G | p.H607R | 25.5 | 0.01 | . | 3 × 10−4 | 4 × 10−4 | |
| 22 | 19233264 | 19233264 | C | A | CLTCL1 | rs782774942 | NM_007098 | c.G1423T | p.A475S | 23.6 | 0.01 | . | . | 6 × 10−5 | |
| 22 | 19234672 | 19234672 | A | G | CLTCL1 | . | NM_007098 | c.T1004C | p.V335A | 25.3 | 0.01 | . | . | . | |
| 11 | 129872957 | 129872957 | G | A | NFRKB | . | NM_006165 | c.C2765T | p.P922L | 31 | 0.01 | . | . | . | |
| 11 | 129874521 | 129874521 | G | A | NFRKB | rs200192480 | NM_006165 | c.C2113T | p.P705S | 23.9 | 0.01 | . | . | 4 × 10−5 | fNTMC (1 family) |
| 11 | 129884816 | 129884816 | G | A | NFRKB | rs755726394 | NM_006165 | c.C746T | p.A249V | 22.9 | 0.01 | . | . | 6 × 10−5 | |
| 22 | 41117808 | 41117808 | C | T | EP300 | . | NM_001429 | c.C716T | p.P239L | 21.3 | 0.01 | . | . | . | |
| 22 | 41137724 | 41137724 | C | T | EP300 | . | NM_001429 | c.C1694T | p.T565I | 24.9 | 0.01 | . | . | . | |
| 22 | 41149147 | 41149147 | C | T | EP300 | rs201480900 | NM_001429 | c.C2351T | p.P784L | 23.2 | 0.01 | . | 8 × 10−5 | 2 × 10−4 | SKCM (1) |
| 16 | 70663765 | 70663765 | G | A | MTSS2 | rs749003640 | NM_138383 | c.C2156T | p.P719L | 24.5 | 0.01 | . | 2 × 10−4 | 1 × 10−3 | KIRP (1) |
| 16 | 70664615 | 70664615 | T | C | MTSS2 | rs147433916 | NM_138383 | c.A1454G | p.D485G | 23.9 | 0.01 | 2 × 10−3 | 2 × 10−4 | 2 × 10−3 | SKCM (1) |
| 16 | 70665044 | 70665044 | C | T | MTSS2 | rs549028223 | NM_138383 | c.G1181A | p.R394Q | 26.1 | 0.01 | . | 5 × 10−5 | 2 × 10−3 | |
| 16 | 70679820 | 70679820 | C | G | MTSS2 | rs768341867 | NM_138383 | c.G348C | p.K116N | 29.1 | 0.01 | . | . | 9 ×10−6 | |
| 3 | 47046509 | 47046509 | C | T | SETD2 | rs766193321 | NM_001349370 | c.G6944A | p.G2315E | 33 | 0.01 | . | 3 × 10−5 | 9 × 10−6 | |
| 3 | 47046543 | 47046543 | G | T | SETD2 | . | NM_001349370 | c.C6910A | p.P2304T | 25.9 | 0.01 | . | . | . | |
| 3 | 47084114 | 47084114 | A | G | SETD2 | rs148097513 | NM_001349370 | c.T5534C | p.M1845T | 25.3 | 0.01 | 9 × 10−4 | 1 × 10−3 | 2 × 10−3 | KIRC (3)–KIRP (1)–SKCM (1) |
| 3 | 47121407 | 47121407 | T | C | SETD2 | rs114719990 | NM_001349370 | c.A3097G | p.T1033A | 23.6 | 0.01 | 2 × 10−3 | 2 × 10−3 | 2 × 10−3 | SKCM (3)–KIRP (1) |
| 3 | 47123308 | 47123308 | C | G | SETD2 | . | NM_001349370 | c.G1196C | p.R399T | 25.8 | 0.01 | . | . | . | |
| 9 | 104114033 | 104114033 | C | T | SMC2 | . | NM_001042550 | c.C1484T | p.T495I | 20.6 | 0.01 | . | . | . | |
| 9 | 104125007 | 104125007 | G | A | SMC2 | rs147960477 | NM_001042550 | c.G2353A | p.A785T | 23 | 0.02 | 9 × 10−4 | 2 × 10−4 | 9 × 10−4 | |
| 9 | 104139220 | 104139220 | A | G | SMC2 | . | NM_001042550 | c.A3499G | p.T1167A | 23.9 | 0.01 | . | . | . | |
| 20 | 2706020 | 2706020 | G | A | EBF4 | rs202097996 | NM_001110514 | c.G329A | p.R110Q | 21 | 0.01 | 9 × 10−4 | 6 × 10−5 | 1 × 10−4 | |
| 20 | 2706211 | 2706211 | C | A | EBF4 | . | NM_001110514 | c.C349A | p.L117M | 23.3 | 0.01 | . | . | . | |
| 20 | 2755749 | 2755749 | G | A | EBF4 | rs369331115 | NM_001110514 | c.G1651A | p.A551T | 32 | 0.01 | . | . | . |
| Pathway ID | Pathway Description | q-Value * | Number of Genes in Pathway | Candidate Genes in Pathway |
|---|---|---|---|---|
| KEGG:05215 | Prostate cancer | 2.8 × 10−3 | 97 | PIK3CD, MTOR, EP300 |
| KEGG:04066 | HIF-1 signaling pathway | 4 × 10−3 | 109 | PIK3CD, MTOR, EP300 |
| KEGG:04935 | Growth hormone synthesis, secretion and action | 5.1 × 10−3 | 118 | PIK3CD, MTOR, EP300 |
| KEGG:04919 | Thyroid hormone signaling pathway | 5.5 × 10−3 | 121 | PIK3CD, MTOR, EP300 |
| WP:WP4018 | Pathways in clear cell renal cell carcinoma | 7.7 × 10−3 | 86 | MTOR, EP300, SETD2 |
| KEGG:04630 | JAK-STAT signaling pathway | 1.3 × 10−2 | 162 | PIK3CD, MTOR, EP300 |
| KEGG:05164 | Influenza A | 1.5 × 10−2 | 169 | PIK3CD, RAE1, EP300 |
| WP:WP3287 | Overview of nanoparticle effects | 1.6 × 10−2 | 19 | PIK3CD, NFRKB |
| KEGG:05167 | Kaposi sarcoma-associated herpesvirus infection | 2.2 × 10−2 | 193 | PIK3CD, MTOR, EP300 |
| WP:WP4217 | Ebola Virus Pathway on Host | 2.6 × 10−2 | 129 | PIK3CD, CLTCL1, EP300 |
| KEGG:04930 | Type II diabetes mellitus | 3.1 × 10−2 | 45 | PIK3CD, MTOR |
| WP:WP4874 | CAMKK2 Pathway | 5 × 10−2 | 33 | MTOR, EP300 |
| WP:WP4241 | Type 2 papillary renal cell carcinoma | 5.3 × 10−2 | 34 | EP300, SETD2 |
| KEGG:04213 | Longevity regulating pathway—multiple species | 5.7 × 10−2 | 61 | PIK3CD, MTOR |
| KEGG:05221 | Acute myeloid leukemia | 6.9 × 10−2 | 67 | PIK3CD, MTOR |
| KEGG:05211 | Renal cell carcinoma | 7.1 × 10−2 | 68 | PIK3CD, EP300 |
| KEGG:05230 | Central carbon metabolism in cancer | 7.5 × 10−2 | 70 | PIK3CD, MTOR |
| KEGG:05016 | Huntington disease | 8.4 × 10−2 | 306 | MTOR, CLTCL1, EP300 |
| KEGG:05214 | Glioma | 8.6 × 10−2 | 75 | PIK3CD, MTOR |
| KEGG:05206 | MicroRNAs in cancer | 8.8 × 10−2 | 310 | PIK3CD, MTOR, EP300 |
| KEGG:05212 | Pancreatic cancer | 8.8 × 10−2 | 76 | PIK3CD, MTOR |
| KEGG:05100 | Bacterial invasion of epithelial cells | 9.1 × 10−2 | 77 | PIK3CD, CLTCL1 |
| KEGG:01521 | EGFR tyrosine kinase inhibitor resistance | 9.5 × 10−2 | 79 | PIK3CD, MTOR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hubert, J.-N.; Suybeng, V.; Vallée, M.; Delhomme, T.M.; Maubec, E.; Boland, A.; Bacq, D.; Deleuze, J.-F.; Jouenne, F.; Brennan, P.; et al. The PI3K/mTOR Pathway Is Targeted by Rare Germline Variants in Patients with Both Melanoma and Renal Cell Carcinoma. Cancers 2021, 13, 2243. https://doi.org/10.3390/cancers13092243
Hubert J-N, Suybeng V, Vallée M, Delhomme TM, Maubec E, Boland A, Bacq D, Deleuze J-F, Jouenne F, Brennan P, et al. The PI3K/mTOR Pathway Is Targeted by Rare Germline Variants in Patients with Both Melanoma and Renal Cell Carcinoma. Cancers. 2021; 13(9):2243. https://doi.org/10.3390/cancers13092243
Chicago/Turabian StyleHubert, Jean-Noël, Voreak Suybeng, Maxime Vallée, Tiffany M. Delhomme, Eve Maubec, Anne Boland, Delphine Bacq, Jean-François Deleuze, Fanélie Jouenne, Paul Brennan, and et al. 2021. "The PI3K/mTOR Pathway Is Targeted by Rare Germline Variants in Patients with Both Melanoma and Renal Cell Carcinoma" Cancers 13, no. 9: 2243. https://doi.org/10.3390/cancers13092243
APA StyleHubert, J.-N., Suybeng, V., Vallée, M., Delhomme, T. M., Maubec, E., Boland, A., Bacq, D., Deleuze, J.-F., Jouenne, F., Brennan, P., McKay, J. D., Avril, M.-F., Bressac-de Paillerets, B., & Chanudet, E. (2021). The PI3K/mTOR Pathway Is Targeted by Rare Germline Variants in Patients with Both Melanoma and Renal Cell Carcinoma. Cancers, 13(9), 2243. https://doi.org/10.3390/cancers13092243







