Effect of Intravenous Chemotherapy Regimen on Globe Salvage Success Rates for Retinoblastoma Based on Disease Class—A Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
Rationale and Objectives
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Methods
2.3. Study Selection
2.4. Data Collection
2.5. Data Synthesis and Analysis
3. Results
3.1. Study Characteristics
3.2. Globe Salvage Rates among All Retinoblastoma Eyes by R-E and ICRB/IIRC Classification
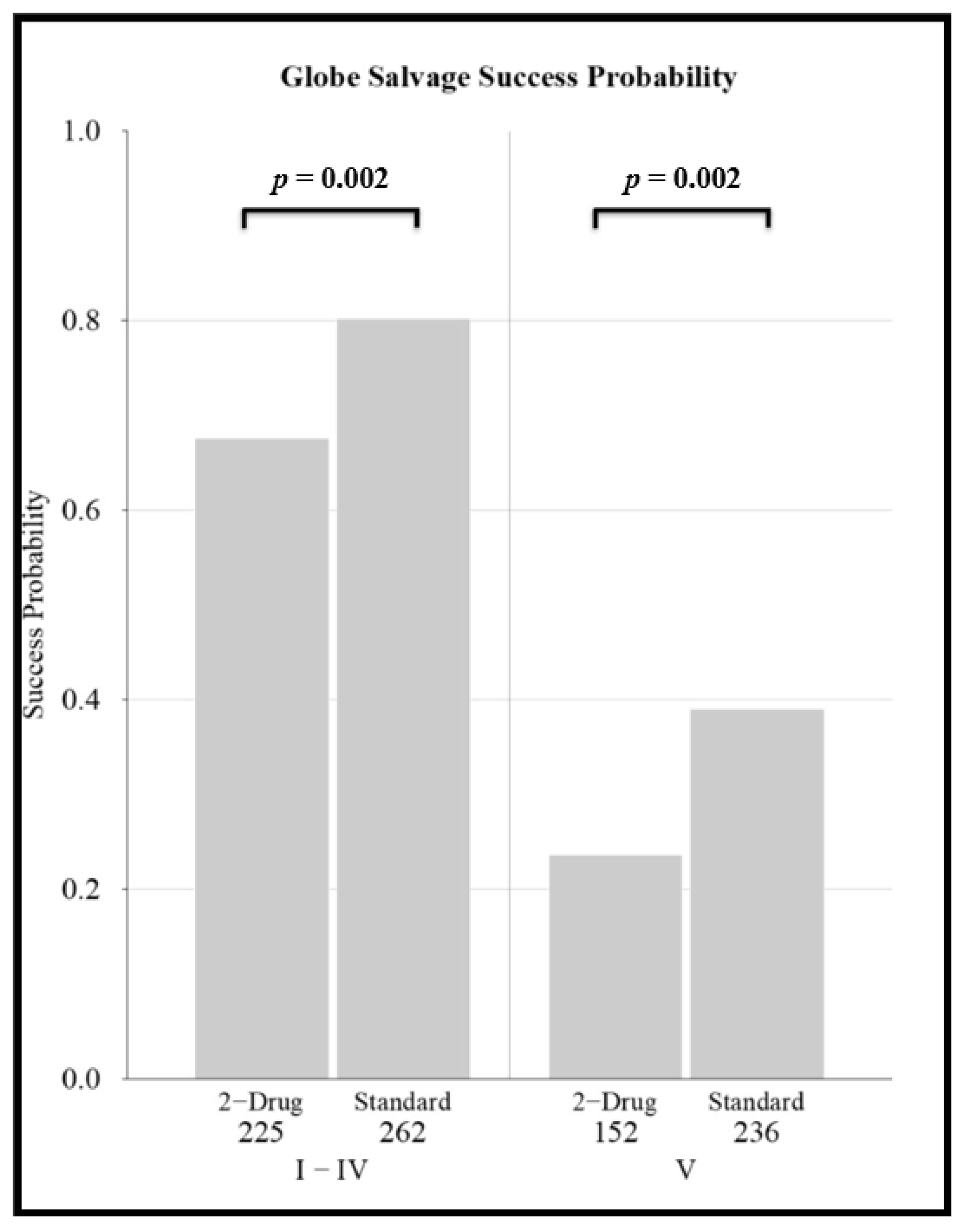
3.3. Globe Salvage Success Rates of Chemotherapy Regimens and the Effect of Disease Classification
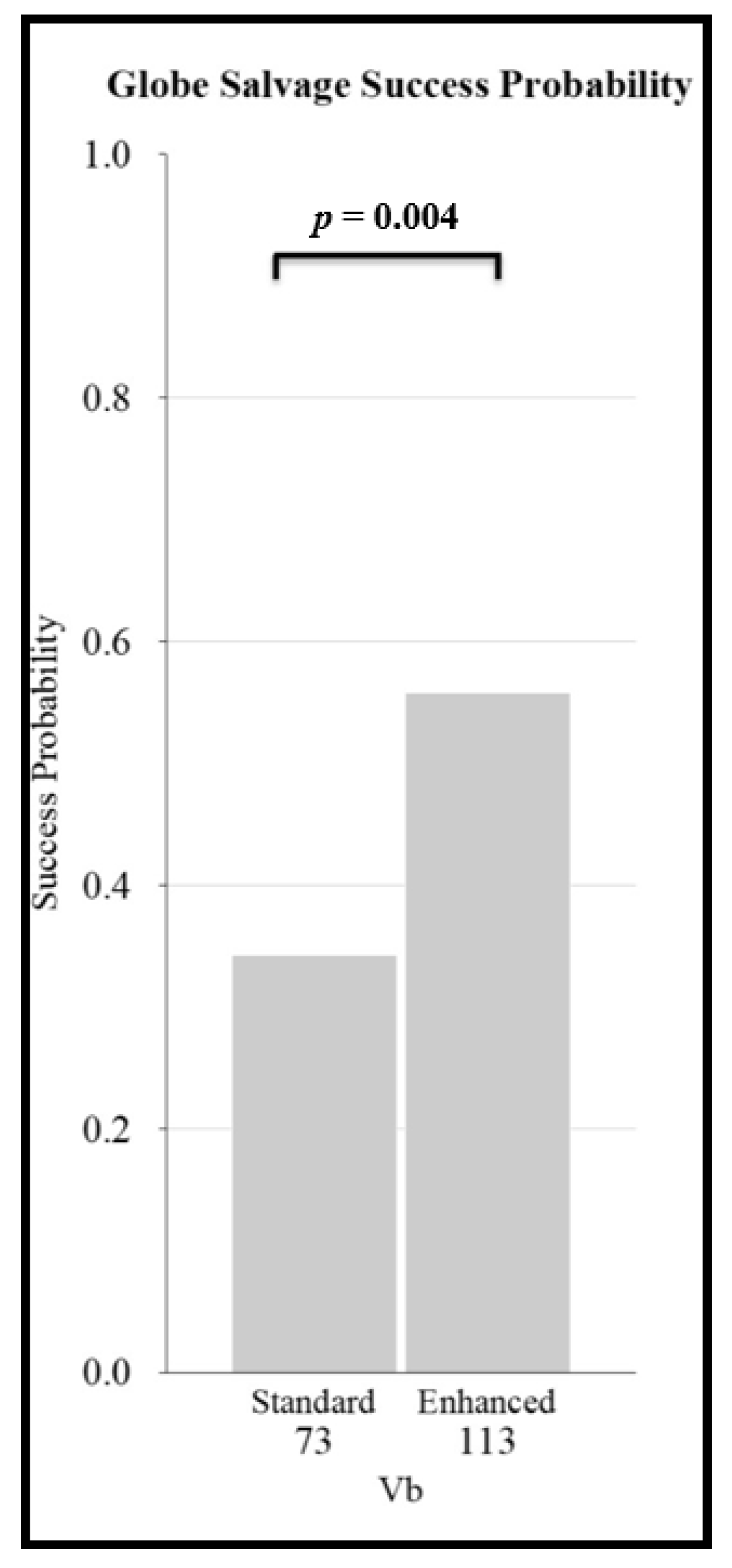
3.4. Individual Patient Level Data: Effect of Patient Age, Eye Classification, and Chemotherapy Regimen on Globe Salvage Success
4. Discussion
4.1. Summary of Evidence
4.2. Limitations and Risks for Bias
5. Conclusions
5.1. General Conclusions
- Establishes a consolidated and evidence-based baseline for globe salvage success rates for each retinoblastoma classification for various intravenous chemoreduction regimens, against which to compare new, emerging, and future therapeutic options.
- Confirms that both the Reese–Ellsworth and International Classification of Retinoblastoma classification schemes are predictive of intravenous chemoreduction success.
- Establishes that older age reduces the chance for successful globe salvage, regardless of disease severity and regardless of chemotherapy regimen.
- Demonstrates that globe salvage success is affected by chemotherapy regimen. For both less severely affected eyes as well as for eyes with advanced disease, globe salvage rates were lower for two-drug regimens. For more severely affected eyes with vitreous seeds, enhanced regimens have higher success rates than standard intravenous chemotherapy, and thus the use of a higher-intensity regimen might be considered in advanced eyes (that are not candidates for IAC).
- The published retinoblastoma literature lacks standardized reporting and is fraught with treatment bias and selection bias. The field would benefit from agreed-upon standard reporting of patient inclusion and outcomes.
5.2. Importance of This Work
Author Contributions
Funding
Conflicts of Interest
References
- Broaddus, E.; Topham, A.; Singh, A.D. Incidence of retinoblastoma in the USA: 1975–2004. Br. J. Ophthalmol. 2009, 93, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Seregard, S.; Lundell, G.; Svedberg, H.; Kivelä, T. Incidence of retinoblastoma from 1958 to 1998 in Northern Europe: Advantages of birth cohort analysis. Ophthalmology 2004, 111, 1228–1232. [Google Scholar] [CrossRef]
- Kingston, J.E.; Hungerford, J.L.; Madreperla, S.A.; Plowman, P.N. Results of combined chemotherapy and radiotherapy for advanced intraocular retinoblastoma. Arch. Ophthalmol. 1996, 114, 1339–1343. [Google Scholar] [CrossRef]
- Gallie, B.L.; Budnig, A.; DeBoer, G.; Thiessen, J.J.; Koren, G.; Verjee, Z.; Ling, V.; Chan, H.S. Chemotherapy with focal therapy can cure intraocular retinoblastoma without radiotherapy. Arch. Ophthalmol. 1996, 114, 1321–1328. [Google Scholar] [CrossRef]
- Murphree, A.L.; Villablanca, J.G.; Deegan, W.F.; Sato, J.K.; Malogolowkin, M.; Fisher, A.; Parker, R.; Reed, E.; Gomer, C.J. Chemotherapy plus local treatment in the management of intraocular retinoblastoma. Arch. Ophthalmol. 1996, 114, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; De Potter, P.; Himelstein, B.P.; Shields, J.A.; Meadows, A.T.; Maris, J.M. Chemoreduction in the initial management of intraocular retinoblastoma. Arch. Ophthalmol. 1996, 114, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Daniels, A.B.; Mukai, S. Modern surgical techniques in the management of retinoblastoma. Int. Ophthalmol. Clin. 2017, 57, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Broaddus, E.; Topham, A.; Singh, A.D. Survival with retinoblastoma in the USA: 1975–2004. Br. J. Ophthalmol. 2009, 93, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Kivela, T. The epidemiological challenge of the most frequent eye cancer: Retinoblastoma, an issue of birth and death. Br. J. Ophthalmol. 2009, 93, 1129–1131. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.A.; Shields, C.L.; Sivalingam, V. Decreasing frequency of enucleation in patients with retinoblastoma. Am. J. Ophthalmol. 1989, 108, 185–188. [Google Scholar] [CrossRef]
- Shields, C.L.; Honavar, S.G.; Meadows, A.T.; Shields, J.A.; Demirci, H.; Naduvilath, T.J. Chemoreduction for unilateral retinoblastoma. Arch. Ophthalmol. 2002, 120, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.A.; Shields, C.L.; Shields, J.A. Trends in the management of retinoblastoma: Evaluation of 1,196 consecutive eyes during 1974 to 2001. J. Pediatr. Ophthalmol. Strabismus 2003, 40, 196–203. [Google Scholar] [CrossRef]
- Berman, E.L.; Donaldson, C.E.; Giblin, M.; Martin, F.J. Outcomes in retinoblastoma, 1974–2005: The Children’s Hospital, Westmead. Clin. Experiment. Ophthalmol. 2007, 35, 5–12. [Google Scholar] [CrossRef]
- Yamane, T.; Kaneko, A.; Mohri, M. The technique of ophthalmic arterial infusion therapy for patients with intraocular retinoblastoma. Int. J. Clin. Oncol. 2004, 9, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Kaliki, S.; Rojanaporn, D.; Al-Dahmash, S.; Bianciotto, C.G.; Shields, J.A. Intravenous and intra-arterial chemotherapy for retinoblastoma: What have we learned? Curr. Opin. Ophthalmol. 2012, 23, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, F.; Shields, C.L. Intravitreal Melphalan for Refractory or Recurrent Vitreous Seeding From Retinoblastoma. Arch. Ophthalmol. 2012, 130, 1268. [Google Scholar] [CrossRef] [PubMed]
- Yousef, Y.A.; Soliman, S.E.; Astudillo, P.P.; Durairaj, P.; Dimaras, H.; Chan, H.S.; Héon, E.; Gallie, B.L.; Shaikh, F. Intra-arterial Chemotherapy for Retinoblastoma. JAMA Ophthalmol. 2016, 134, 584. [Google Scholar] [CrossRef]
- Abramson, D.H.; Marr, B.P.; Francis, J.H.; Dunkle, I.J.; Fabius, A.W.; Brodie, S.E.; Mondesire-Crump, I.; Gobin, Y.P. Simultaneous Bilateral Ophthalmic Artery Chemosurgery for Bilateral Retinoblastoma (Tandem Therapy). PLoS ONE 2016, 11, e0156806. [Google Scholar]
- Abramson, D.H.; Shields, C.L.; Munier, F.L.; Chantada, G.L. Treatment of retinoblastoma in 2015: Agreement and disagreement. JAMA Ophthalmol. 2015, 133, 1341–1347. [Google Scholar] [CrossRef]
- Munier, F.L.; Mosimann, P.; Puccinelli, F.; Gaillard, M.C.; Stathopoulos, C.; Houghton, S.; Bergin, C.; Beck-Popovic, M. First-line intra-arterial versus intravenous chemotherapy in unilateral sporadic group D retinoblastoma: Evidence of better visual outcomes, ocular survival and shorter time to success with intra-arterial delivery from retrospective review of 20 years of treatment. Br. J. Ophthalmol. 2017, 101, 1086–1093. [Google Scholar]
- Munier, F.L.; Gaillard, M.C.; Balmer, A.; Soliman, S.; Podilsky, G.; Moulin, A.P.; Beck-Popovic, M. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: From prohibition to conditional indications. Br. J. Ophthalmol. 2012, 96, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J.; Smith, B.D. Evaluating the risk of extraocular tumour spread following intravitreal injection therapy for retinoblastoma: A systematic review. Br. J. Ophthalmol. 2013, 97, 1231–1236. [Google Scholar] [CrossRef]
- Francis, J.H.; Abramson, D.H.; Gaillard, M.C.; Marr, B.P.; Beck-Popovic, M.; Munier, F.L. The classification of vitreous seeds in retinoblastoma and response to intravitreal melphalan. Ophthalmology 2015, 122, 1173–1179. [Google Scholar] [CrossRef]
- Francis, J.H.; Brodie, S.E.; Marr, B.; Zabor, E.C.; Mondesire-Crump, I.; Abramson, D.H. Efficacy and toxicity of intravitreous chemotherapy for retinoblastoma: Four-Year experience. Ophthalmology 2017, 124, 488–495. [Google Scholar] [CrossRef]
- Santapuram, P.R.; Schremp, E.A.; Friedman, D.L.; Koyama, T.; Froehler, M.T.; Daniels, A.B. Adverse events, treatment burden, and outcomes of intravenous versus intra-arterial chemotherapy for retinoblastoma. Ophthalmol. Retina 2020. Online ahead of print. [Google Scholar] [CrossRef]
- Shields, C.L.; Jorge, R.; Say, E.A.; Magrath, G.; Alset, A.; Caywood, E.; Leahey, A.M.; Jabbour, P.; Shields, J.A. Unilateral retinoblastoma managed with intravenous chemotherapy versus intra-arterial chemotherapy. Outcomes based on the international classification of retinoblastoma. Asia Pac. J. Ophthalmol. 2016, 5, 97–103. [Google Scholar] [CrossRef]
- Abramson, D.H.; Daniels, A.B.; Marr, B.P.; Francis, J.H.; Brodie, S.E.; Dunkel, I.J.; Gobin, Y.P. Intra-arterial chemotherapy (ophthalmic artery chemosurgery) for group D retinoblastoma. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.H.; Levin, A.M.; Zabor, E.C.; Gobin, Y.P.; Abramson, D.H. Ten-year experience with ophthalmic artery chemosurgery: Ocular and recurrence-free survival. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Antoneli, C.B.G.; Ribeiro, K.C.B.; Steinhorst, F.; Novaes, P.E.; Chojniak, M.M.; Malogolowkin, M. Treatment of retinoblastoma patients with chemoreduction plus local therapy: Experience of the AC Camargo Hospital, Brazil. J. Pediatr. Hematol. Oncol. 2006, 28, 342–345. [Google Scholar] [CrossRef]
- Schiavetti, A.; Hadjistilianou, T.; Clerico, A.; Bonci, E.; Ragni, G.; Castello, M.A. Conservative therapy in intraocular retinoblastoma: Response/recurrence rate. J. Pediatr. Hematol. Oncol. 2005, 27, 3–6. [Google Scholar] [CrossRef]
- Künkele, A.; Jurklies, C.; Wieland, R.; Lohmann, D.; Bornfeld, N.; Eggert, A.; Schulte, J.H. Chemoreduction improves eye retention in patients with retinoblastoma: A report from the German Retinoblastoma Reference Centre. Br. J. Ophthalmol. 2013, 97, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yu, Y.S.; Khwarg, S.I.; Choi, H.S.; Shin, H.Y.; Ahn, H.S. Clinical result of prolonged primary chemotherapy in retinoblastoma patients. Korean J. Ophthalmol. 2003, 17, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Brichard, B.; De Bruycker, J.J.; De Potter, P.; Neven, B.; Vermylen, C.; Cornu, G. Combined chemotherapy and local treatment in the management of intraocular retinoblastoma. Med. Pediatr. Oncol. 2002, 38, 411–415. [Google Scholar] [CrossRef]
- Greenwald, M.J.; Strauss, L.C. Treatment of intraocular retinoblastoma with carboplatin and etoposide chemotherapy. Ophthalmology 1996, 103, 1989–1997. [Google Scholar] [CrossRef]
- Rodriguez-Galindo, C.; Wilson, M.W.; Haik, B.G.; Merchant, T.E.; Billups, C.A.; Shah, N.; Cain, A.; Langston, J.; Lipson, M.; Kun, L.E.; et al. Treatment of intraocular retinoblastoma with vincristine and carboplatin. J. Clin. Oncol. 2003, 21, 2019–2025. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.W.; Haik, B.G.; Liu, T.; Merchant, T.E.; Rodriguez-Galindo, C. Effect on ocular survival of adding early intensive focal treatments to a two-drug chemotherapy regimen in patients with retinoblastoma. Am. J. Ophthalmol. 2005, 140, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, I.J.; Lee, T.C.; Shi, W.; Beaverson, K.L.; Novetsky, D.; Lyden, D.; Finlay, J.L.; McCormick, B.; Abramson, D.H. A phase II trial of carboplatin for intraocular retinoblastoma. Pediatr. Blood Cancer 2007, 49, 643–648. [Google Scholar] [CrossRef]
- Lumbroso-Le Rouic, L.; Aerts, I.; Hajage, D.; Lévy-Gabriel, C.; Savignoni, A.; Algret, N.; Cassoux, N.; Bertozzi, A.I.; Esteve, M.; Doz, F.; et al. Conservative treatment of retinoblastoma: A prospective phase II randomized trial of neoadjuvant chemotherapy followed by local treatments and chemothermotherapy. Eye 2016, 30, 46–52. [Google Scholar] [CrossRef]
- Gündüz, K.; Shields, C.L.; Shields, J.A.; Meadows, T.A.; Gross, N.; Cater, J.; Needle, M. The outcome of chemoreduction treatment in patients with Reese-Ellsworth group V retinoblastoma. Arch. Ophthalmol. 1998, 116, 1613–1617. [Google Scholar] [CrossRef][Green Version]
- Levy, C.; Doz, F.; Quintana, E.; Pacquement, H.; Michon, J.; Schlienger, P.; Validire, P.; Asselain, B.; Desjardins, L.; Zucker, J.M. Role of chemotherapy alone or in combination with hyperthermia in the primary treatment of intraocular retinoblastoma: Preliminary results. Br. J. Ophthalmol. 1998, 82, 1154–1158. [Google Scholar] [CrossRef]
- Beck, M.N.; Balmer, A.; Dessing, C.; Pica, A.; Munier, F. First-Line chemotherapy with local treatment can prevent external-beam irradiation and enucleation in low-stage intraocular retinoblastoma. J. Clin. Oncol. 2000, 18, 2881–2887. [Google Scholar] [CrossRef]
- Wilson, M.W.; Rodriguez-Galindo, C.; Haik, B.G.; Moshfeghi, D.M.; Merchant, T.E.; Pratt, C.B. Multiagent chemotherapy as neoadjuvant treatment for multifocal intraocular retinoblastoma. Ophthalmology 2001, 108, 2106–2115. [Google Scholar] [CrossRef]
- Manjandavida, F.P.; Honavar, S.G.; Reddy, V.A.P.; Khanna, R. Management and outcome of retinoblastoma with vitreous seeds. Ophthalmology 2014, 121, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.H.; Sohn, W.Y.; Sung, K.W.; Jung, H.L.; Koo, H.H.; Oh, S.Y.; Kang, S.W. Chemoreduction followed by local therapy and adjuvant chemotherapy for advanced intraocular retinoblastoma: A pilot study in a single center. J. Korean Med. Sci. 2002, 17, 817–822. [Google Scholar] [CrossRef]
- Menon, B.S.; Juraida, E.; Alagaratnam, J.; Mohammad, M.; Ibrahim, H.; George, T.M.; Ariffin, H.; Ho, C.; Khuzaiah, R.; Peng, L.H. Chemoreduction for intraocular retinoblastoma in Malaysia. J. Pediatr. Hematol. Oncol. 2007, 29, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Bartuma, K.; Pal, N.; Kosek, S.; Holm, S.; All-Ericsson, C. A 10-year experience of outcome in chemotherapy-treated hereditary retinoblastoma. Acta Ophthalmol. 2014, 92, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.E.; Sa, H.S.; Koo, H.H.; Yoo, K.H.; Sung, K.W.; Ham, D.I. Clinical manifestations and treatment of retinoblastoma in Korea. Br. J. Ophthalmol. 2008, 92, 1180–1184. [Google Scholar] [CrossRef]
- Shin, J.Y.; Kim, J.H.; Yu, Y.S.; Khwarg, S.I.; Choung, H.K.; Shin, H.Y.; Ahn, H.S. Eye-preserving therapy in retinoblastoma: Prolonged primary chemotherapy alone or combined with local therapy. Korean J. Ophthalmol. 2010, 24, 219–224. [Google Scholar] [CrossRef]
- Zage, P.E.; Reitman, A.J.; Seshadri, R.; Weinstein, J.L.; Mets, M.B.; Zeid, J.L.; Greenwald, M.J.; Strauss, L.C.; Goldman, S. Outcomes of a two-drug chemotherapy regimen for intraocular retinoblastoma. Pediatr. Blood Cancer 2008, 50, 567–572. [Google Scholar] [CrossRef]
- Cohen, V.M.L.; Kingston, J.; Hungerford, J.L. The success of primary chemotherapy for group D heritable retinoblastoma. Br. J. Ophthalmol. 2009, 93, 887–890. [Google Scholar] [CrossRef]
- Berry, J.L.; Jubran, R.; Kim, J.W.; Wong, K.; Bababeygy, S.R.; Almarzouki, H.; Lee, T.C.; Murphree, A.L. Long-term outcomes of Group D eyes in bilateral retinoblastoma patients treated with chemoreduction and low-dose IMRT salvage. Pediatr. Blood Cancer 2013, 60, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Mashayekhi, A.; Au, A.K.; Czyz, C.; Leahey, A.; Meadows, A.T.; Shields, J.A. The international classification of retinoblastoma predicts chemoreduction success. Ophthalmology 2006, 113, 2276–2280. [Google Scholar] [CrossRef] [PubMed]
- Schefler, A.C.; Cicciarelli, N.; Feuer, W.; Toledano, S.; Murray, T.G. Macular retinoblastoma: Evaluation of tumor control, local complications, and visual outcomes for eyes treated with chemotherapy and repetitive foveal laser ablation. Ophthalmology 2007, 114, 162–169. [Google Scholar] [CrossRef]
- Friedman, D.L.; Krailo, M.; Villaluna, D.; Gombos, D.; Langholz, B.; Jubran, R.; Shields, C.; Murphree, L.; O’Brien, J.; Kessel, S.; et al. Systemic neoadjuvant chemotherapy for Group B intraocular retinoblastoma (ARET0331): A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2017, 64. [Google Scholar] [CrossRef]
- Scelfo, C.; Francis, J.H.; Khetan, V.; Jenkins, T.; Marr, B.; Abramson, D.H.; Shields, C.L.; Pe’er, J.; Munier, F.; Berry, J.; et al. An international survey of classification and treatment choices for group D retinoblastoma. Int. J. Ophthalmol. 2017, 10, 961–967. [Google Scholar]
- Novetsky, D.E.; Abramson, D.H.; Kim, J.W.; Dunkel, I.J. Published international classification of retinoblastoma (ICRB) definitions contain inconsistencies—An analysis of impact. Ophthalmic Genet. 2009, 30, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Stacey, A.W.; Clarke, B.; Moraitis, C.; Fabian, I.D.; Smith, V.; Sagoo, M.S.; Reddy, M.A. The Incidence of Binocular Visual Impairment and Blindness in Children with Bilateral Retinoblastoma. Ocul. Oncol. Pathol. 2019, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]


| Author(s) | Year | Regimen | Total Eyes | Patient Level Data | Reese-Ellsworth Classification (R-E) Number of Eyes | International Classification of RB (ICRB)/International Intraocular RB Classification (IIRC) Number of Eyes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | A | B | C | D | E | |||||
| Brichard et al | 2002 [33] | Standard /Reduced | 24 | Yes | 4 | 3 | 5 | 0 | 12 | - | - | - | - | - |
| Hee Yoo et al | 2002 [44] | Standard /Enhanced | 10 | Yes | 0 | 0 | 0 | 2 | 8 | - | - | - | - | - |
| Kim et al | 2003 [32] | Standard /Enhanced | 27 | Yes | 2 | 7 | 5 | 7 | 6 | - | - | - | - | - |
| Menon et al | 2007 [45] | Standard /Enhanced | 25 | Yes | 1 | 5 | 11 | 7 | 1 | - | - | - | - | - |
| Bartuma et al | 2014 [46] | Standard | 46 | Yes | - | - | - | - | - | 8 | 25 | 1 | 11 | 1 |
| Greenwald et al | 1996 [34] | Reduced | 11 | Yes | 0 | 1 | 3 | 1 | 6 | - | - | - | - | - |
| Levy et al | 1998 [40] | Reduced | 38 | Yes | 2 | 1 | 12 | 3 | 20 | - | - | - | - | - |
| Beck et al | 2000 [41] | Reduced | 33 | Yes | 5 | 10 | 3 | 1 | 14 | - | - | - | - | - |
| Wilson et al | 2001 [42] | Reduced | 34 | Yes | 5 | 11 | 2 | 2 | 14 | - | - | - | - | - |
| Rodriguez-Galindo et al | 2003 [35] | Reduced | 43 | Yes | 7 | 12 | 5 | 3 | 16 | - | - | - | - | - |
| Wilson et al | 2005 [36] | Reduced | 27 | Yes | 4 | 1 | 2 | 0 | 20 | - | - | - | - | - |
| Dunkel et al | 2007 [37] | Reduced | 43 | Yes | 13 | 5 | 13 | 2 | 10 | - | - | - | - | - |
| Gallie et al | 1996 [4] | Enhanced | 38 | Yes a | 9 | 7 | 4 | 2 | 16 | - | - | - | - | - |
| Manjandavida et al | 2014 [43] | Enhanced | 101 | Yes a | 0 | 0 | 0 | 0 | 101 | 0 | 0 | 21 | 40 | 40 |
| Murphree et al | 1996 [5] | Standard /Reduced | 73 | No | 18 | 16 | 4 | 4 | 31 | - | - | - | - | - |
| Gündüz et al | 1998 [39] | Standard | 27 | No | 0 | 0 | 0 | 0 | 27 | - | - | - | - | - |
| Antoneli et al | 2006 [29] | Standard | 145 | No | 22 | 16 | 13 | 11 | 83 | - | - | - | - | - |
| Shields et al | 2006 [52] | Standard | 249 | No | 27 | 53 | 78 | 37 | 54 | 23 | 96 | 21 | 109 | 0 |
| Schefler et al | 2007 [53] | Standard | 44 | No | 1 | 6 | 3 | 5 | 29 | - | - | - | - | - |
| Cohen et al | 2009 [50] | Standard | 18 | No | - | - | - | - | - | 0 | 0 | 0 | 18 | 0 |
| Berry et al | 2013 [51] | Standard | 55 | No | - | - | - | - | - | 0 | 0 | 0 | 55 | 0 |
| Schiavetti et al | 2005 [30] | Reduced | 58 | No | 10 | 16 | 9 | 6 | 17 | - | - | - | - | - |
| Zage et al | 2008 [49] | Reduced | 48 | No | 6 | 7 | 9 | 1 | 25 | 7 | 15 | 8 | 18 | 0 |
| Lumbroso-Le Rouic et al | 2016 [38] | Reduced | 65 | No | 11 | 15 | 25 | 5 | 9 | 3 | 32 | 11 | 19 | 0 |
| Chung et al | 2008 [47] | Enhanced | 80 | No | 4 | 7 | 8 | 8 | 53 | 6 | 19 | 6 | 30 | 0 |
| Young Shin et al | 2010 [48] | Enhanced | 65 | No | 7 | 15 | 18 | 15 | 10 | 8 | 14 | 0 | 42 | 1 |
| Künkele et al | 2013 [31] | Enhanced | 56 | No | - | - | - | - | - | 4 | 40 | 6 | 6 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniels, A.B.; Patel, S.N.; Milam, R.W.; Kohanim, S.; Friedman, D.L.; Koyama, T. Effect of Intravenous Chemotherapy Regimen on Globe Salvage Success Rates for Retinoblastoma Based on Disease Class—A Meta-Analysis. Cancers 2021, 13, 2216. https://doi.org/10.3390/cancers13092216
Daniels AB, Patel SN, Milam RW, Kohanim S, Friedman DL, Koyama T. Effect of Intravenous Chemotherapy Regimen on Globe Salvage Success Rates for Retinoblastoma Based on Disease Class—A Meta-Analysis. Cancers. 2021; 13(9):2216. https://doi.org/10.3390/cancers13092216
Chicago/Turabian StyleDaniels, Anthony B., Shriji N. Patel, Ronald W. Milam, Sahar Kohanim, Debra L. Friedman, and Tatsuki Koyama. 2021. "Effect of Intravenous Chemotherapy Regimen on Globe Salvage Success Rates for Retinoblastoma Based on Disease Class—A Meta-Analysis" Cancers 13, no. 9: 2216. https://doi.org/10.3390/cancers13092216
APA StyleDaniels, A. B., Patel, S. N., Milam, R. W., Kohanim, S., Friedman, D. L., & Koyama, T. (2021). Effect of Intravenous Chemotherapy Regimen on Globe Salvage Success Rates for Retinoblastoma Based on Disease Class—A Meta-Analysis. Cancers, 13(9), 2216. https://doi.org/10.3390/cancers13092216





