Prognosis of Acute Ischaemic Stroke Patients with Cancer: A National Inpatient Sample Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Inclusion Criteria
2.2. Statistical Analysis
2.2.1. Outcomes
2.2.2. Exposures and Confounders
2.2.3. Descriptive Statistics
2.2.4. Association between Prevalent Cancer and Odds of Receiving Revascularisation Therapy
2.2.5. Association between Non-Metastatic and Metastatic Cancer and In-Hospital Outcomes
2.2.6. Association between Non-Metastatic and Metastatic Cancer and In-Hospital Outcomes amongst Patients Undergoing Endovascular Thrombectomy
2.2.7. Association between the Five Most Common Primary Cancer Types and In-Hospital Outcomes
3. Results
3.1. Descriptive Statistics
3.2. Association between Prevalent Cancer and Odds of Receiving Revascularisation Therapy
3.3. Association between Non-Metastatic and Metastatic Cancer and In-Hospital Outcomes
3.4. Association between Non-Metastatic and Metastatic Cancer and In-Hospital Outcomes amongst Patients Undergoing Endovascular Thrombectomy
3.5. Association between the Five Most Common Primary Cancer Types and In-Hospital Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Navi, B.B.; Iadecola, C. Ischemic stroke in cancer patients: A review of an underappreciated pathology. Ann. Neurol. 2018, 83, 873–883. [Google Scholar] [CrossRef]
- Navi, B.B.; Reiner, A.S.; Kamel, H.; Iadecola, C.; Elkind, M.S.V.; Panageas, K.S.; DeAngelis, L.M. Association between incident cancer and subsequent stroke. Ann. Neurol. 2015, 77, 291–300. [Google Scholar] [CrossRef]
- Wilbers, J.; Sondag, L.; Mulder, S.; Siegerink, B.; van Dijk, E.J. Cancer prevalence higher in stroke patients than in the general population: The Dutch String-of-Pearls Institute (PSI) Stroke study. Eur. J. Neurol. 2020, 27, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Kneihsl, M.; Enzinger, C.; Wunsch, G.; Khalil, M.; Culea, V.; Urbanic-Purkart, T.; Payer, F.; Niederkorn, K.; Fazekas, F.; Gattringer, T. Poor short-term outcome in patients with ischaemic stroke and active cancer. J. Neurol. 2016, 263, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Corraini, P.; Szepligeti, S.K.; Henderson, V.W.; Ording, A.G.; Horvath-Puho, E.; Sorensen, H.T. Comorbidity and the increased mortality after hospitalization for stroke: A population-based cohort study. J. Thromb. Haemost. 2018, 16, 242–252. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Murray, V.; Berge, E.; del Zoppo, G.J. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst. Rev. 2014, CD000213. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.J.; Mitchell, P.J.; Demchuk, A.M.; Davalos, A.; Majoie, C.B.L.M.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta- analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- Hacke, W.; Kaste, M.; Bluhmki, E.; Brozman, M.; Davalos, A.; Guidetti, D.; Larrue, V.; Lees, K.R.; Medeghri, Z.; Machnig, T.; et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 2008, 359, 1317–1329. [Google Scholar] [CrossRef]
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995, 333, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Sandercock, P.; Wardlaw, J.M.; Lindley, R.I.; Dennis, M.; Cohen, G.; Murray, G.; Innes, K.; Venables, G.; Czlonkowska, A.; Kobayashi, A.; et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): A randomised controlled trial. Lancet 2012, 379, 2352–2363. [Google Scholar] [PubMed]
- Bluhmki, E.; Chamorro, A.; Davalos, A.; Machnig, T.; Sauce, C.; Wahlgren, N.; Wardlaw, J.; Hacke, W. Stroke treatment with alteplase given 3.0–4.5 h after onset of acute ischaemic stroke (ECASS III): Additional outcomes and subgroup analysis of a randomised controlled trial. Lancet. Neurol. 2009, 8, 1095–1102. [Google Scholar] [CrossRef]
- Inohara, T.; Liang, L.; Kosinski, A.S.; Smith, E.E.; Schwamm, L.H.; Hernandez, A.F.; Bhatt, D.L.; Fonarow, G.C.; Peterson, E.D.; Xian, Y. Thrombolytic therapy in older acute ischemic stroke patients with gastrointestinal malignancy or recent bleeding. Eur. Stroke J. 2020, 5, 47–55. [Google Scholar] [CrossRef]
- Weeda, E.R.; Bohm, N. Association between comorbid cancer and outcomes among admissions for acute ischemic stroke receiving systemic thrombolysis. Int. J. Stroke 2019, 14, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.B.; Karanth, S.; Shah, S.; Shastri, A.; Rao, C.P.V.; Bershad, E.M.; Suarez, J.I. Thrombolysis for acute ischemic stroke in patients with cancer: A population study. Stroke 2013, 44, 3573–3576. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Jung, C.; Hyoung Kim, J.; Se Choi, B.; Jung Bae, Y.; Sunwoo, L.; Geol Woo, H.; Young Chang, J.; Joon Kim, B.; Han, M.-K.; et al. Procedural and clinical outcomes of endovascular recanalization therapy in patients with cancer-related stroke. Interv. Neuroradiol. 2018, 24, 520–528. [Google Scholar] [CrossRef]
- Cho, B.-H.; Yoon, W.; Kim, J.-T.; Choi, K.-H.; Kang, K.-W.; Lee, J.-H.; Cho, K.-H.; Park, M.-S. Outcomes of endovascular treatment in acute ischemic stroke patients with current malignancy. Neurol. Sci. 2020, 41, 379–385. [Google Scholar] [CrossRef]
- Lee, D.; Lee, D.H.; Suh, D.C.; Kwon, H.S.; Jeong, D.-E.; Kim, J.-G.; Lee, J.-S.; Kim, J.S.; Kang, D.-W.; Jeon, S.-B.; et al. Intra-arterial thrombectomy for acute ischaemic stroke patients with active cancer. J. Neurol. 2019, 266, 2286–2293. [Google Scholar] [CrossRef]
- Mohamed, M.O.; Kirchhof, P.; Vidovich, M.; Savage, M.; Rashid, M.; Kwok, C.S.; Thomas, M.; El Omar, O.; Al Ayoubi, F.; Fischman, D.L.; et al. Effect of Concomitant Atrial Fibrillation on In-Hospital Outcomes of Non- ST-Elevation-Acute Coronary Syndrome-Related Hospitalizations in the United States. Am. J. Cardiol. 2019, 124, 465–475. [Google Scholar] [CrossRef]
- (HCUP) Healthcare Cost and Utilization Project. NIS Database Documentation [Internet]; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2018. Available online: https://www.hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp (accessed on 30 January 2021).
- (HCUP) Healthcare Cost and Utilization Project. Checklist for Working with the NIS [Internet]; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2017. Available online: www.hcup-us.ahrq.gov/db/nation/nis/nischecklist.jsp (accessed on 30 January 2021).
- (HCUP) Healthcare Cost and Utilization Project. HCUP NIS Description of Data Elements [Internet]; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2008. Available online: www.hcup-us.ahrq.gov/db/vars/nis_stratum/nisnote.jsp (accessed on 30 January 2021).
- Houchens, R.; Ross, D.; Elixhauser, A. Final Report on Calculating National Inpatient Sample (NIS) Variances for Data Years 2012 and Later; Healthcare Cost and Utilization Project (HCUP): Rockville, MD, USA, 2015.
- (HCUP) Healthcare Cost and Utilization Project. HCUP NIS Description of Data Elements [Internet]; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2008. Available online: https://www.hcup-us.ahrq.gov/db/vars/died/nisnote.jsp (accessed on 30 January 2021).
- (HCUP) Healtcare Cost and Utilization Project. HCUP NIS Description of Data Elements [Internet]; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2008. Available online: https://www.hcup-us.ahrq.gov/db/vars/los/nisnote.jsp (accessed on 30 January 2021).
- Myint, P.K.; Sheng, S.; Xian, Y.; Matsouaka, R.A.; Reeves, M.J.; Saver, J.L.; Bhatt, D.L.; Fonarow, G.C.; Schwamm, L.H.; Smith, E.E. Shock Index Predicts Patient-Related Clinical Outcomes in Stroke. J. Am. Heart Assoc. 2018, 7, e007581. [Google Scholar] [CrossRef]
- (HCUP) Healthcare Cost and Utilization Project. HCUP NIS Description of Data Elements [Internet]; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2008. Available online: www.hcup-us.ahrq.gov/db/vars/dispuniform/nisnote.jsp (accessed on 30 January 2021).
- Healthcare Cost and Utilization Project (HCUP). Clinical Classifications Software Refined (CCSR); Agency for Healthcare Research and Quality: Rockville, MD, USA, 2020.
- (HCUP) Healthcare Cost and Utilization Project. Tools Archive for Elixhauser Comorbidity Software Refined for ICD-10-CM [Internet]; The Agency for Healthcare Research and Quality: Rockville, MD, USA, 2020. Available online: https://www.hcup-us.ahrq.gov/toolssoftware/comorbidityicd10/comorbidity_icd10_archive.jsp (accessed on 30 January 2021).
- Bharadwaj, A.; Potts, J.; Mohamed, M.O.; Parwani, P.; Swamy, P.; Lopez-Mattei, J.C.; Rashid, M.; Kwok, C.S.; Fischman, D.L.; Vassiliou, V.S.; et al. Acute myocardial infarction treatments and outcomes in 6.5 million patients with a current or historical diagnosis of cancer in the USA. Eur. Heart J. 2020, 41, 2183–2193. [Google Scholar] [CrossRef]
- Cutting, S.; Wettengel, M.; Conners, J.J.; Ouyang, B.; Busl, K. Three-Month Outcomes Are Poor in Stroke Patients with Cancer Despite Acute Stroke Treatment. J. Stroke Cerebrovasc. Dis. 2017, 26, 809–815. [Google Scholar] [CrossRef]
- Yoo, J.; Nam, H.S.; Kim, Y.D.; Lee, H.S.; Heo, J.H. Short-Term Outcome of Ischemic Stroke Patients With Systemic Malignancy. Stroke 2019, 50, 507–511. [Google Scholar] [CrossRef]
- Navi, B.B.; Singer, S.; Merkler, A.E.; Cheng, N.T.; Stone, J.B.; Kamel, H.; Iadecola, C.; Elkind, M.S.V.; DeAngelis, L.M. Cryptogenic subtype predicts reduced survival among cancer patients with ischemic stroke. Stroke 2014, 45, 2292–2297. [Google Scholar] [CrossRef] [PubMed]
- Navi, B.B.; Singer, S.; Merkler, A.E.; Cheng, N.T.; Stone, J.B.; Kamel, H.; Iadecola, C.; Elkind, M.S.V.; DeAngelis, L.M. Recurrent thromboembolic events after ischemic stroke in patients with cancer. Neurology 2014, 83, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.B.; Biffi, A.; Falcone, G.J.; Sansing, L.H.; Torres Lopez, V.; Navi, B.B.; Roh, D.J.; Mandava, P.; Hanley, D.F.; Ziai, W.C.; et al. Antiplatelet Therapy After Spontaneous Intracerebral Hemorrhage and Functional Outcomes. Stroke 2019, 50, 3057–3063. [Google Scholar] [CrossRef] [PubMed]
- Sobolewski, P.; Brola, W.; Szczuchniak, W.; Fudala, M.; Sobota, A. Safety of intravenous thrombolysis for acute ischaemic stroke including concomitant neoplastic disease sufferers—Experience from Poland. Int. J. Clin. Pract. 2015, 69, 666–673. [Google Scholar] [CrossRef]
- Masrur, S.; Abdullah, A.R.; Smith, E.E.; Hidalgo, R.; El-Ghandour, A.; Rordorf, G.; Schwamm, L.H. Risk of thrombolytic therapy for acute ischemic stroke in patients with current malignancy. J. Stroke Cerebrovasc. Dis. 2011, 20, 124–130. [Google Scholar] [CrossRef]
- Huang, S.; Lu, X.; Tang, L.V.; Hu, Y. Efficacy and safety of intravenous thrombolysis for acute ischemic stroke in cancer patients: A systemic review and meta-analysis. Am. J. Transl. Res. 2020, 12, 4795–4806. [Google Scholar]
- Arboix, A.; Alió, J. Cardioembolic stroke: Clinical features, specific cardiac disorders and prognosis. Curr. Cardiol. Rev. 2010, 6, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Pana, T.A.; McLernon, D.J.; Mamas, M.A.; Bettencourt-Silva, J.H.; Metcalf, A.K.; Potter, J.F.; Myint, P.K. Individual and Combined Impact of Heart Failure and Atrial Fibrillation on Ischemic Stroke Outcomes. Stroke 2019, 50, 183. [Google Scholar] [CrossRef] [PubMed]
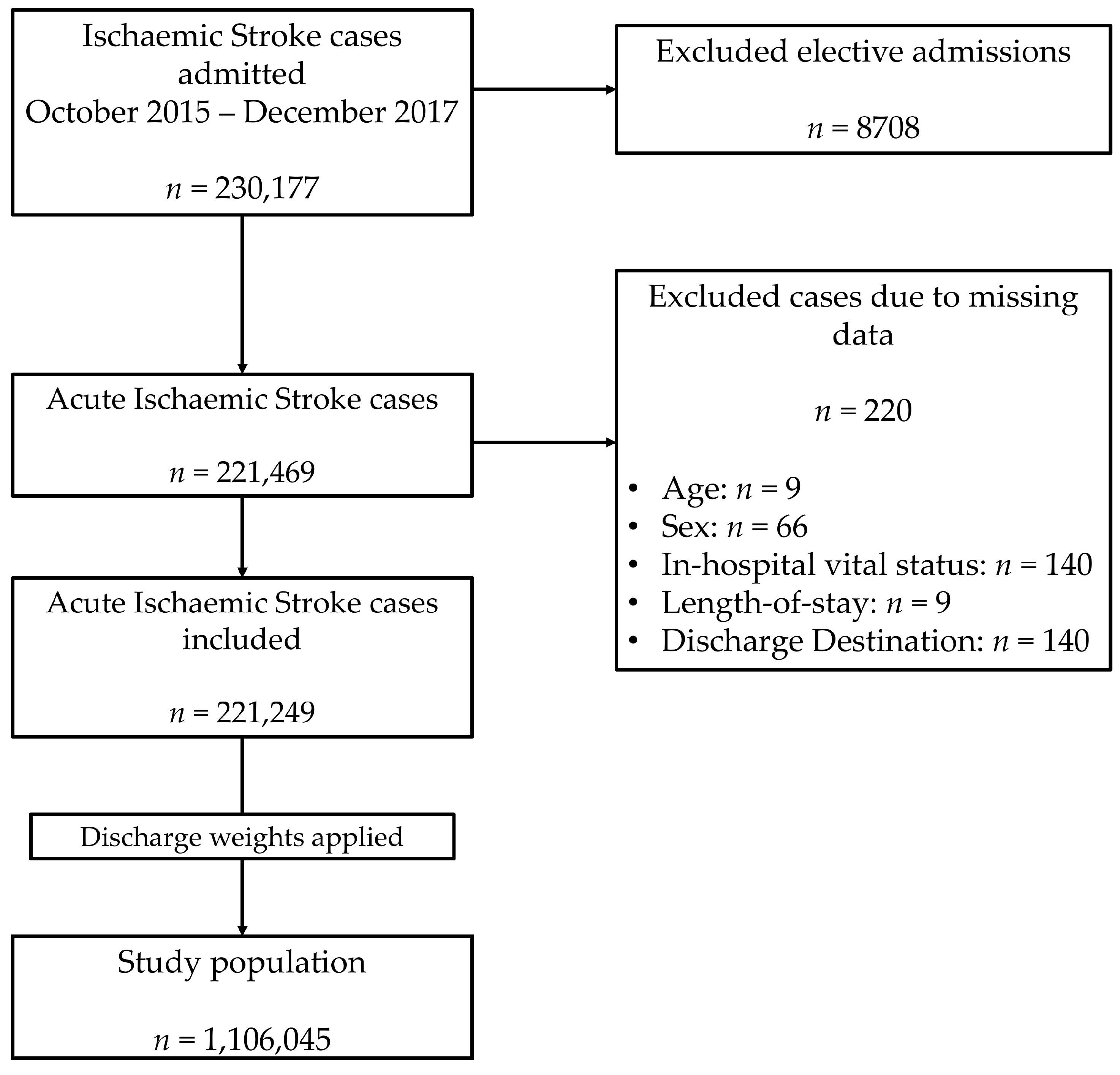
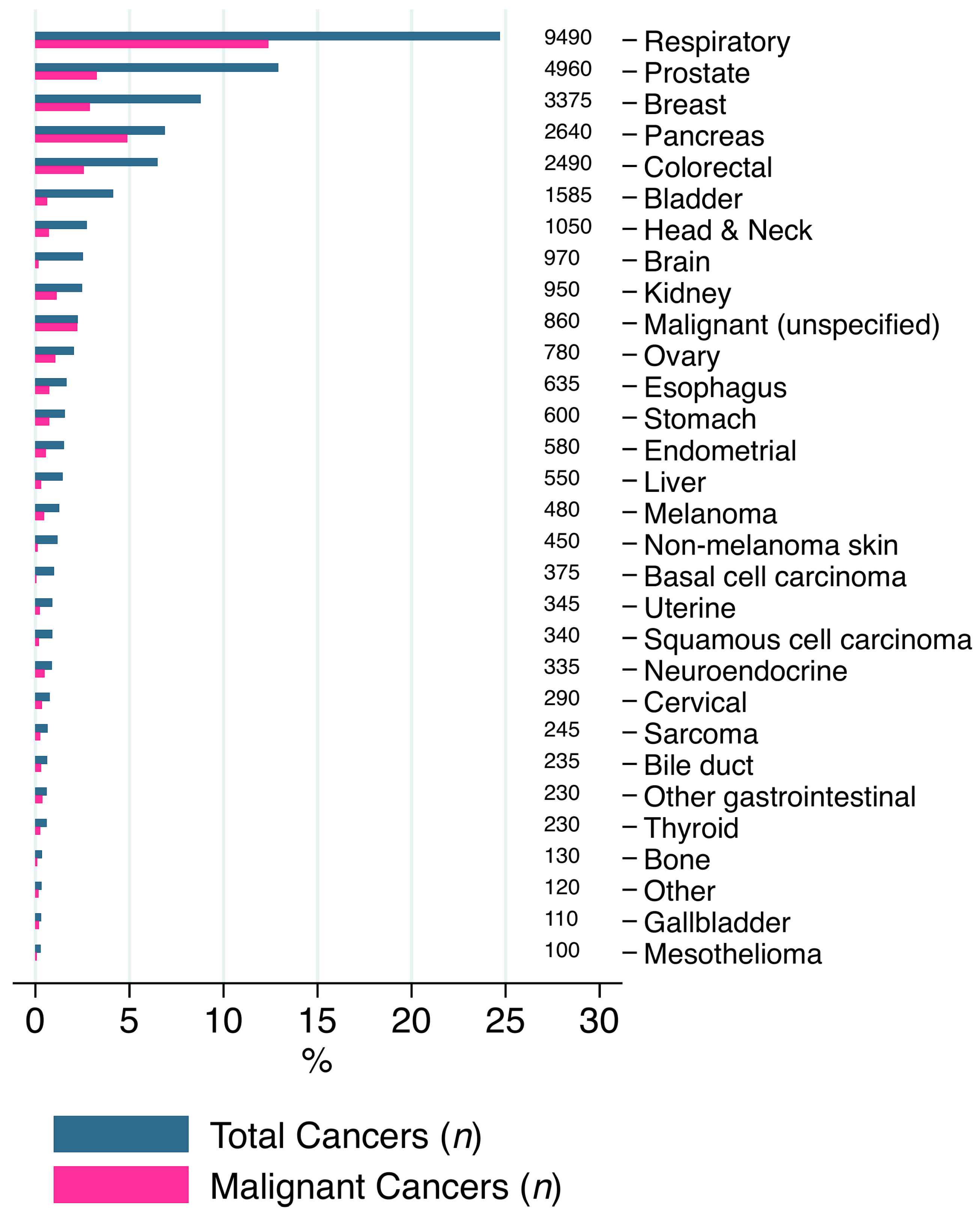
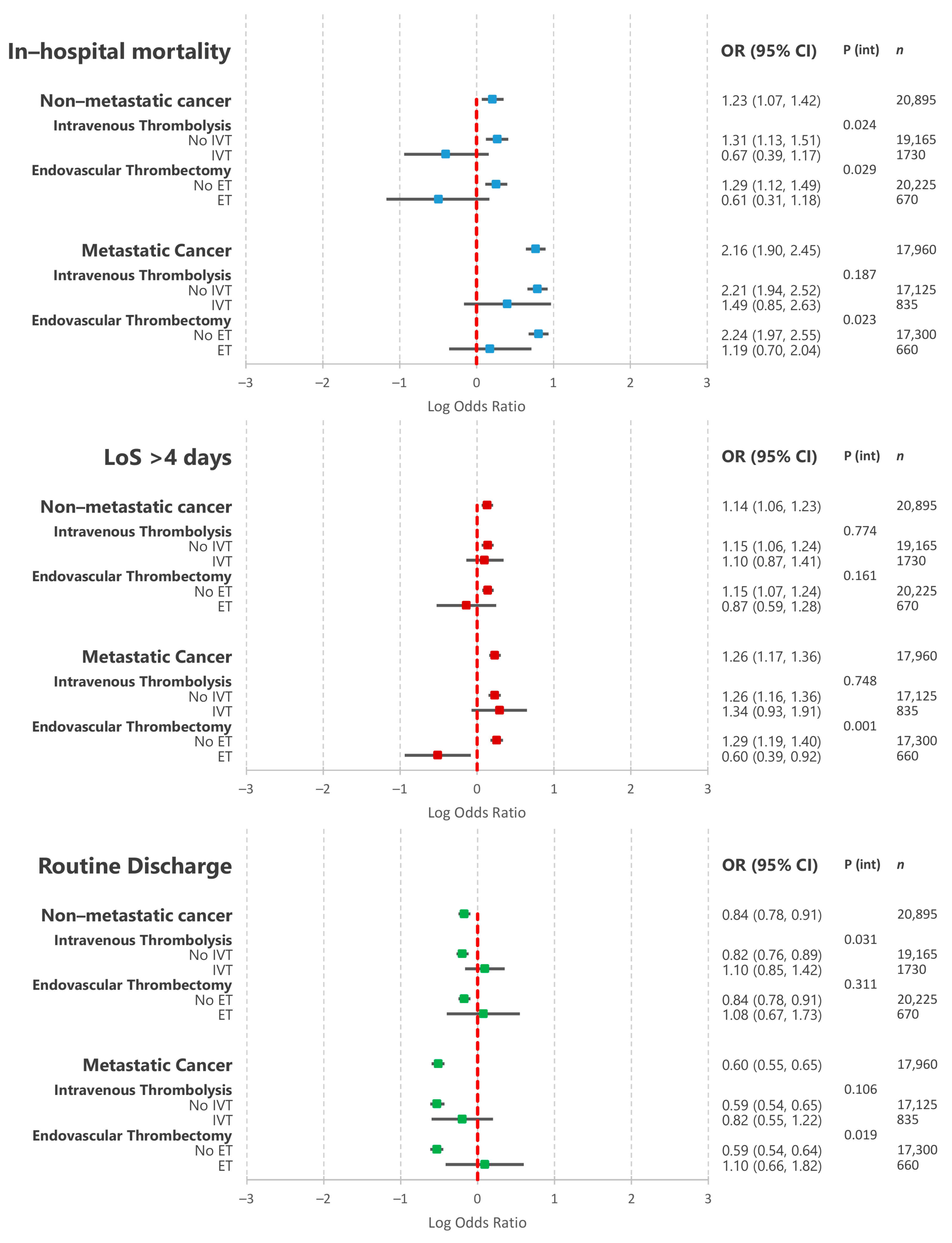

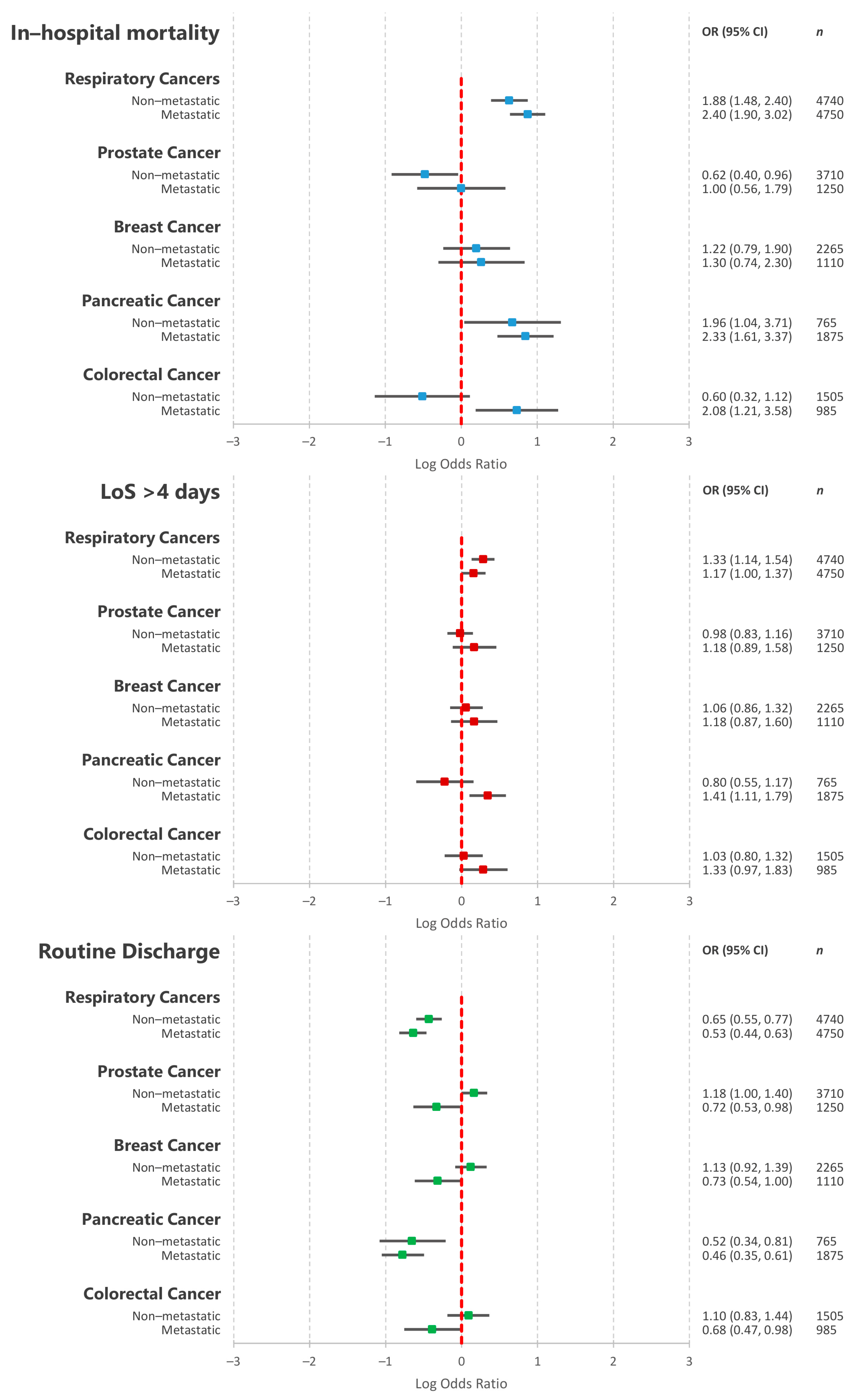
| Variation | All | No Active Cancer | Non-Metastatic Cancer | Metastatic Cancer | p Value |
|---|---|---|---|---|---|
| n | 1,106,045 | 1,067,190 | 20,895 | 17,960 | |
| Age (years), median (IQR) | 72 (61–82) | 71 (60–82) | 75 (67–83) | 70 (62–78) | <0.001 |
| Length-of-stay (days), median (IQR) | 3 (2–6) | 3 (2–6) | 4 (2–7) | 4 (2–7) | <0.001 |
| Sex Female, n (%) | 557,595 (50.41) | 538,635 (50.47) | 9795 (46.88) | 9165 (51.03) | <0.001 |
| Ethnicity | <0.001 | ||||
| White | 735,330 (66.48) | 707,550 (66.30) | 15,005 (71.81) | 12,775 (71.13) | |
| Black | 183,090 (16.55) | 177,790 (16.66) | 2880 (13.78) | 2420 (13.47) | |
| Hispanic | 84,950 (7.68) | 82,700 (7.75) | 1240 (5.93) | 1010 (5.62) | |
| Asian or Pacific Islander | 31,635 (2.86) | 30,500 (2.86) | 530 (2.54) | 605 (3.37) | |
| Native American | 4700 (0.42) | 4570 (0.43) | 60 (0.29) | 70 (0.39) | |
| Other | 27,460 (2.48) | 26,535 (2.49) | 410 (1.96) | 515 (2.87) | |
| ELIXHAUSER CO-MORBIDITIES, n (%) | |||||
| Congestive Heart Failure | 172,170 (15.57) | 166,950 (15.64) | 3225 (15.43) | 1995 (11.11) | <0.001 |
| Valvular Disease | 110,540 (9.99) | 106,640 (9.99) | 2300 (11.01) | 1600 (8.91) | 0.008 |
| Pulmonary Circulation Disease | 8460 (0.76) | 6710 (0.63) | 555 (2.66) | 1195 (6.65) | <0.001 |
| Peripheral Vascular Disease | 112,065 (10.13) | 107,955 (10.12) | 2470 (11.82) | 1640 (9.13) | <0.001 |
| Paralysis | 112,895 (10.21) | 108,920 (10.21) | 2260 (10.82) | 1715 (9.55) | 0.178 |
| Other Neurological Disorders | 6620 (0.60) | 6275 (0.59) | 210 (1.01) | 135 (0.75) | 0.001 |
| Chronic Pulmonary Disease | 174,180 (15.75) | 165,690 (15.53) | 4795 (22.95) | 3695 (20.57) | <0.001 |
| Diabetes (without chronic complications) | 210,220 (19.01) | 203,765 (19.09) | 3520 (16.85) | 2935 (16.34) | <0.001 |
| Diabetes (with chronic complications) | 214,400 (19.38) | 209,135 (19.60) | 3095 (14.81) | 2170 (12.08) | <0.001 |
| Hypothyroidism | 159,160 (14.39) | 153,810 (14.41) | 2950 (14.12) | 2400 (13.36) | 0.193 |
| Renal Failure | 181,950 (16.45) | 175,960 (16.49) | 3670 (17.56) | 2320 (12.92) | <0.001 |
| Liver Disease | 18,310 (1.66) | 17,275 (1.62) | 550 (2.63) | 485 (2.70) | <0.001 |
| Peptic Ulcer Disease | 7695 (0.70) | 7400 (0.69) | 175 (0.84) | 120 (0.67) | 0.527 |
| Acquired Immune Deficiency Syndrome | 2395 (0.22) | 2280 (0.21) | 100 (0.48) | 15 (0.08) | <0.001 |
| Lymphoma | 5315 (0.48) | 4915 (0.46) | 220 (1.05) | 180 (1.00) | <0.001 |
| Rheumatoid Arthritis/Collagen Vascular Disease | 30,150 (2.73) | 29,240 (2.74) | 520 (2.49) | 390 (2.17) | 0.075 |
| Coagulopathy | 41,405 (3.74) | 37,105 (3.48) | 1560 (7.47) | 2740 (15.26) | <0.001 |
| Obesity | 145,465 (13.15) | 142,575 (13.36) | 1775 (8.49) | 1115 (6.21) | <0.001 |
| Weight loss | 44,030 (3.98) | 39,685 (3.72) | 1795 (8.59) | 2550 (14.20) | <0.001 |
| Fluid and electrolyte disorders | 246,680 (22.30) | 235,750 (22.09) | 5200 (24.89) | 5730 (31.90) | <0.001 |
| Anaemia (chronic blood loss) | 4025 (0.36) | 3590 (0.34) | 240 (1.15) | 195 (1.09) | <0.001 |
| Anaemia (deficiency) | 133,005 (12.03) | 123,720 (11.59) | 4375 (20.94) | 4910 (27.34) | <0.001 |
| Alcohol abuse | 49,375 (4.46) | 48,080 (4.51) | 800 (3.83) | 495 (2.76) | <0.001 |
| Drug abuse | 28,985 (2.62) | 28,365 (2.66) | 355 (1.70) | 265 (1.48) | <0.001 |
| Psychoses | 26,255 (2.37) | 25,490 (2.39) | 440 (2.11) | 325 (1.81) | 0.041 |
| Depression | 124,635 (11.27) | 120,280 (11.27) | 2330 (11.15) | 2025 (11.28) | 0.971 |
| Hypertension | 946,140 (85.54) | 916,430 (85.87) | 16,975 (81.24) | 12,735 (70.91) | <0.001 |
| PROCEDURES, n (%) | |||||
| Thrombectomy | 34,420 (3.11) | 33,090 (3.10) | 670 (3.21) | 660 (3.67) | 0.139 |
| Thrombolysis | 103,600 (9.37) | 101,035 (9.47) | 1730 (8.28) | 835 (4.65) | <0.001 |
| OUTCOMES, n (%) | |||||
| In-hospital mortality | 43,545 (3.94) | 40,545 (3.80) | 1230 (5.89) | 1770 (9.86) | <0.001 |
| Length-of-stay >4 days | 380,605 (34.41) | 363,430 (34.05) | 8680 (41.54) | 8495 (47.30) | <0.001 |
| Routine Discharge | 394,105 (35.99) | 384,490 (36.39) | 5600 (26.94) | 4015 (22.52) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pana, T.A.; Mohamed, M.O.; Mamas, M.A.; Myint, P.K. Prognosis of Acute Ischaemic Stroke Patients with Cancer: A National Inpatient Sample Study. Cancers 2021, 13, 2193. https://doi.org/10.3390/cancers13092193
Pana TA, Mohamed MO, Mamas MA, Myint PK. Prognosis of Acute Ischaemic Stroke Patients with Cancer: A National Inpatient Sample Study. Cancers. 2021; 13(9):2193. https://doi.org/10.3390/cancers13092193
Chicago/Turabian StylePana, Tiberiu A., Mohamed O. Mohamed, Mamas A. Mamas, and Phyo K. Myint. 2021. "Prognosis of Acute Ischaemic Stroke Patients with Cancer: A National Inpatient Sample Study" Cancers 13, no. 9: 2193. https://doi.org/10.3390/cancers13092193
APA StylePana, T. A., Mohamed, M. O., Mamas, M. A., & Myint, P. K. (2021). Prognosis of Acute Ischaemic Stroke Patients with Cancer: A National Inpatient Sample Study. Cancers, 13(9), 2193. https://doi.org/10.3390/cancers13092193







