Risk Stratification of Cholangiocarcinoma Patients Presenting with Jaundice: A Retrospective Analysis from a Tertiary Referral Center
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
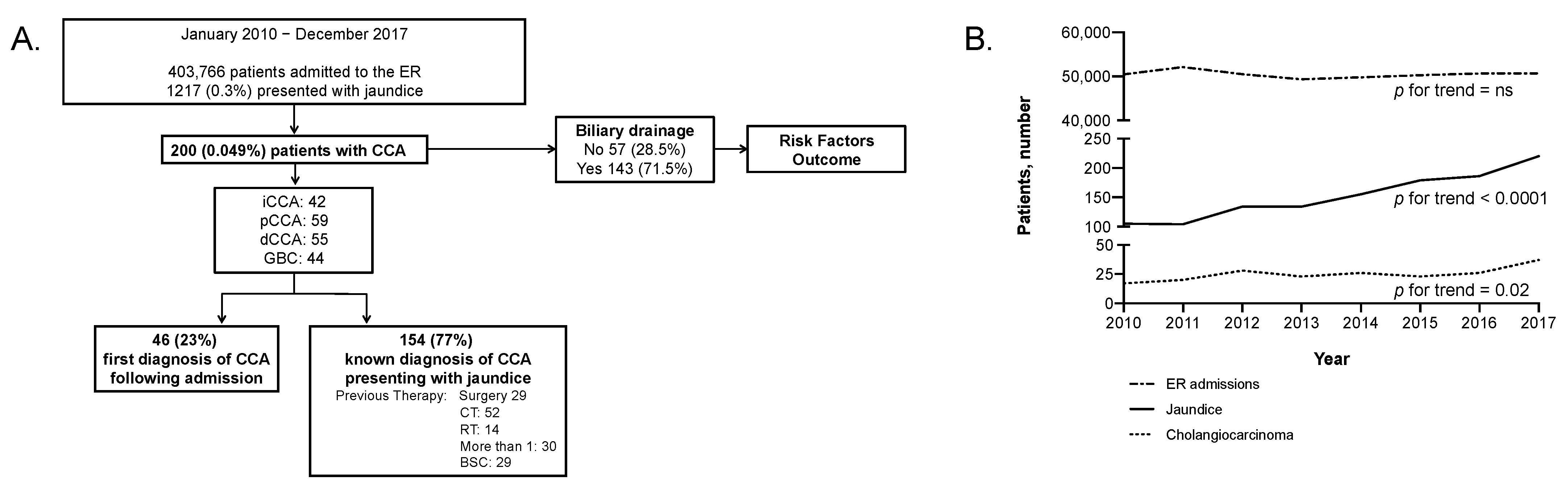
2.1. Baseline Characteristics
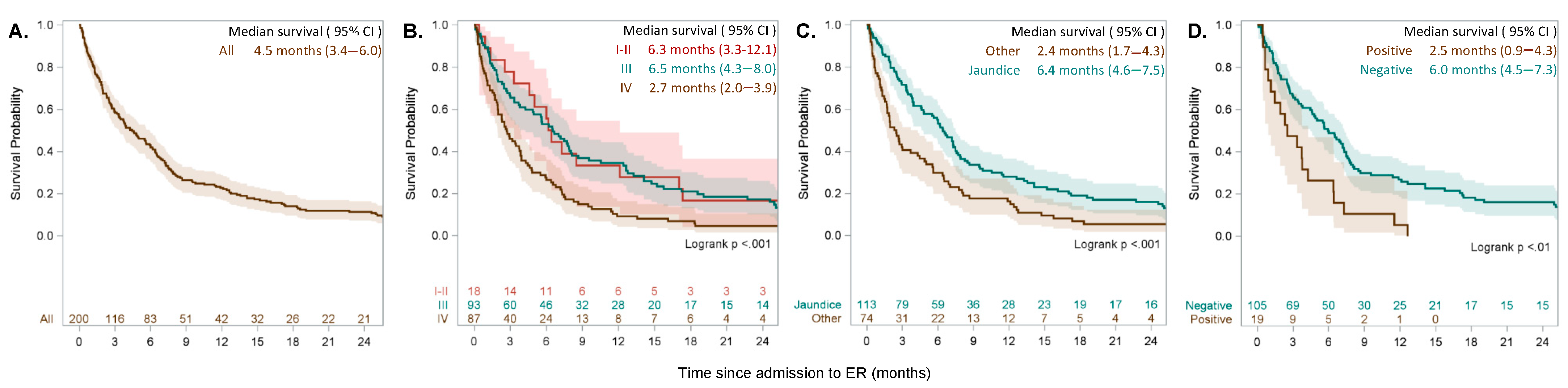
2.2. Outcome and Prognostic Parameters
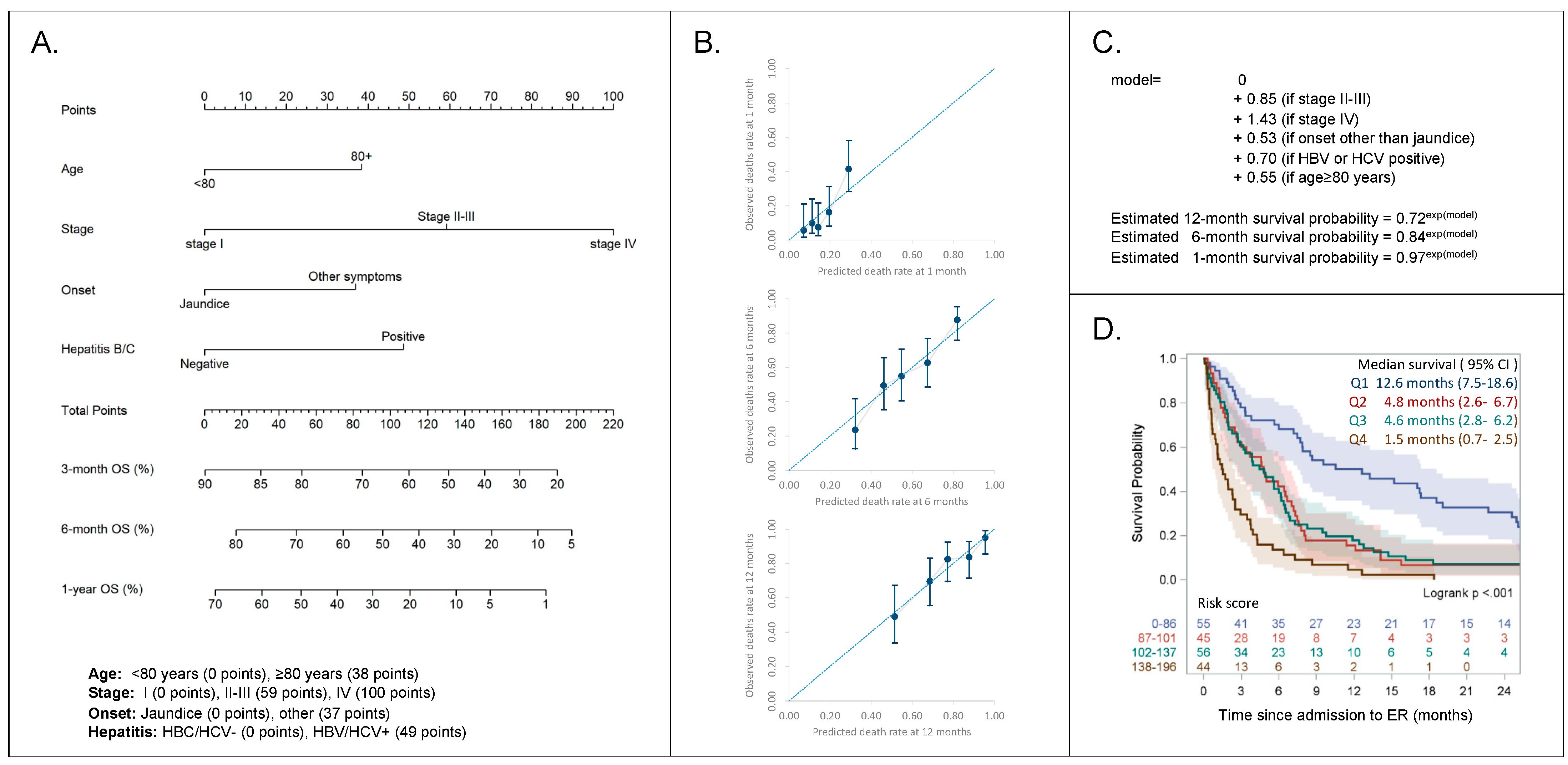
2.3. Risk Stratification
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banales:, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Bertuccio, P.; Malvezzi, M.; Carioli, G.; Hashim, D.; Boffetta, P.; El-Serag, H.B.; La Vecchia, C.; Negri, E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J. Hepatol. 2019, 71, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Islami, F.; Bray, F.; Jemal, A. Worldwide Burden of and Trends in Mortality from Gallbladder and Other Biliary Tract Cancers. Clin. Gastroenterol. Hepatol. 2018, 16, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Taylor-Robinson, S.D.; Toledano, M.B.; Beck, A.; Elliott, P.; Thomas, H.C. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J. Hepatol. 2002, 37, 806–813. [Google Scholar] [CrossRef]
- Clements, O.; Eliahoo, J.; Kim, J.U.; Taylor-Robinson, S.D.; Khan, S.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Hepatol. 2020, 72, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.R.; Oh, J.K.; Masuyer, E.; Curado, M.P.; Bouvard, V.; Fang, Y.Y.; Wiangnon, S.; Sripa, B.; Hong, S.T. Epidemiology of cholangiocarcinoma: An update focusing on risk factors. Cancer Sci. 2010, 101, 579–585. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Y.; Li, B.; Huang, J.; Wu, L.; Xu, D.; Yang, J.; He, J. Hepatitis viruses infection and risk of intrahepatic cholangiocarcinoma: Evidence from a meta-analysis. BMC Cancer 2012, 12, 289. [Google Scholar] [CrossRef]
- Shaib, Y.H.; El-Serag, H.B.; Davila, J.A.; Morgan, R.; McGlynn, K.A. Risk factors of intrahepatic cholangiocarcinoma in the United States: A case-control study. Gastroenterology 2005, 128, 620–626. [Google Scholar] [CrossRef]
- Petrick, J.L.; Yang, B.; Altekruse, S.F.; Van Dyke, A.L.; Koshiol, J.; Graubard, B.I.; McGlynn, K.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: A population-based study in SEER-Medicare. PLoS ONE 2017, 12, e0186643. [Google Scholar] [CrossRef]
- Claessen, M.M.; Vleggaar, F.P.; Tytgat, K.M.; Siersema, P.D.; van Buuren, H.R. High lifetime risk of cancer in primary sclerosing cholangitis. J. Hepatol. 2009, 50, 158–164. [Google Scholar] [CrossRef]
- Kelley, R.K.; Bridgewater, J.; Gores, G.J.; Zhu, A.X. Systemic therapies for intrahepatic cholangiocarcinoma. J. Hepatol. 2020, 72, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef]
- Alvaro, D.; Bragazzi, M.C.; Benedetti, A.; Fabris, L.; Fava, G.; Invernizzi, P.; Marzioni, M.; Nuzzo, G.; Strazzabosco, M.; Stroffolini, T.; et al. Cholangiocarcinoma in Italy: A national survey on clinical characteristics, diagnostic modalities and treatment. Results from the “Cholangiocarcinoma” committee of the Italian Association for the Study of Liver disease. Dig. Liver Dis. 2011, 43, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Davidson, B.R.; Goldin, R.D.; Heaton, N.; Karani, J.; Pereira, S.P.; Rosenberg, W.M.; Tait, P.; Taylor-Robinson, S.D.; Thillainayagam, A.V.; et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: An update. Gut 2012, 61, 1657–1669. [Google Scholar] [CrossRef]
- Rizzo, A.; Ricci, A.D.; Frega, G.; Palloni, A.; S, D.E.L.; Abbati, F.; Mollica, V.; Tavolari, S.; M, D.I.M.; Brandi, G. How to Choose Between Percutaneous Transhepatic and Endoscopic Biliary Drainage in Malignant Obstructive Jaundice: An Updated Systematic Review and Meta-analysis. In Vivo 2020, 34, 1701–1714. [Google Scholar] [CrossRef]
- Coelen, R.J.S.; Roos, E.; Wiggers, J.K.; Besselink, M.G.; Buis, C.I.; Busch, O.R.C.; Dejong, C.H.C.; van Delden, O.M.; van Eijck, C.H.J.; Fockens, P.; et al. Endoscopic versus percutaneous biliary drainage in patients with resectable perihilar cholangiocarcinoma: A multicentre, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2018, 3, 681–690. [Google Scholar] [CrossRef]
- Lamarca, A.; Benafif, S.; Ross, P.; Bridgewater, J.; Valle, J.W. Cisplatin and gemcitabine in patients with advanced biliary tract cancer (ABC) and persistent jaundice despite optimal stenting: Effective intervention in patients with luminal disease. Eur. J. Cancer 2015, 51, 1694–1703. [Google Scholar] [CrossRef]
- La Vecchia, C.; Negri, E.; Cavalieri d’Oro, L.; Franceschi, S. Liver cirrhosis and the risk of primary liver cancer. Eur. J. Cancer Prev. 1998, 7, 315–320. [Google Scholar] [CrossRef]
- Patel, T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2002, 2, 10. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis patients with HIV/HCV co-infection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | All n (%) | iCCA n (%) | pCCA n (%) | dCCA n (%) | GBC n (%) |
|---|---|---|---|---|---|
| All | 200 (100) | 42 (100) | 59 (100) | 55 (100) | 44 (100) |
| Gender | |||||
| Male | 106 (53.0) | 19 (45.2) | 35 (59.3) | 30 (54.5) | 22 (50.0) |
| Female | 94 (47.0) | 23 (54.8) | 24 (40.7) | 25 (45.5) | 22 (50.0) |
| Age | |||||
| <50 | 12 (6.0) | 5 (11.9) | 4 (6.8) | 3 (5.5) | - |
| 50–59 | 25 (12.5) | 5 (11.9) | 7 (11.9) | 6 (10.9) | 7 (15.9) |
| 60–69 | 63 (31.5) | 16 (38.1) | 18 (30.5) | 13 (23.6) | 16 (36.4) |
| 70–79 | 70 (35.0) | 11 (26.2) | 24 (40.7) | 20 (36.4) | 15 (34.1) |
| 80+ | 30 (15.0) | 5 (11.9) | 6 (10.2) | 13 (23.6) | 6 (13.6) |
| Bilirubin, mean ± SD (mg/dL) | 12.4 ± 3.0 | 10.2 ± 5.5 | 12.7 ± 2.5 | 17.3 ± 2.5 | 9.7 ± 1.7 |
| Ca19.9, mean ± SD (IU/mL) | 995 ± 219 | 892 ± 102 | 1023 ± 285 | 945 ± 382 | 1123 ± 107 |
| CEA, mean ± SD (ng/mL) | 25 ± 19 | 24 ± 12 | 32 ± 27 | 29 ± 18 | 18 ± 11 |
| Stage | |||||
| I | 6 (3.0) | 2 (4.8) | 2 (3.4) | - | 2 (4.5) |
| II | 12 (6.0) | 1 (2.4) | 7 (11.9) | 3 (5.5) | 1 (2.3) |
| III | 93 (46.5) | 16 (38.1) | 33 (55.9) | 31 (56.4) | 13 (29.5) |
| IV | 87 (43.5) | 23 (54.8) | 16 (27.1) | 21 (38.2) | 27 (61.4) |
| N/a | 2 (1.0) | - | 1 (1.7) | - | 1 (2.3) |
| Diagnosis of CCA | |||||
| First diagnosis | 46 (23.0) | 4 (9.5) | 19 (32.2) | 13 (23.6) | 10 (22.7) |
| Previously known CCA | 154 (77.0) | 38 (90.5) | 40 (67.8) | 42 (76.4) | 34 (77.3) |
| If previous, treatment * | |||||
| Surgery | 29 (14.5) | 8 (19.0) | 3 (5.1) | 7 (12.7) | 11 (25.0) |
| CT | 52 (26.0) | 17 (40.5) | 8 (13.6) | 13 (23.6) | 14 (31.8) |
| RT | 14 (7.0) | 3 (7.1) | 3 (5.1) | 5 (9.1) | 3 (6.8) |
| Best Supportive Care | 29 (14.5) | 6 (14.3) | 8 (13.6) | 4 (7.3) | 11 (25.0) |
| Onset | |||||
| Jaundice (bilirubin > 3.5 mg/dL) | 113 (56.5) | 11 (26.2) | 41 (69.5) | 38 (69.1) | 23 (52.3) |
| Laboratory | 27 (13.5) | 14 (33.3) | 5 (8.5) | 1 (1.8) | 7 (15.9) |
| Weight loss | 11 (5.5) | 2 (4.8) | 5 (8.5) | 1 (1.8) | 3 (6.8) |
| Cholangitis | 12 (6.0) | 1 (2.4) | 3 (5.1) | 4 (7.3) | 4 (9.1) |
| Other | 37 (18.5) | 14 (33.3) | 5 (8.5) | 11 (20) | 7 (15.9) |
| Lithiasis | |||||
| No | 132 (66.0) | 34 (81) | 47 (79.7) | 33(60.0) | 18 (40.9) |
| Yes | 68 (34.0) | 8 (19.0) | 12 (20.3) | 22 (40.0) | 26 (59.1) |
| HCV-Ab or HBsAg | |||||
| Negative | 105 (52.5) | 21 (50.0) | 31 (52.5) | 32 (58.2) | 21 (47.7) |
| Positive | 19 (9.5) | 11 (26.2) | 4 (6.8) | 3 (5.5) | 1 (2.3) |
| N/a | 76 (38.0) | 10 (23.8) | 24 (40.7) | 20 (36.4) | 22 (50.0) |
| Alcohol | |||||
| No | 187 (93.5) | 39 (92.9) | 57 (96.6) | 51 (92.7) | 40 (90.9) |
| Yes | 13 (6.5) | 3 (7.1) | 2 (3.4) | 4 (7.3) | 4 (9.1) |
| Comorbidities | |||||
| Hypertension | 92 (46.0) | 17 (40.5) | 33 (55.9) | 20 (36.4) | 22 (50.0) |
| Cirrhosis | 10 (5.0) | 6 (14.3) | 1 (1.7) | 1 (1.8) | 2 (4.5) |
| Diabetes | 35 (17.5) | 8 (19.0) | 10 (16.9) | 9 (16.4) | 8 (18.2) |
| Obesity | 20 (10) | 4 (9.5) | 3 (5.1) | 5 (9.1) | 8 (18.2) |
| Histology | |||||
| No | 39 (19.5) | 6 (14.3) | 18 (30.5) | 7 (12.8) | 8 (18.2) |
| Yes | 161 (80.5) | 36 (85.7) | 41 (69.5) | 48 (87.3) | 36 (81.8) |
| Biliary drainage after ER | |||||
| No | 57 (28.5) | 23 (54.8) | 11 (18.6) | 14 (25.5) | 9 (20.5) |
| Yes | 143 (71.5) | 19 (45.2) | 48 (81.4) | 41 (74.5) | 35 (79.5) |
| If yes, type of drainage | |||||
| PTBD | 60 (41.9) | 16 (84.2) | 9 (18.7) | 3 (7.3) | 32 (91.4) |
| Endoscopy | 83 (58.1) | 3 (15.8) | 39 (81.3) | 38 (92.7) | 3 (8.6) |
| Variable | Comparison Groups | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Site | Extra-hepatic (vs. iCCA) | 0.77 (0.53–1.11) | 0.16 | ||
| Gallbladder (vs. iCCA) | 1.13 (0.73–1.74) | 0.57 | |||
| Gender | Female (vs. male) | 1.21 (0.91–1.61) | 0.19 | ||
| Age | ≥80 years (vs. <80 years) | 1.32 (0.89–1.96) | 0.17 | 1.73 (1.15–2.62) | 0.009 |
| Stage | II (vs. I) | 2.28 (0.78–6.72) | 0.13 | 2.67 (0.88–8.05) | 0.08 |
| III (vs. I) | 2.06 (0.81–5.20) | 0.13 | 2.31 (0.89–5.95) | 0.08 | |
| IV (vs. I) | 3.87 (1.52–9.84) | 0.005 | 4.20 (1.61–11.0) | 0.003 | |
| First presentation | Yes (vs. no) | 0.75 (0.53–1.06) | 0.10 | ||
| Previous Surgery | Yes (vs. no) | 1.13 (0.75–1.69) | 0.57 | ||
| Previous CT | Yes (vs. no) | 1.27 (0.91–1.76) | 0.16 | ||
| Previous RT | Yes (vs. no) | 1.91 (1.10–3.30) | 0.02 | ||
| Previous palliative | Yes (vs. no) | 1.46 (0.96–2.20) | 0.07 | ||
| Disease onset | Other (vs. jaundice) | 1.72 (1.27–2.33) | 0.0004 | 1.71 (1.25–2.33) | 0.0008 |
| Lithiasis | Yes (vs. no) | 0.99 (0.73–1.35) | 0.96 | ||
| Hepatitis | Positive (vs. negative) | 2.10 (1.27–3.47) | 0.004 | 2.00 (1.20–3.33) | 0.008 |
| Alcohol | Yes (vs. no) | 0.65 (0.36–1.18) | 0.15 | ||
| Body mass index | Overweight (vs. normal) | 0.85 (0.41–1.79) | 0.68 | ||
| Obese (vs. normal) | 0.50 (0.20–1.30) | 0.16 | |||
| Hypertension | Yes (vs. no) | 0.89 (0.66–1.19) | 0.42 | ||
| Cirrhosis | Yes (vs. no) | 1.08 (0.57–2.05) | 0.82 | ||
| Diabetes | Yes (vs. no) | 0.98 (0.68–1.43) | 0.93 | ||
| Histology | CTM (vs. histology) | 1.21 (0.70–2.10) | 0.50 | ||
| No (vs. histology) | 1.40 (0.96–2.06) | 0.08 | |||
| Biliary drainage after ER | Yes (vs. no) | 0.73 (0.53–1.01) | 0.05 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lleo, A.; Colapietro, F.; Maisonneuve, P.; Aloise, M.; Craviotto, V.; Ceriani, R.; Rimassa, L.; Badalamenti, S.; Donadon, M.; Pedicini, V.; et al. Risk Stratification of Cholangiocarcinoma Patients Presenting with Jaundice: A Retrospective Analysis from a Tertiary Referral Center. Cancers 2021, 13, 2070. https://doi.org/10.3390/cancers13092070
Lleo A, Colapietro F, Maisonneuve P, Aloise M, Craviotto V, Ceriani R, Rimassa L, Badalamenti S, Donadon M, Pedicini V, et al. Risk Stratification of Cholangiocarcinoma Patients Presenting with Jaundice: A Retrospective Analysis from a Tertiary Referral Center. Cancers. 2021; 13(9):2070. https://doi.org/10.3390/cancers13092070
Chicago/Turabian StyleLleo, Ana, Francesca Colapietro, Patrick Maisonneuve, Monia Aloise, Vincenzo Craviotto, Roberto Ceriani, Lorenza Rimassa, Salvatore Badalamenti, Matteo Donadon, Vittorio Pedicini, and et al. 2021. "Risk Stratification of Cholangiocarcinoma Patients Presenting with Jaundice: A Retrospective Analysis from a Tertiary Referral Center" Cancers 13, no. 9: 2070. https://doi.org/10.3390/cancers13092070
APA StyleLleo, A., Colapietro, F., Maisonneuve, P., Aloise, M., Craviotto, V., Ceriani, R., Rimassa, L., Badalamenti, S., Donadon, M., Pedicini, V., Repici, A., Di Tommaso, L., Voza, A., Torzilli, G., & Aghemo, A. (2021). Risk Stratification of Cholangiocarcinoma Patients Presenting with Jaundice: A Retrospective Analysis from a Tertiary Referral Center. Cancers, 13(9), 2070. https://doi.org/10.3390/cancers13092070












