Defining an Ultra-Low Risk Group in Asymptomatic IgM Monoclonal Gammopathy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics
2.2. Bone Marrow Evaluation
2.3. MYD88 L265P Mutation Analysis
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
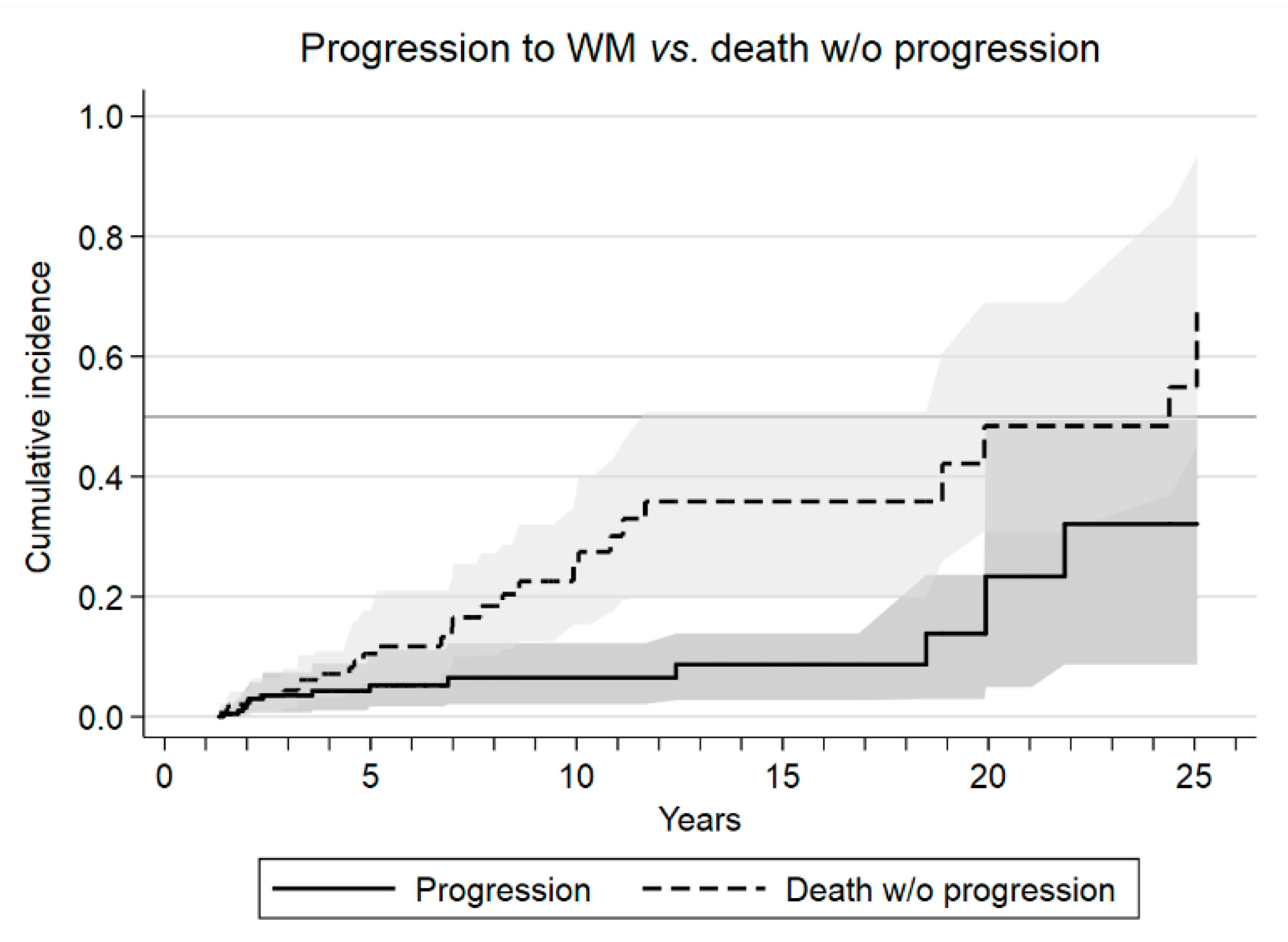
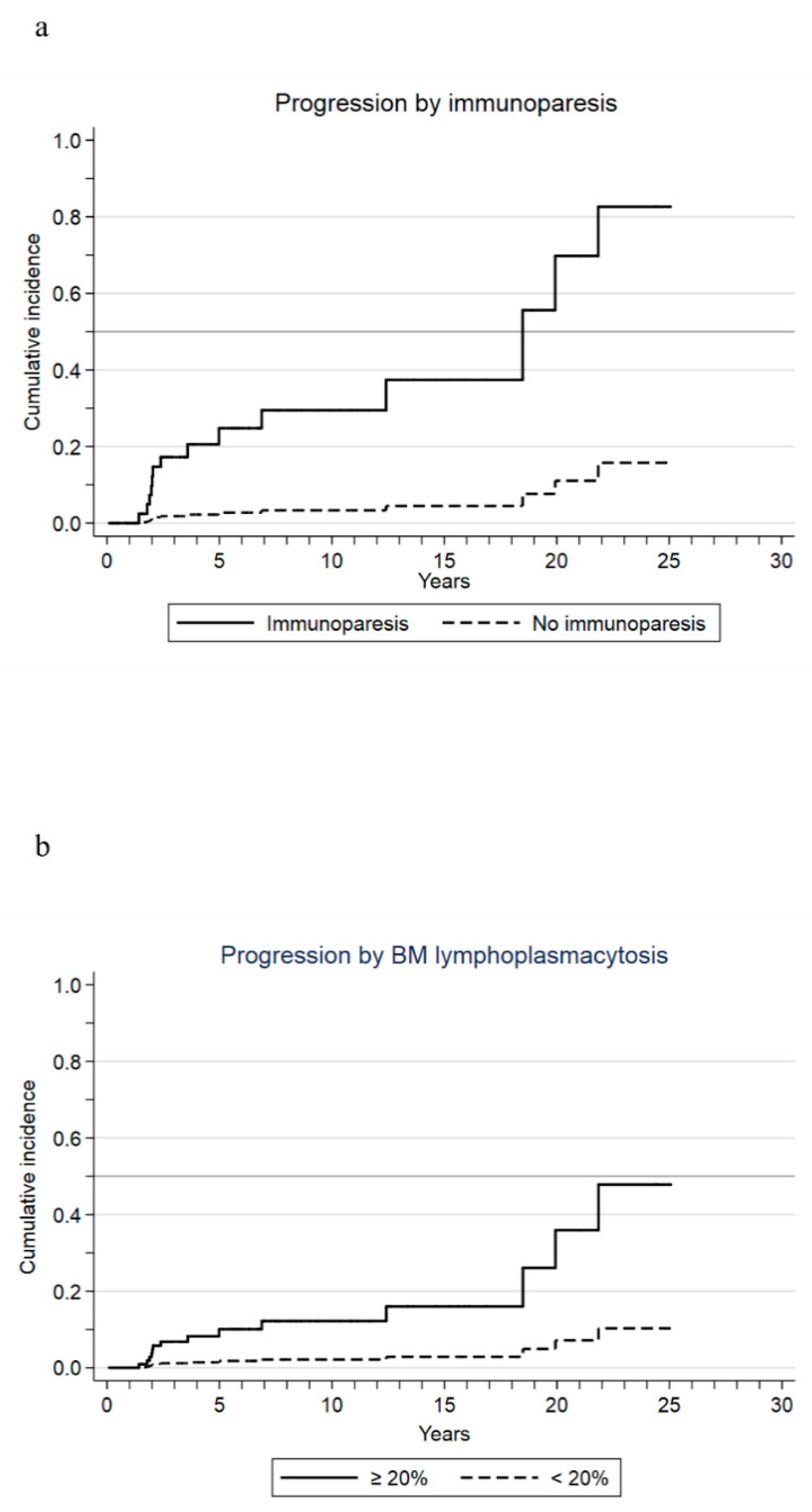
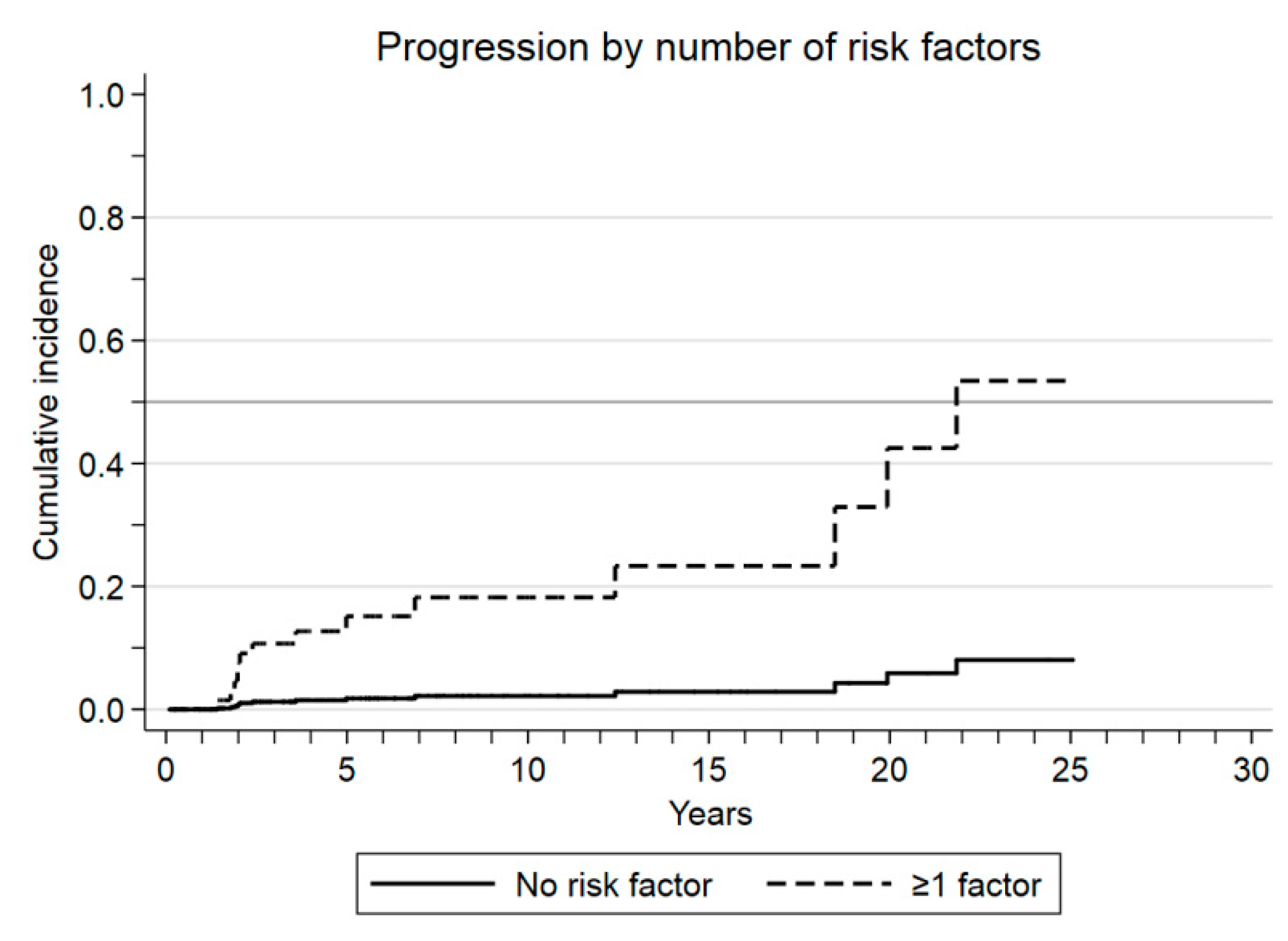
3.2. Outcomes and Risk Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Paiva, B.; San Miguel, J.F.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Owen, R.G.; Treon, S.P.; Al-Katib, A.; Fonseca, R.; Greipp, P.R.; McMaster, M.L.; Morra, E.; Pangalis, G.A.; San Miguel, J.F.; Branagan, A.R.; et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: Consensus Panel Recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin. Oncol. 2003, 30, 110–115. [Google Scholar] [CrossRef]
- Kyle, R.A.; Benson, J.T.; Larson, D.R.; Therneau, T.M.; Dispenzieri, A.; Kumar, S.; Melton, L.J.; Rajkumar, S.V. Progression in smoldering Waldenstrom macroglobulinemia: Long-term results. Blood 2012, 119, 4462–4466. [Google Scholar] [CrossRef]
- Bustoros, M.; Sklavenitis-Pistofidis, R.; Kapoor, P.; Liu, C.J.; Kastritis, E.; Zanwar, S.; Fell, G.; Abeykoon, J.P.; Hornburg, K.; Ghobrial, I.M.; et al. Progression Risk Stratification of Asymptomatic Waldenström Macroglobulinemia. J. Clin. Oncol. 2019, 37, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Larson, D.R.; Therneau, T.M.; Dispenzieri, A.; Kumar, S.; Cerhan, J.R.; Rajkumar, S.V. Long-Term Follow-up of Monoclonal Gammopathy of Undetermined Significance. N. Engl. J. Med. 2018, 378, 241–249. [Google Scholar] [CrossRef]
- Go, R.S.; Rajkumar, S.V. How I manage monoclonal gammopathy of undetermined significance. Blood 2018, 131, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Baldini, L.; Goldaniga, M.; Guffanti, A.; Broglia, C.; Cortelazzo, S.; Rossi, A.; Morra, E.; Colombi, M.; Callea, V.; Gobbi, P.; et al. Immunoglobulin M Monoclonal Gammopathies of Undetermined Significance and Indolent Waldenström’s Macroglobulinemia Recognize the Same Determinants of Evolution Into Symptomatic Lymphoid Disorders: Proposal for a Common Prognostic Scoring System. J. Clin. Oncol. 2005, 23, 4662–4668. [Google Scholar] [CrossRef] [PubMed]
- Kyle, R.A.; Therneau, T.M.; Rajkumar, S.V.; Remstein, E.D.; Offord, J.R.; Larson, D.R.; Melton, L.J. Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Blood 2003, 102, 3759–3764. [Google Scholar] [CrossRef] [PubMed]
- Turesson, I.; Kovalchik, S.A.; Pfeiffer, R.M.; Kristinsson, S.Y.; Goldin, L.R.; Drayson, M.T.; Landgren, O. Monoclonal gammopathy of undetermined significance and risk of lymphoid and myeloid malignancies: 728 cases followed up to 30 years in Sweden. Blood 2014, 123, 338–345. [Google Scholar] [CrossRef]
- Leblond, V.; Kastritis, E.; Advani, R.; Ansell, S.M.; Buske, C.; Castillo, J.J.; Dimopoulos, M.A. Treatment recommendations from the Eighth International Workshop on Waldenstroms Macroglobulinemia. Blood 2016, 128, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A. Waldenström macroglobulinemia: 2019 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2019, 94, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Hunter, Z.R.; Yang, G.; Zhou, Y.; Cao, Y.; Liu, X.; Treon, S.P. MYD88 L265P in Waldenstrom macroglobulinemia, immunoglobulin M monoclonal gammopathy, and other B-cell lymphoproliferative disorders using conventional and quantitative allele-specific polymerase chain reaction. Blood 2013, 121, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Grambsch, P.M.; Therneau, T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Fine, J.P.; Gray, R.J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Mallett, S.; Royston, P.; Waters, R.; Dutton, S.; Altman, D.G. Reporting performance of prognostic models in cancer: A review. BMC Med. 2010, 8, 21. [Google Scholar] [CrossRef]
- Paiva, B.; Montes, M.C.; Garcia-Sanz, R.; Ocio, E.M.; Alonso, J.; de Las Heras, N.; San Miguel, J.F. Multiparameter flow cytometry for the identification of the Waldenström’s clone in IgM-MGUS and Waldenström’s Macroglobulinemia: New criteria for differential diagnosis and risk stratification. Leukemia 2014, 28, 166–173. [Google Scholar] [CrossRef]
- PPaiva, B.; Corchete, L.A.; Vidriales, M.B.; García-Sanz, R.; Perez, J.J.; Aires-Mejia, I.; San Miguel, J.F. The cellular origin and malignant transformation of Waldenström macroglobulinemia. Blood 2015, 125, 2370–2380. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Kastritis, E.; Owen, R.G.; Kyle, R.A.; Landgren, O.; Morra, E.; Treon, S.P. Treatment recommendations for patients with Waldenstrom macroglobulinemia (WM) and related disorders: IWWM-7 consensus. Blood 2014, 124, 1404–1411. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Kastritis, E. How I treat Waldenström macroglobulinemia. Blood 2019, 134, 2022–2035. [Google Scholar] [CrossRef]
- Alexanian, R.; Weber, D.; Delasalle, K.; Cabanillas, F.; Dimopoulos, M. Asymptomatic Waldenstrom’s macroglobulinemia. Semin. Oncol. 2003, 30, 206–210. [Google Scholar] [CrossRef]
- Varettoni, M.; Zibellini, S.; Boveri, E.; Klersy, C.; Candido, C.; Rattotti, S.; Ferretti, V.V.; Defrancesco, I.; Nizzoli, M.E.; Arcaini, L.; et al. A risk-stratification model based on the initial concentration of the serum monoclonal protein and MYD 88 mutation status identifies a subset of patients with IgM monoclonal gammopathy of undetermined significance at high risk of progression to Waldenström macroglobulinaemia or other lymphoproliferative disorders. Br. J. Haematol. 2019, 187, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Vos, J.M.; Tsakmaklis, N.; Patterson, C.J.; Meid, K.; Castillo, J.J.; Brodsky, P.; Ganz, T.; Pals, S.T.; Kersten, M.J.; Hunter, Z.R.; et al. CXCL13 levels are elevated in patients with Waldenström macroglobulinemia, and are predictive of major response to ibrutinib. Haematologica 2017, 102, e452–e455. [Google Scholar] [CrossRef] [PubMed]
- Hunter, Z.R.; Manning, R.J.; Hanzis, C.; Ciccarelli, B.T.; Ioakimidis, L.; Patterson, C.J.; Lewicki, M.C.; Tseng, H.; Gong, P.; Treon, S.P.; et al. IgA and IgG hypogammaglobulinemia in Waldenstrom’s macroglobulinemia. Haematologica 2010, 95, 470–475. [Google Scholar] [CrossRef]
- Morra, E.; Cesana, C.; Klersy, C.; Varettoni, M.; Cavanna, L.; Canesi, B.; Tresoldi, E.; Barbarano, L.; Lazzarino, M. Predictive variables for malignant transformation in 452 patients with asymptomatic IgM monoclonal gammopathy. Semin. Oncol. 2003, 30, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, A.; Rajkumar, S.V.; Buadi, F.K.; Binder, M.; Gertz, M.A.; Lacy, M.Q.; Dispenzieri, A.; Dingli, D.; Fonder, A.L.; Kumar, S.K.; et al. Risk stratification of smoldering multiple myeloma incorporating revised IMWG diagnostic criteria. Blood Cancer J. 2018, 8, 59. [Google Scholar] [CrossRef]
- De Larrea, C.F.; Isola, I.; Pereira, A.; Cibeira, M.T.; Magnano, L.; Tovar, N.; Rodríguez-Lobato, L.-G.; Calvo, X.; Aróstegui, J.I.; Rosiñol, L.; et al. Evolving M-protein pattern in patients with smoldering multiple myeloma: Impact on early progression. Leukemia 2018, 32, 1427–1434. [Google Scholar] [CrossRef]
- Correa, J.G.; Cibeira, M.T.; Tovar, N.; Isola, I.; Pedrosa, F.; Díaz, T.; Lozano, E.; Magnano, L.; Rosiñol, L.; de Larrea, C.F.; et al. Prevalence and prognosis implication of MYD88 L265P mutation in IgM monoclonal gammopathy of undetermined significance and smouldering Waldenström macroglobulinaemia. Br. J. Haematol. 2017, 179, 849–851. [Google Scholar] [CrossRef]
- Varettoni, M.; Zibellini, S.; Defrancesco, I.; Ferretti, V.V.; Rizzo, E.; Malcovati, L.; Galli, A.; Della Porta, M.G.; Boveri, E.; Cazzola, M.; et al. Pattern of somatic mutations in patients with Waldenström macroglobulinemia or IgM monoclonal gammopathy of undetermined significance. Haematologica 2017, 102, 2077–2085. [Google Scholar] [CrossRef]
- Treon, S.P.; Cao, Y.; Xu, L.; Yang, G.; Liu, X.; Hunter, Z.R. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenstrom macroglobulinemia. Blood 2014, 123, 2791–2796. [Google Scholar] [CrossRef]
- Schuster, N.A.; Hoogendijk, E.O.; Kok, A.A.; Twisk, J.W.; Heymans, M.W. Ignoring competing events in the analysis of survival data may lead to biased results: A nonmathematical illustration of competing risk analysis. J. Clin. Epidemiol. 2020, 122, 42–48. [Google Scholar] [CrossRef] [PubMed]
| Median (IQR a) | Frequencies | |
|---|---|---|
| Serum M-protein (g/L) | 12.2 (9.1–14.7) | ≥12 in 53.2% ≥15 in 23.4% |
| Serum IgM (g/L) | 5.4 (3.5–11) | <30 in 97% |
| Calcium (mg/dL) | 9.5 (9.2–9.8) | >10.5 in 3.2% |
| Creatinine (mg/dL) | 0.9 (0.8–1) | >2 in 0.6% |
| Hemoglobin (g/dL) | 13.4 (12.2–14.6) | <12 in 15.8% |
| Platelets (×109/L) | 237 (194–291) | <100 in 1.2% |
| β2-microglobulin (mg/dL) | 2.3 (1.9–3.1) | ≥4 in 7% |
| Albumin (g/L) | 43 (40–45) | ≤35 in 4.1% |
| Lymphoplasmacytic infiltration | 16 (11–24) | |
| Immunoparesis b (%) | 14/167 (8.4) | |
| Abnormal serum FLC ratio c (%) | 42/92 (45.7) | |
| MYD88 L265P mutation d (%) | 84/160 (52.5) | |
| Progressive disease (%) | 14/171 (8.2) |
| IgM MGUS a (%) n = 64 | SWM b (%) n = 107 | p-Value | |
|---|---|---|---|
| Serum M-protein (g/L) ≥12 g/L | 12.1 51 (48) | 11.7 37 (58) | 0.6 0.1 |
| Hemoglobin (g/dL) | 13.3 | 13.4 | 0.8 |
| Platelets (×109/L) | 255 | 245 | 0.4 |
| β2-microglobulin (mg/dL) ≥4 mg/dL | 2.9 6/60 (10) | 2.4 5/96 (5) | 0.1 0.25 |
| Albumin (g/L) ≤35 g/L | 42.3 3 (5) | 42.5 4 (4) | 0.7 0.7 |
| Immunoparesis | 4/63 (6) | 10/104 (10) | 0.4 |
| Abnormal serum FLC ratio | 10/32 (31) | 32/60 (53) | 0.04 |
| MYD88 L265P mutation | 18/60 (30) | 66/100 (66) | <0.001 |
| Adverse Variables | Subhazard Ratio (95% CI a) | p-Value |
| Immunoparesis | 10.2 (4.2–24.8) | <0.001 |
| Lymphoplasmacytic infiltrate ≥20% in the bone marrow aspirate | 6.0 (1.6–22.1) | 0.007 |
| Risk Model | Subhazard Ratio (95% CI) | p-Value |
| Good prognosis group (no risk factors) | 0.1 (0.02–0.5) | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, D.F.; Pereira, A.; Tovar, N.; Cibeira, M.T.; Magnano, L.; Rozman, M.; López-Guerra, M.; Colomer, D.; Martín-Antonio, B.; Jiménez-Segura, R.; et al. Defining an Ultra-Low Risk Group in Asymptomatic IgM Monoclonal Gammopathy. Cancers 2021, 13, 2055. https://doi.org/10.3390/cancers13092055
Moreno DF, Pereira A, Tovar N, Cibeira MT, Magnano L, Rozman M, López-Guerra M, Colomer D, Martín-Antonio B, Jiménez-Segura R, et al. Defining an Ultra-Low Risk Group in Asymptomatic IgM Monoclonal Gammopathy. Cancers. 2021; 13(9):2055. https://doi.org/10.3390/cancers13092055
Chicago/Turabian StyleMoreno, David F., Arturo Pereira, Natalia Tovar, María Teresa Cibeira, Laura Magnano, María Rozman, Mónica López-Guerra, Dolors Colomer, Beatriz Martín-Antonio, Raquel Jiménez-Segura, and et al. 2021. "Defining an Ultra-Low Risk Group in Asymptomatic IgM Monoclonal Gammopathy" Cancers 13, no. 9: 2055. https://doi.org/10.3390/cancers13092055
APA StyleMoreno, D. F., Pereira, A., Tovar, N., Cibeira, M. T., Magnano, L., Rozman, M., López-Guerra, M., Colomer, D., Martín-Antonio, B., Jiménez-Segura, R., Isola, I., Rodríguez-Lobato, L. G., Oliver-Caldés, A., Mena, M. P., Rosiñol, L., Bladé, J., & Fernández de Larrea, C. (2021). Defining an Ultra-Low Risk Group in Asymptomatic IgM Monoclonal Gammopathy. Cancers, 13(9), 2055. https://doi.org/10.3390/cancers13092055







