Influencers of the Decision to Undergo Contralateral Prophylactic Mastectomy among Women with Unilateral Breast Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Before your contralateral prophylactic mastectomy, how would you have described your concern about developing breast cancer?
- 4□
- Very concerned
- 3□
- Concerned
- 2□
- Not very concerned
- 1□
- Not concerned at all
- At the time of your prophylactic mastectomy, what was your marital status?
- 1□
- Married
- 2□
- Living together but unmarried
- 3□
- Separated or divorced
- 4□
- Widowed
- 5□
- Single, never married
- What were your reasons for having a contralateral prophylactic mastectomy? Please check all that apply.
- 1□
- Uncomfortably large breasts
- 2□
- Concerns about appearance
- 3□
- Family history of breast cancer
- 4□
- Prevent breast cancer
- 5□
- Other, please specify: __________________________________________
- Which statement (s) best describes the decision about your contalateral prophylactic mastectomy? Choose all that apply.
- 1□
- I made the final decision to have surgery.
- 2□
- I made the final decision to have surgery after seriously considering my doctor’s opinion.
- 3□
- My doctor and I shared responsibility for the final decision to have surgery.
- 4□
- My doctor made the final decision about my surgery, but seriously considered my opinion.
- 5□
- My doctor made the final decision about my surgery.
- 6□
- I made the final decision to have surgery after seriously considering my partner’s opinion.
- 7□
- My partner made the final decision about my surgery.
- Media Influence: Please choose one number to indicate whether or not the media had influenced your decision making to undergo prophylactic mastectomy.
Not At All A Little Bit Some-What Quite A Bit Very Much 1 2 3 4 5 - Thinking back to six months after your prophylactic mastectomy, how satisfied were you with your decision to have the surgery?
- 1□
- Very dissatisfied
- 2□
- Dissatisfied
- 3□
- Neither Satisfied or Dissatisfied
- 4□
- Satisfied
- 5□
- Very satisfied
- Did you have breast reconstruction after your prophylactic mastectomy? Breast reconstruction is a surgical procedure in which the breasts are recreated using implants or tissue from the body.
- 0□
- No.
- 1□
- Yes, done in a separate surgery after the prophylactic mastectomy
- 2□
- Yes, done along with prophylactic mastectomy
- I “yes” Have you had surgery to revise or repair your reconstruction?
- 0□
- No
- 1□
- Yes, one or two times
- 2□
- Yes, multiple times
- 9.
- Below is a list of statements that describe aspects of women’s lives, including thoughts about your body and sexuality.
Please Choose One Number to Indicate How True Each Statement Has Been for You during the Past 30 Days. FREQUENCY Not At All A Little Bit Some-What Quite A Bit Very Much - a.
- I am able to enjoy life.
1 2 3 4 5 - b.
- I am content with the quality of my life right now.
1 2 3 4 5 - c.
- I feel self-conscious about my appearance.
1 2 3 4 5 - d.
- I am happy with my current weight.
1 2 3 4 5 - e.
- I am satisfied with my appearance when dressed.
1 2 3 4 5 - f.
- I find it difficult to look at myself naked.
1 2 3 4 5 - g.
- I am embarrassed for others to see my body.
1 2 3 4 5 - h.
- I am able to feel like a woman.
1 2 3 4 5 - i.
- I feel sexually attractive.
1 2 3 4 5 - j.
- I am satisfied with my sex life.
1 2 3 4 5
- 10.
- What was your age at the time of prophylactic mastectomy?
- 1□
- 20 to 30 years old
- 2□
- 31 to 40 years old
- 3□
- 41 to 50 years old
- 4□
- 51 to 60 years old
- 11.
- To what race/ethnic group do you belong? Please check all that apply.
- 1□
- Asian or Pacific Islander, please specify: ________________________
- 2□
- Black or African American
- 3□
- Hispanic/Latino, please specify:________________________
- 4□
- Native American or Alaskan Native
- 5□
- White or Caucasian
- 9□
- Other, please specify: ________________________
- 12.
- What is the highest level of education you have completed?
- 1□
- Less than or some high school
- 2□
- High school or GED
- 3□
- Trade or technical school
- 4□
- Junior college, or some college
- 5□
- College graduate
- 6□
- Postgraduate work or degree
- 13.
- On what date did you complete this questionnaire?____/____/____ month/day/year)
- 14.
- How long ago was your prophylactic mastectomy? (Please insert the number of years)___________Years ago.
- 15.
- Overall, how satisfied are you now with your decision to have contralateral prophylactic mastectomy?
- 1□
- Very dissatisfied
- 2□
- Dissatisfied
- 3□
- Neither Satisfied or Dissatisfied
- 4□
- Satisfied
- 5□
- Very satisfied
- 16.
- What one thing do you wish you had known before your prophylactic mastectomy?________________________________________________________________________________________________________________________________________________________________________________________________________________________
References
- Lizarraga, I.M.; Sugg, S.L.; Weigel, R.J.; Scott-Conner, C.E. Review of risk factors for the development of contralateral breast cancer. Am. J. Surg. 2013, 206, 704–708. [Google Scholar] [CrossRef]
- Gao, X.; Fisher, S.G.; Emami, B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: A population-based study. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 1038–1045. [Google Scholar] [CrossRef]
- Jin, J. Women With Breast Cancer Who Opt for Contralateral Prophylactic Mastectomy May Overestimate Future Risk. JAMA 2013, 310, 1548. [Google Scholar] [CrossRef]
- Metcalfe, K.; Lynch, H.T.; Ghadirian, P.; Tung, N.; Olivotto, I.; Warner, E.; Olopade, O.I.; Eisen, A.; Weber, B.; McLennan, J. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J. Clin. Oncol. 2004, 22, 2328–2335. [Google Scholar] [CrossRef]
- Boughey, J.C.; Attai, D.J.; Chen, S.L.; Cody, H.S.; Dietz, J.R.; Feldman, S.M.; Greenberg, C.C.; Kass, R.B.; Landercasper, J.; Lemaine, V. Contralateral prophylactic mastectomy (CPM) consensus statement from the American Society of Breast Surgeons: Data on CPM outcomes and risks. Ann. Surg. Oncol. 2016, 23, 3100–3105. [Google Scholar] [CrossRef]
- Teoh, V.; Tasoulis, M.-K.; Gui, G. Contralateral prophylactic mastectomy in women with unilateral breast cancer who are genetic carriers, have a strong family history or are just young at presentation. Cancers 2020, 12, 140. [Google Scholar] [CrossRef]
- Guliano, A.E.; Boolbol, S.; Degnim, A.; Kuerer, H.; Leitch, A.M.; Morrow, M. Society of Surgical Oncology: Position statement on prophylactic mastectomy. approved by the society of surgical oncology executive council, March 2007. Ann. Surg. Oncol. 2007, 14, 2425–2427. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.M.; Freedman, R.A.; Sagara, Y.; Aydogan, F.; Barry, W.T.; Golshan, M. Growing Use of Contralateral Prophylactic Mastectomy Despite no Improvement in Long-term Survival for Invasive Breast Cancer. Ann Surg 2017, 265, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.M.; Tracy, M.S.; Meyer, M.E.; Sepucha, K.; Gelber, S.; Hirshfield-Bartek, J.; Troyan, S.; Morrow, M.; Schapira, L.; Come, S.E. Perceptions, knowledge, and satisfaction with contralateral prophylactic mastectomy among young women with breast cancer: A cross-sectional survey. Ann. Intern. Med. 2013, 159, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.M.; Sepucha, K.; Ruddy, K.J.; Tamimi, R.M.; Gelber, S.; Meyer, M.E.; Schapira, L.; Come, S.E.; Borges, V.F.; Golshan, M. Local therapy decision-making and contralateral prophylactic mastectomy in young women with early-stage breast cancer. Ann. Surg. Oncol. 2015, 22, 3809–3815. [Google Scholar] [CrossRef]
- Elsayegh, N.; Webster, R.D.; Gutierrez Barrera, A.M.; Lin, H.; Kuerer, H.M.; Litton, J.K.; Bedrosian, I.; Arun, B.K. Contralateral prophylactic mastectomy rate and predictive factors among patients with breast cancer who underwent multigene panel testing for hereditary cancer. Cancer med. 2018, 7, 2718–2726. [Google Scholar] [CrossRef]
- Nash, R.; Goodman, M.; Lin, C.C.; Freedman, R.A.; Dominici, L.S.; Ward, K.; Jemal, A. State Variation in the Receipt of a Contralateral Prophylactic Mastectomy Among Women Who Received a Diagnosis of Invasive Unilateral Early-Stage Breast Cancer in the United States, 2004-2012. JAMA Surg 2017, 152, 648–657. [Google Scholar] [CrossRef]
- Lostumbo, L.; Carbine, N.E.; Wallace, J. Prophylactic mastectomy for the prevention of breast cancer. The Cochrane Database Syst. Rev. 2010, Cd002748. [Google Scholar] [CrossRef]
- Brewster, A.M.; Parker, P.A. Current knowledge on contralateral prophylactic mastectomy among women with sporadic breast cancer. Oncologist 2011, 16, 935–941. [Google Scholar] [CrossRef]
- Brown, S.L.; Salmon, P. Reconciling the theory and reality of shared decision-making: A "matching" approach to practitioner leadership. Health Expect. Int. J. Public Particip. Health Care Health policy 2019, 22, 275–283. [Google Scholar] [CrossRef]
- Sinha, A.K.; Patel, J.R.; Shen, Y.; Ueno, N.T.; Giordano, S.H.; Tripathy, D.; Lopez, D.S.; Barcenas, C.H. Location of receipt of initial treatment and outcomes in long-term breast cancer survivors. PLoS ONE 2017, 12, e0170081. [Google Scholar] [CrossRef] [PubMed]
- Geiger, A.M.; West, C.N.; Nekhlyudov, L.; Herrinton, L.J.; Liu, I.L.; Altschuler, A.; Rolnick, S.J.; Harris, E.L.; Greene, S.M.; Elmore, J.G.; et al. Contentment with quality of life among breast cancer survivors with and without contralateral prophylactic mastectomy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A language and environment for statistical computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 22 April 2021).
- Lenth, R. emmeans: Estimated Marginal Means, aka Least-Squares Means. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 22 April 2021).
- Cumming, G. The New Statistics:Why and How. Psychol. Sci. 2014, 25, 7–29. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C. catseyes: Create Catseye Plots Illustrating the Normal Distribution of the Means. Available online: https://CRAN.R-project.org/package=catseyes (accessed on 22 April 2021).
- Bouchard-Fortier, A.; Baxter, N.N.; Sutradhar, R.; Fernandes, K.; Camacho, X.; Graham, P.; Quan, M.L. Contralateral prophylactic mastectomy in young women with breast cancer: A population-based analysis of predictive factors and clinical impact. Curr Oncol 2018, 25, e562–e568. [Google Scholar] [CrossRef]
- King, T.A.; Sakr, R.; Patil, S.; Gurevich, I.; Stempel, M.; Sampson, M.; Morrow, M. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 2158–2164. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Hunt, K.K.; Arun, B.K.; Bedrosian, I.; Barrera, A.G.; Do, K.-A.; Kuerer, H.M.; Babiera, G.V.; Mittendorf, E.A.; Ready, K. Factors affecting the decision of breast cancer patients to undergo contralateral prophylactic mastectomy. Cancer Prev. Res. 2010, 3, 1026–1034. [Google Scholar] [CrossRef]
- Elsayegh, N.; Profato, J.; Barrera, A.M.; Lin, H.; Kuerer, H.M.; Ardic, C.; Litton, J.K.; Tripathy, D.; Arun, B.K. Predictors that Influence Election of Contralateral Prophylactic Mastectomy among Women with Ductal Carcinoma in Situ who are BRCA-Negative. J. Cancer 2015, 6, 610–615. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tuttle, T.M.; Habermann, E.B.; Grund, E.H.; Morris, T.J.; Virnig, B.A. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: A trend toward more aggressive surgical treatment. J. Clin. Oncol. 2007, 25, 5203–5209. [Google Scholar] [CrossRef] [PubMed]
- Bedrosian, I.; Hu, C.-Y.; Chang, G.J. Population-based study of contralateral prophylactic mastectomy and survival outcomes of breast cancer patients. J. Natl. Cancer Inst. 2010, 102, 401–409. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.K.; Schaid, D.J.; Myers, J.L.; Grant, C.S.; Donohue, J.H.; Woods, J.E.; Frost, M.H.; Johnson, J.L.; Sitta, D.L.; Slezak, J.M. Efficacy of contralateral prophylactic mastectomy in women with a personal and family history of breast cancer. J. Clin. Oncol. 2001, 19, 3938–3943. [Google Scholar] [CrossRef]
- Yao, K.; Sisco, M.; Bedrosian, I. Contralateral prophylactic mastectomy: Current perspectives. Int. J. Womens Health 2016, 8, 213–223. [Google Scholar] [CrossRef]
- Herrinton, L.J.; Barlow, W.E.; Yu, O.; Geiger, A.M.; Elmore, J.G.; Barton, M.B.; Harris, E.L.; Rolnick, S.; Pardee, R.; Husson, G. Efficacy of prophylactic mastectomy in women with unilateral breast cancer: A cancer research network project. J. Clin. Oncol. 2005, 23, 4275–4286. [Google Scholar] [CrossRef]
- Pesce, C.; Liederbach, E.; Wang, C.; Lapin, B.; Winchester, D.J.; Yao, K. Contralateral prophylactic mastectomy provides no survival benefit in young women with estrogen receptor-negative breast cancer. Ann. Surg. Oncol. 2014, 21, 3231–3239. [Google Scholar] [CrossRef]
- Kruper, L.; Kauffmann, R.M.; Smith, D.D.; Nelson, R.A. Survival analysis of contralateral prophylactic mastectomy: A question of selection bias. Ann. Surg. Oncol. 2014, 21, 3448–3456. [Google Scholar] [CrossRef]
- Frost, M.H.; Slezak, J.M.; Tran, N.V.; Williams, C.I.; Johnson, J.L.; Woods, J.E.; Petty, P.M.; Donohue, J.H.; Grant, C.S.; Sloan, J.A. Satisfaction after contralateral prophylactic mastectomy: The significance of mastectomy type, reconstructive complications, and body appearance. J. Clin. Oncol. 2005, 23, 7849–7856. [Google Scholar] [CrossRef] [PubMed]
- Rueth, N.; Tuttle, T. Positive, Negative, and Disparate—Women’s Differing Long-Term Psychosocial Experiences of Bilateral or Contralateral Prophylactic Mastectomy. Breast Dis. Year Book Q. 2009, 3, 294–295. [Google Scholar] [CrossRef]
- Altschuler, A.; Nekhlyudov, L.; Rolnick, S.J.; Greene, S.M.; Elmore, J.G.; West, C.N.; Herrinton, L.J.; Harris, E.L.; Fletcher, S.W.; Emmons, K.M.; et al. Positive, negative, and disparate--women’s differing long-term psychosocial experiences of bilateral or contralateral prophylactic mastectomy. Breast J. 2008, 14, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Frost, M.H.; Hoskin, T.L.; Hartmann, L.C.; Degnim, A.C.; Johnson, J.L.; Boughey, J.C. Contralateral prophylactic mastectomy: Long-term consistency of satisfaction and adverse effects and the significance of informed decision-making, quality of life, and personality traits. Ann. Surg. Oncol. 2011, 18, 3110. [Google Scholar] [CrossRef]
- Elwyn, G.; Frosch, D.; Thomson, R.; Joseph-Williams, N.; Lloyd, A.; Kinnersley, P.; Cording, E.; Tomson, D.; Dodd, C.; Rollnick, S.; et al. Shared decision making: A model for clinical practice. J. Gen. Intern. Med. 2012, 27, 1361–1367. [Google Scholar] [CrossRef]
- Ridd, M.; Shaw, A.; Lewis, G.; Salisbury, C. The patient-doctor relationship: A synthesis of the qualitative literature on patients’ perspectives. Br. J. Gen. Pract. 2009, 59, e116–133. [Google Scholar] [CrossRef] [PubMed]
- Sacks, G.D.; Morrow, M. Addressing the Dilemma of Contralateral Prophylactic Mastectomy With Behavioral Science. J. Clin. Oncol. 2021. [Google Scholar] [CrossRef] [PubMed]
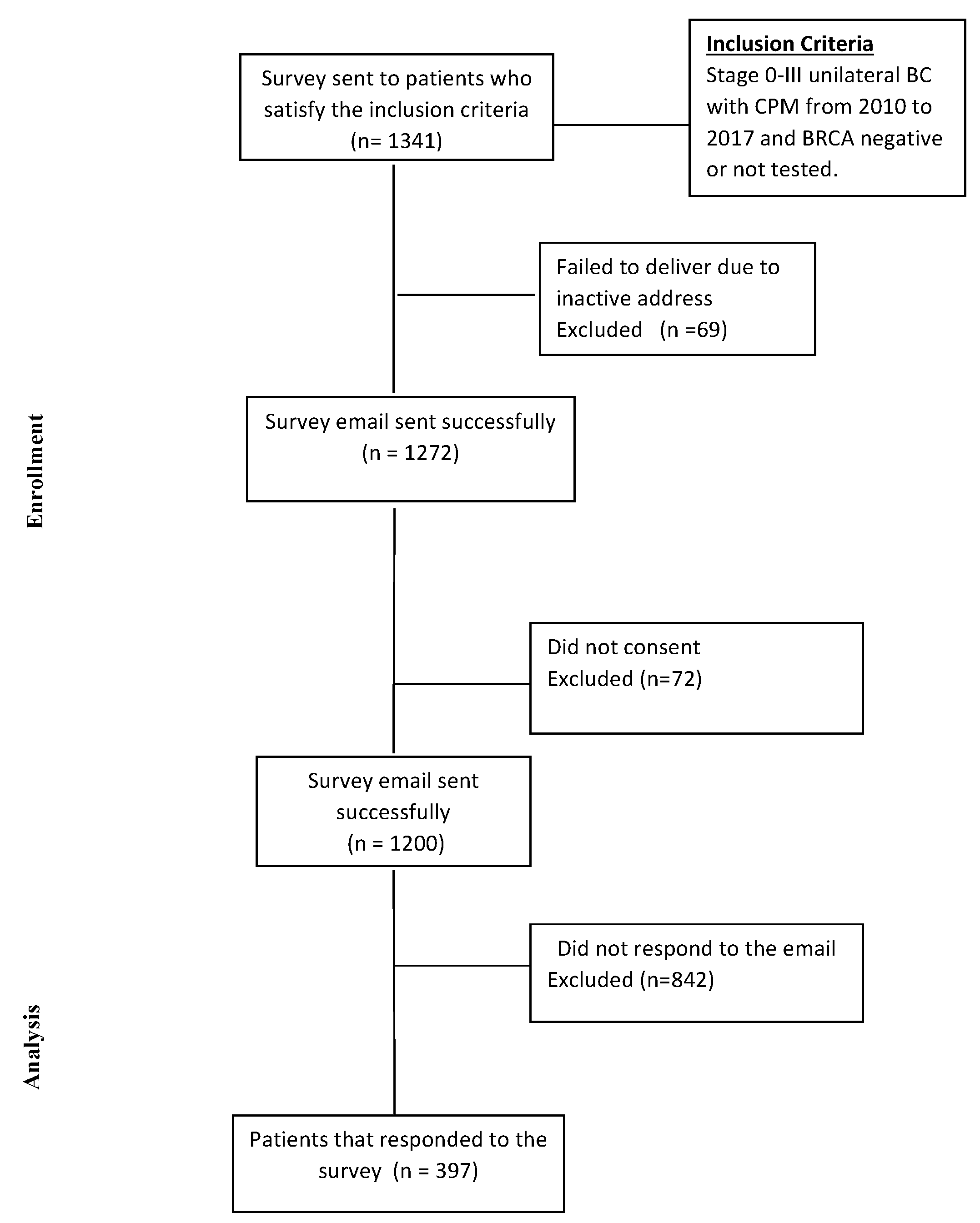
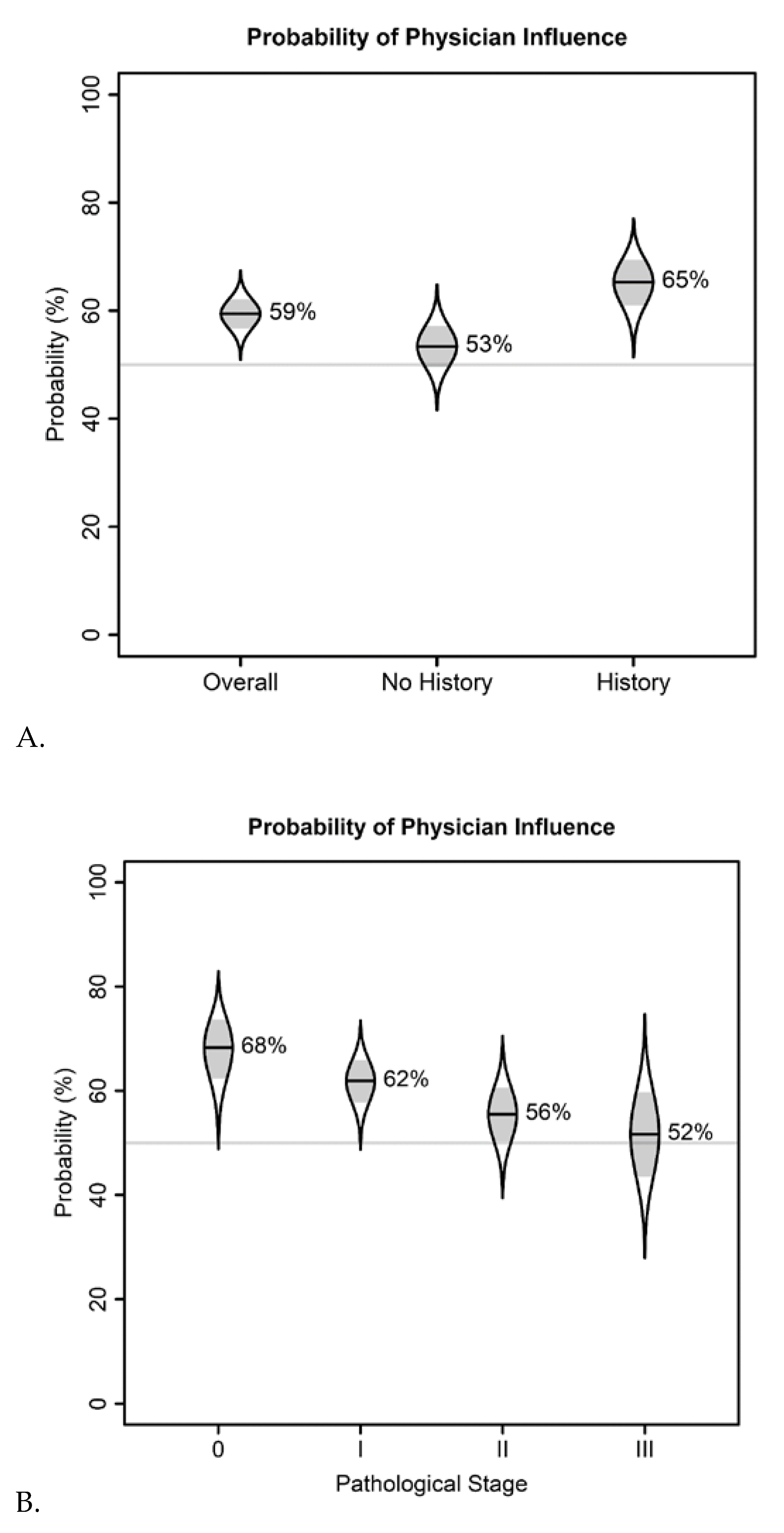
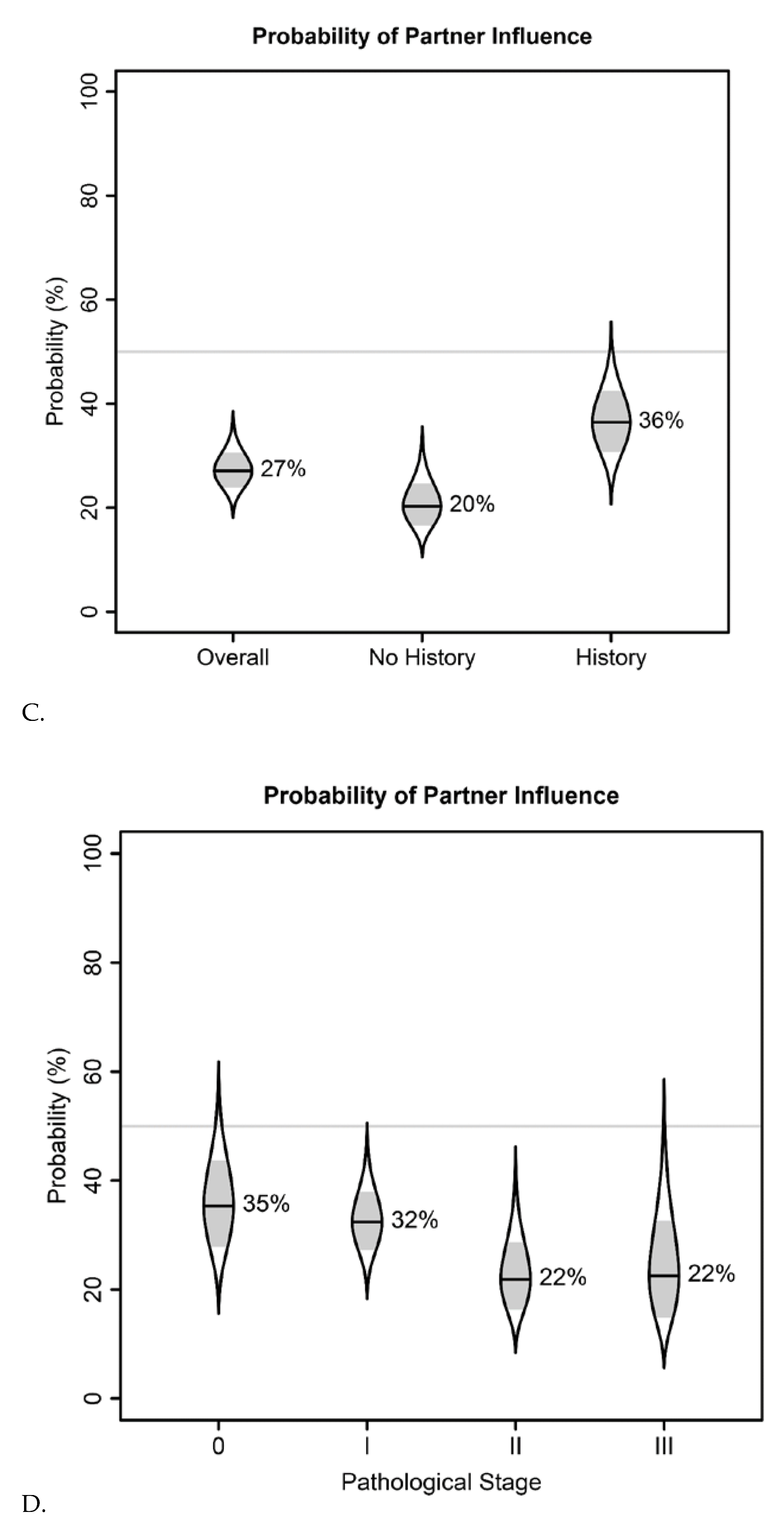

| Demographic | N (%) |
|---|---|
| Age at Diagnosis of Breast Cancer | |
| 20 to 30 | 16 (4) |
| 31 to 40 | 104 (26) |
| 41 to 50 | 169 (43) |
| 51 to 60 | 108 (27) |
| Race | |
| Asian or Pacific Islander | 14 (4) |
| Black or African American | 15 (4) |
| Hispanic/Latino | 35 (9) |
| Native American or Alaskan | 1 (0) |
| White or Caucasian | 328 (83) |
| Other | 4 (1) |
| Education Level | |
| Less than or some high school | 0 (0) |
| High school or general educational development | 29 (7) |
| Trade or technical school | 13 (3) |
| Junior college or some college | 64 (16) |
| College graduate | 132 (33) |
| Post-graduate work or degree | 120 (30) |
| No response | 39 (10) |
| Marital status | |
| Married | 305 (77) |
| Living together but not married | 12 (3) |
| Separated or divorced | 28 (7) |
| Widowed | 10 (3) |
| Never married | 18 (5) |
| No response | 24 (6) |
| Stage | |
| I | 152 (38) |
| II | 181 (46) |
| III | 64 (16) |
| Grade | |
| I | 28 (7) |
| II | 160 (40) |
| III | 209 (53) |
| Family History | |
| Reported | 170 (43) |
| Not reported | 227 (57) |
| Total participants responded to survey | 397(100) |
| Decision | N (%) | Physician | Partner | Media |
|---|---|---|---|---|
| I made the final decision to have surgery. | 201 (54) | X | X | X |
| I made the final decision to have surgery after seriously considering my doctor’s opinion. | 165 (44) | X | ||
| My doctor and I shared responsibility for the final decision to have surgery. | 60 (16) | X | ||
| My doctor made the final decision about my surgery, but seriously considered my opinion. | 2 (1) | X | ||
| My doctor made the final decision about my surgery. | 4 (1) | X | ||
| I made the final decision to have surgery after seriously considering my partner’s opinion. | 59 (16) | X | ||
| My partner made the final decision about my surgery. | 1 (0) | X | ||
| Media Influence: Please choose one number to indicate whether or not the media had influenced your decision making to undergo prophylactic mastectomy (count indicates any influence other than “Not at all”) | 37 (10) | X | ||
| Count with influence other than self (combines multiple questions) | 203 (59) | 53 (28) | 36 (17) | |
| Total | 373 | 343 | 189 | 213 |
| Physician Influence | Partner Influence | Media Influence | |
|---|---|---|---|
| Overall probability of influence on cpm decision (95% CI) | 59% (54–65%) | 27% (21–34%) | 16% (11–22%) |
| p = 0.0006 | p < 0.0001 | p < 0.0001 | |
| Odds of influence on CPM decision given family history of breast cancer (95% CI) | 1.64 (1.05–2.57) | 2.25 (1.17–4.34) | 1.23 (0.59–2.58) |
| p = 0.029 | p = 0.015 | p = 0.059 |
| Influencers | Influenced Decision to Undergo CPM | p-Value |
|---|---|---|
| Partners | 28% | <0.0001 |
| Physicians | 59% | 0.006 |
| Media | 17% | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singareeka Raghavendra, A.; Alameddine, H.F.; Andersen, C.R.; Selber, J.C.; Brewster, A.M.; Barcenas, C.H.; Caudle, A.S.; Arun, B.K.; Tripathy, D.; Ibrahim, N.K. Influencers of the Decision to Undergo Contralateral Prophylactic Mastectomy among Women with Unilateral Breast Cancer. Cancers 2021, 13, 2050. https://doi.org/10.3390/cancers13092050
Singareeka Raghavendra A, Alameddine HF, Andersen CR, Selber JC, Brewster AM, Barcenas CH, Caudle AS, Arun BK, Tripathy D, Ibrahim NK. Influencers of the Decision to Undergo Contralateral Prophylactic Mastectomy among Women with Unilateral Breast Cancer. Cancers. 2021; 13(9):2050. https://doi.org/10.3390/cancers13092050
Chicago/Turabian StyleSingareeka Raghavendra, Akshara, Hala F. Alameddine, Clark R. Andersen, Jesse C. Selber, Abenaa M. Brewster, Carlos H. Barcenas, Abigail S. Caudle, Banu K. Arun, Debu Tripathy, and Nuhad K. Ibrahim. 2021. "Influencers of the Decision to Undergo Contralateral Prophylactic Mastectomy among Women with Unilateral Breast Cancer" Cancers 13, no. 9: 2050. https://doi.org/10.3390/cancers13092050
APA StyleSingareeka Raghavendra, A., Alameddine, H. F., Andersen, C. R., Selber, J. C., Brewster, A. M., Barcenas, C. H., Caudle, A. S., Arun, B. K., Tripathy, D., & Ibrahim, N. K. (2021). Influencers of the Decision to Undergo Contralateral Prophylactic Mastectomy among Women with Unilateral Breast Cancer. Cancers, 13(9), 2050. https://doi.org/10.3390/cancers13092050






