pH-Responsive Lipid Nanocapsules: A Promising Strategy for Improved Resistant Melanoma Cell Internalization
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Formulation and Characterization of Nanoparticles
2.1.1. Blank Formulation of LNC (BLK)
2.1.2. Fluorescent LNC Formulation
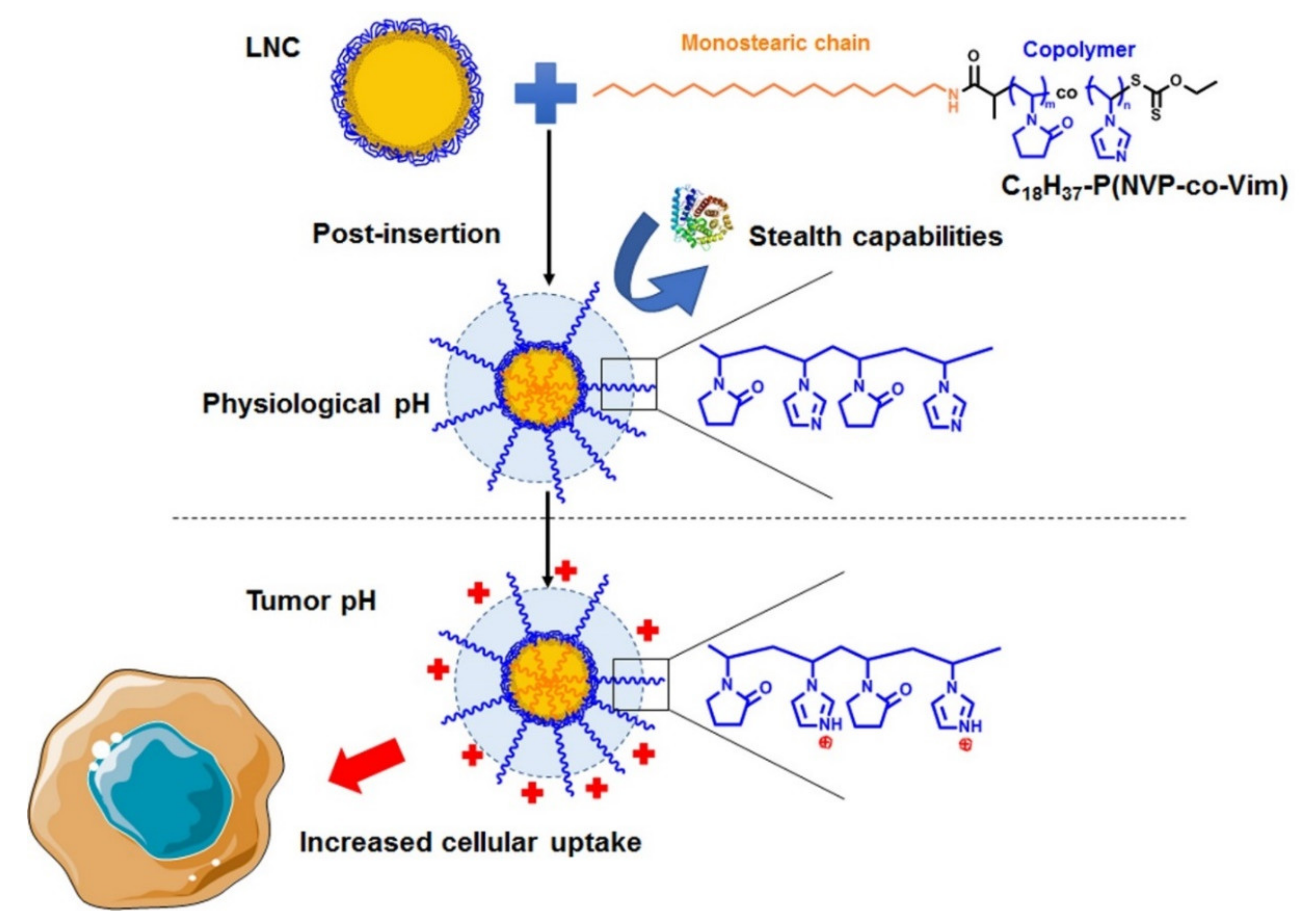
2.1.3. Polymer Post-Insertion
2.1.4. Size and Zeta Potential Measurements
2.1.5. Stability
2.1.6. Endosome Buffering Effect
2.2. Cell Culture
2.3. Resazurin Cell Viability Assay
2.4. Fluorescence-Activated Cell Sorting (FACS): Internalization
2.5. Fluorescence-Activated Cell Sorting (FACS): Internalization Pathway
2.6. Confocal Microscopy
2.7. Complement Activation
2.8. Statistical Analysis
3. Results
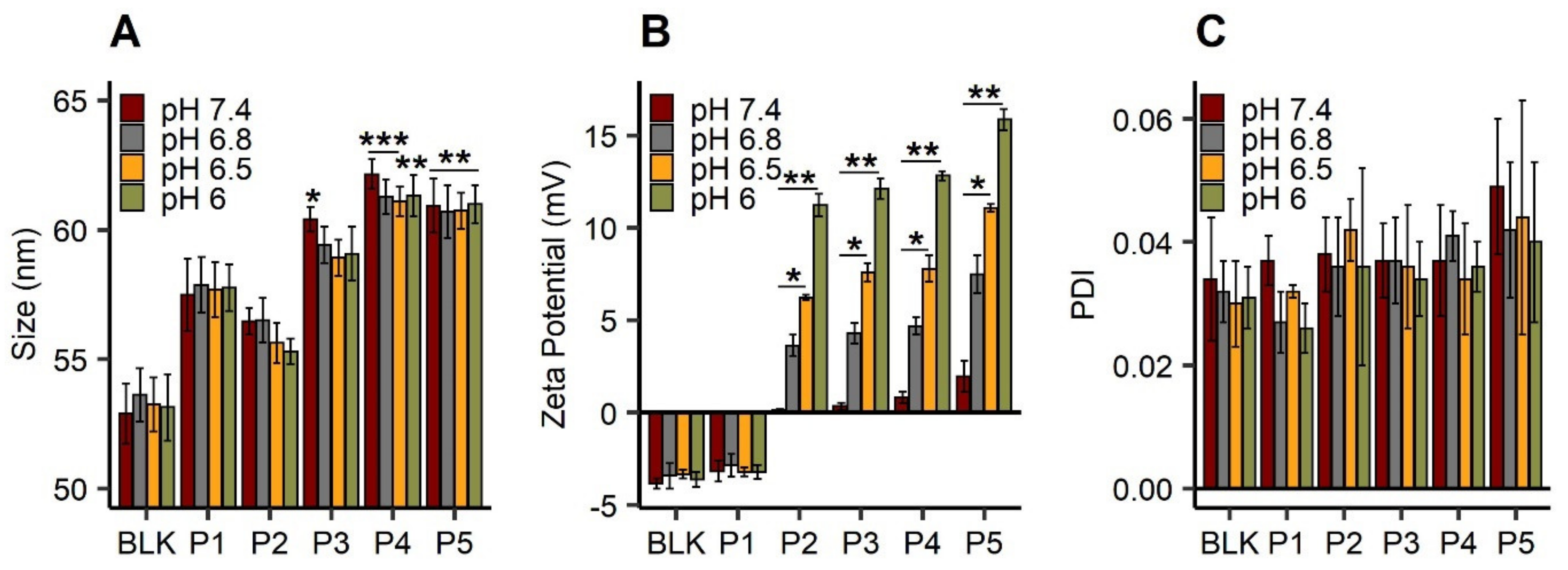
3.1. Polymer Post-Insertion and Switch Charge Capacities of Modified LNC
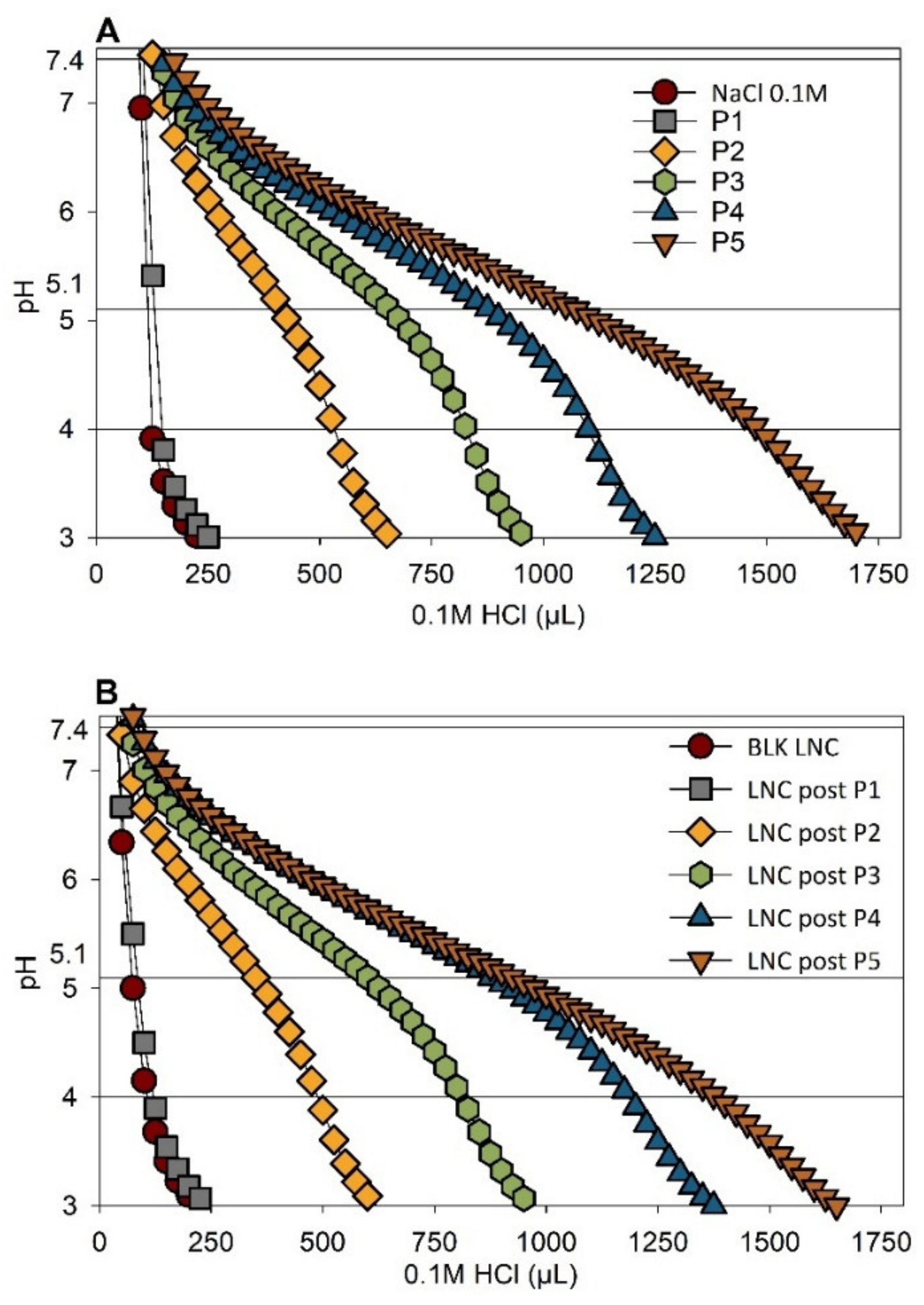
3.2. Buffering Effect of pH-Responsive LNC
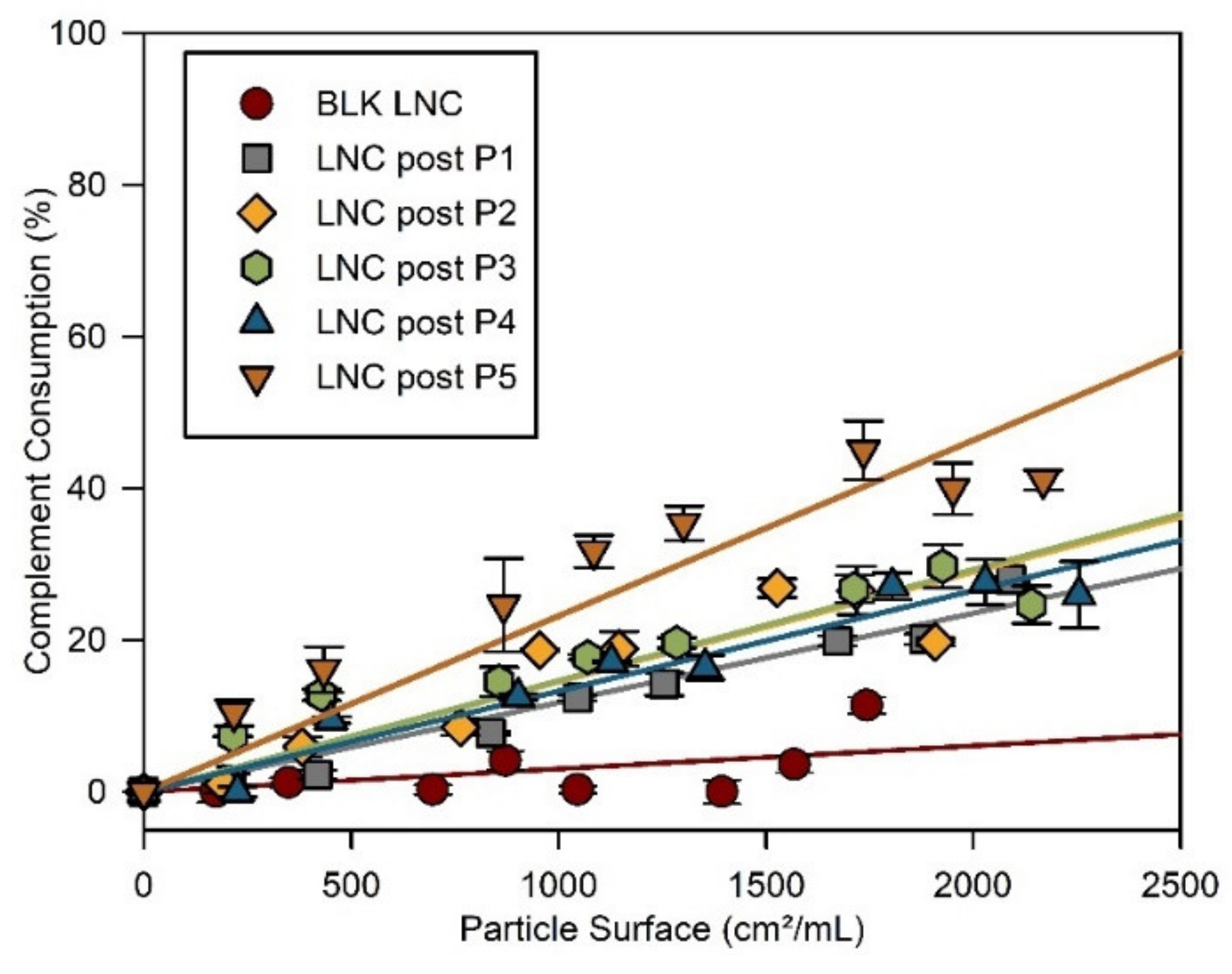
3.3. Stealth Properties of LNC
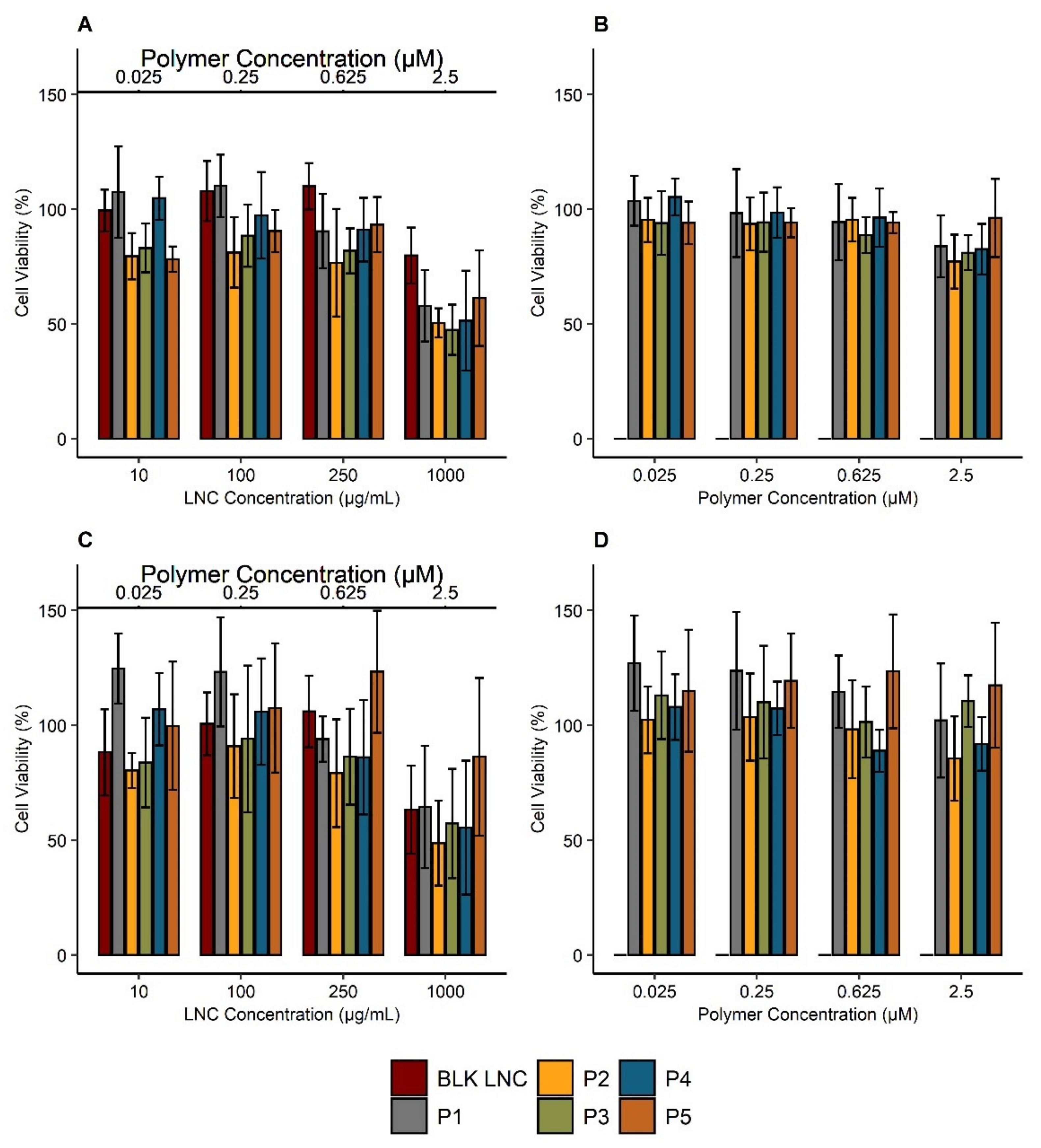
3.4. Impact of Polymers and Modified LNC on Cell Viability
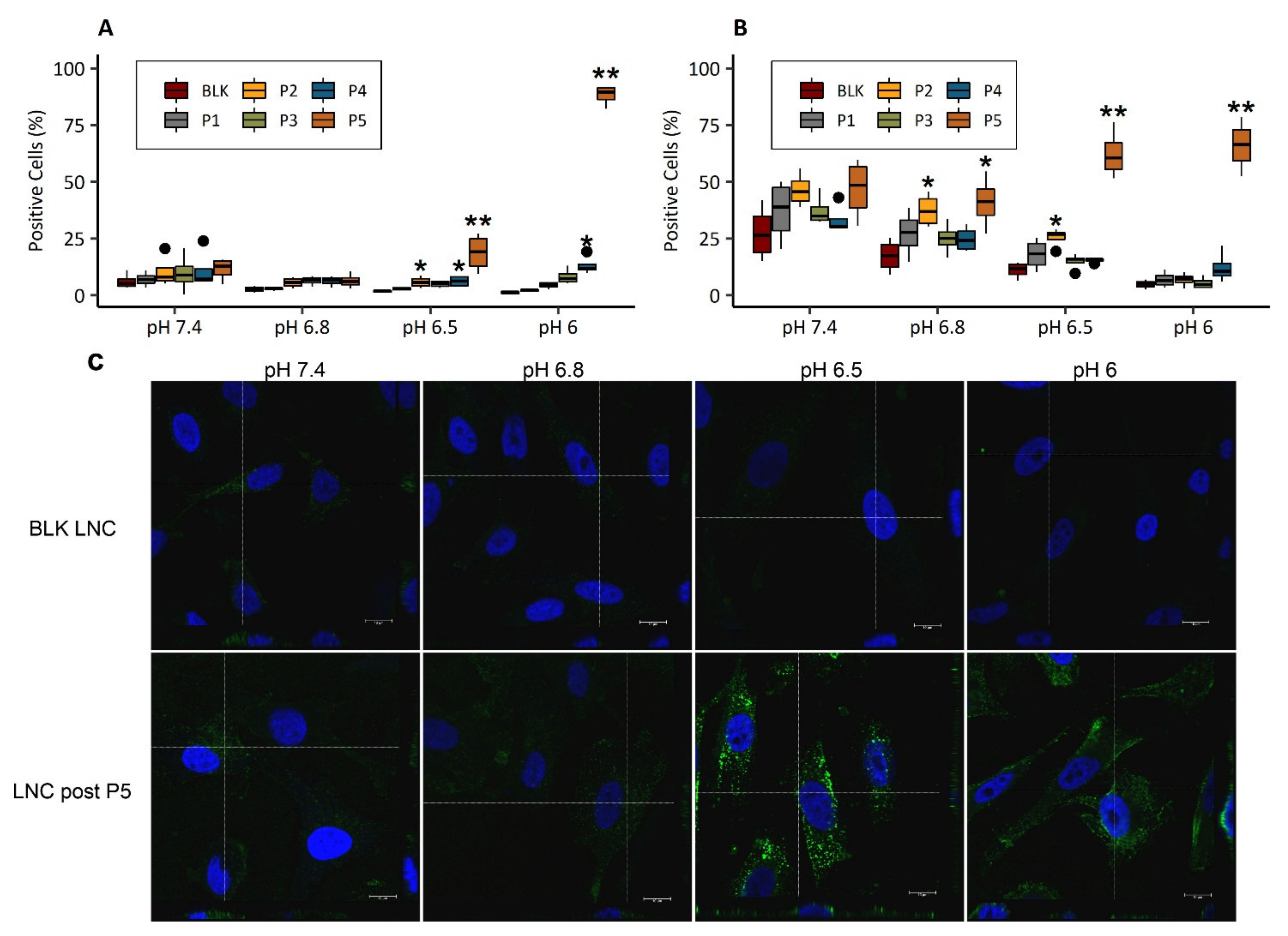
3.5. Cell Uptake of pH-Responsive LNC
3.5.1. pH-Dependent Cellular Uptake
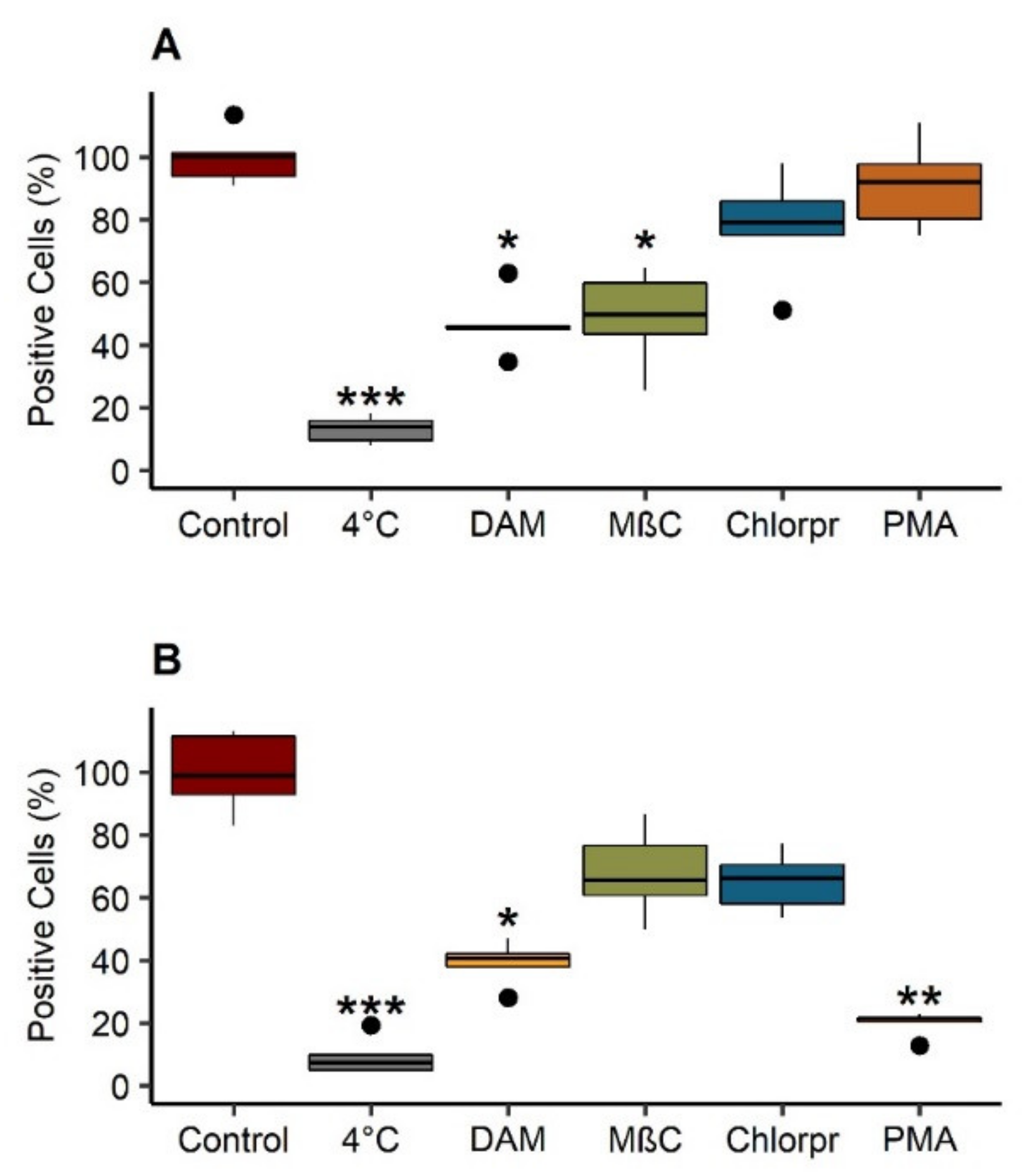
3.5.2. Internalization Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Larkin, J.; Hodi, F.S.; Wolchok, J.D. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 1270–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.S.; Tammam, S.N.; Shetab Boushehri, M.A.; Lamprecht, A. MDR in cancer: Addressing the underlying cellular alterations with the use of nanocarriers. Pharmacol. Res. 2017, 126, 2–30. [Google Scholar] [CrossRef]
- Pautu, V.; Mellinger, A.; Resnier, P.; Lepeltier, E.; Martin, L.; Boussemart, L.; Letournel, F.; Passirani, C.; Clere, N. Melanoma tumour vasculature heterogeneity: From mice models to human. J. Cancer Res. Clin. Oncol. 2019, 145, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Shain, A.H.; Bastian, B.C. From melanocytes to melanomas. Nat. Rev. Cancer 2016, 16, 345–358. [Google Scholar] [CrossRef]
- Erin, N.; Grahovac, J.; Brozovic, A.; Efferth, T. Tumor microenvironment and epithelial mesenchymal transition as targets to overcome tumor multidrug resistance. Drug Resist. Updates 2020, 53, 100715. [Google Scholar] [CrossRef]
- Assaraf, Y.G.; Brozovic, A.; Gonçalves, A.C.; Jurkovicova, D.; Linē, A.; Machuqueiro, M.; Saponara, S.; Sarmento-Ribeiro, A.B.; Xavier, C.P.R.; Vasconcelos, M.H. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist. Updates 2019, 46, 100645. [Google Scholar] [CrossRef]
- Ludwig, R.J.; Boehme, B.; Podda, M.; Henschler, R.; Jager, E.; Tandi, C.; Boehncke, W.-H.; Zollner, T.M.; Kaufmann, R.; Gille, J. Endothelial P-Selectin as a Target of Heparin Action in Experimental Melanoma Lung Metastasis. Cancer Res. 2004, 64, 2743–2750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasiliev, Y.M. Chitosan-based vaccine adjuvants: Incomplete characterization complicates preclinical and clinical evaluation. Expert Rev. Vaccines 2015, 14, 37–53. [Google Scholar] [CrossRef]
- Jaiswal, A.R.; Liu, A.J.; Pudakalakatti, S.; Dutta, P.; Jayaprakash, P.; Bartkowiak, T.; Ager, C.R.; Wang, Z.-Q.; Reuben, A.; Cooper, Z.A.; et al. Melanoma Evolves Complete Immunotherapy Resistance through the Acquisition of a Hypermetabolic Phenotype. Cancer Immunol. Res. 2020, 8, 1365–1380. [Google Scholar] [CrossRef]
- Simon, S.; Roy, D.; Schindler, M. Intracellular pH and the control of multidrug resistance. Proc. Natl. Acad. Sci. USA 1994, 91, 1128–1132. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.-Y.; Xu, Y.-M.; Lau, A.T.Y. Recent Progress of Nanocarrier-Based Therapy for Solid Malignancies. Cancers 2020, 12, 2783. [Google Scholar] [CrossRef] [PubMed]
- Lepeltier, E.; Rijo, P.; Rizzolio, F.; Popovtzer, R.; Petrikaite, V.; Assaraf, Y.G.; Passirani, C. Nanomedicine to target multidrug resistant tumors. Drug Resist. Updates 2020, 52, 100704. [Google Scholar] [CrossRef]
- Heurtault, B.; Saulnier, P.; Pech, B.; Proust, J.E.; Benoit, J.P. A novel phase inversion-based process for the preparation of lipid nanocarriers. Pharm. Res. 2002, 19, 875–880. [Google Scholar] [CrossRef]
- Garcion, E.; Lamprecht, A.; Heurtault, B.; Paillard, A.; Aubert-Pouessel, A.; Denizot, B.; Menei, P.; Benoît, J.-P. A new generation of anticancer, drug-loaded, colloidal vectors reverses multidrug resistance in glioma and reduces tumor progression in rats. Mol. Cancer Ther. 2006, 5, 1710–1722. [Google Scholar] [CrossRef] [Green Version]
- Lamprecht, A.; Benoit, J.-P. Etoposide nanocarriers suppress glioma cell growth by intracellular drug delivery and simultaneous P-glycoprotein inhibition. J. Control. Release 2006, 112, 208–213. [Google Scholar] [CrossRef]
- Fathy Abd-Ellatef, G.-E.; Gazzano, E.; Chirio, D.; Ragab Hamed, A.; Belisario, D.C.; Zuddas, C.; Peira, E.; Rolando, B.; Kopecka, J.; Assem Said Marie, M.; et al. Curcumin-Loaded Solid Lipid Nanoparticles Bypass P-Glycoprotein Mediated Doxorubicin Resistance in Triple Negative Breast Cancer Cells. Pharmaceutics 2020, 12, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushik, L.; Srivastava, S.; Panjeta, A.; Chaudhari, D.; Ghadi, R.; Kuche, K.; Malik, R.; Preet, S.; Jain, S.; Raza, K. Exploration of docetaxel palmitate and its solid lipid nanoparticles as a novel option for alleviating the rising concern of multi-drug resistance. Int. J. Pharm. 2020, 578, 119088. [Google Scholar] [CrossRef] [PubMed]
- Allard, E.; Passirani, C.; Garcion, E.; Pigeon, P.; Vessières, A.; Jaouen, G.; Benoit, J.P. Lipid nanocapsules loaded with an organometallic tamoxifen derivative as a novel drug-carrier system for experimental malignant gliomas. J. Control. Release 2008, 130, 146–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allard, E.; Huynh, N.T.; Vessières, A.; Pigeon, P.; Jaouen, G.; Benoit, J.P.; Passirani, C. Dose effect activity of ferrocifen-loaded lipid nanocapsules on a 9L-glioma model. Int. J. Pharm. 2009, 379, 317–323. [Google Scholar] [CrossRef]
- Allard, E.; Jarnet, D.; Vessières, A.; Vinchon-Petit, S.; Jaouen, G.; Benoit, J.P.; Passirani, C. Local delivery of ferrociphenol lipid nanocapsules followed by external radiotherapy as a synergistic treatment against intracranial 9L glioma xenograft. Pharm. Res. 2010, 27, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.T.; Morille, M.; Bejaud, J.; Legras, P.; Vessieres, A.; Jaouen, G.; Benoit, J.-P.; Passirani, C. Treatment of 9L Gliosarcoma in Rats by Ferrociphenol-Loaded Lipid Nanocapsules Based on a Passive Targeting Strategy via the EPR Effect. Pharm. Res. 2011, 28, 3189–3198. [Google Scholar] [CrossRef] [Green Version]
- Huynh, N.T.; Passirani, C.; Allard-Vannier, E.; Lemaire, L.; Roux, J.; Garcion, E.; Vessieres, A.; Benoit, J.P. Administration-dependent efficacy of ferrociphenol lipid nanocapsules for the treatment of intracranial 9L rat gliosarcoma. Int. J. Pharm. 2012, 423, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Laine, A.L.; Huynh, N.T.; Clavreul, A.; Balzeau, J.; Béjaud, J.; Vessieres, A.; Benoit, J.P.; Eyer, J.; Passirani, C. Brain tumour targeting strategies via coated ferrociphenol lipid nanocapsules. Eur. J. Pharm. Biopharm. 2012, 81, 690–693. [Google Scholar] [CrossRef] [Green Version]
- Lainé, A.L.; Adriaenssens, E.; Vessières, A.; Jaouen, G.; Corbet, C.; Desruelles, E.; Pigeon, P.; Toillon, R.A.; Passirani, C. The in vivo performance of ferrocenyl tamoxifen lipid nanocapsules in xenografted triple negative breast cancer. Biomaterials 2013, 34, 6949–6956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lainé, A.L.; Clavreul, A.; Rousseau, A.; Tétaud, C.; Vessieres, A.; Garcion, E.; Jaouen, G.; Aubert, L.; Guilbert, M.; Benoit, J.P.; et al. Inhibition of ectopic glioma tumor growth by a potent ferrocenyl drug loaded into stealth lipid nanocapsules. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Guan, S.; Gan, Z.; Zhang, G.; Yu, Q. Polymeric Micelles with Uniform Surface Properties and Tunable Size and Charge: Positive Charges Improve Tumor Accumulation. Biomacromolecules 2016, 17, 1801–1810. [Google Scholar] [CrossRef]
- Miller, C.R.; Bondurant, B.; McLean, S.D.; McGovern, K.A.; O’Brien, D.F. Liposome-cell interactions in vitro: Effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomes. Biochemistry 1998, 37, 12875–12883. [Google Scholar] [CrossRef]
- Corbo, C.; Molinaro, R.; Taraballi, F.; Toledano Furman, N.E.; Sherman, M.B.; Parodi, A.; Salvatore, F.; Tasciotti, E. Effects of the protein corona on liposome-liposome and liposome-cell interactions. Int. J. Nanomed. 2016, 11, 3049–3063. [Google Scholar] [CrossRef] [Green Version]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Priya James, H.; John, R.; Alex, A.; Anoop, K.R. Smart polymers for the controlled delivery of drugs-a concise overview. Acta Pharm. Sin. B 2014, 4, 120–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Fujita, Y.; Onishi, N.; Ogawara, K.-i.; Nakayama, H.; Mukai, T. Preparation and characterization of lipid emulsions containing styrene maleic acid copolymer for the development of pH-responsive drug carriers. Chem. Phys. Lipids 2020, 232, 104954. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.L.; Chen, Y.C.; Ou, T.W.; Chen, H.H.; Tsai, H.C.; Wen, C.J.; Lo, C.L.; Wey, S.P.; Lin, K.J.; Yen, T.C.; et al. Multifunctional hollow nanoparticles based on graft-diblock copolymers for doxorubicin delivery. Biomaterials 2011, 32, 2213–2221. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Lagunes, E.; Ruiz, J.C.; Varca, G.H.C.; Bucio, E. Synthesis and characterization of stimuli-responsive polypropylene containing N-vinylcaprolactam and N-vinylimidazole obtained by ionizing radiation. Mater. Sci. Eng. 2016, 67, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, Y.; Tsutsumi, Y.; Yoshioka, Y.; Kamada, H.; Yamamoto, Y.; Kodaira, H.; Tsunoda, S.; Okamoto, T.; Mukai, Y.; Shibata, H.; et al. The use of PVP as a polymeric carrier to improve the plasma half-life of drugs. Biomaterials 2004, 25, 3259–3266. [Google Scholar] [CrossRef]
- Ting, J.M.; Navale, T.S.; Bates, F.S.; Reineke, T.M. Precise Compositional Control and Systematic Preparation of Multimonomeric Statistical Copolymers. ACS Macro Lett. 2013, 2, 770–774. [Google Scholar] [CrossRef]
- Zhong, Z.; Feijen, J.; Lok, M.C.; Hennink, W.E.; Christensen, L.V.; Yockman, J.W.; Kim, Y.H.; Kim, S.W. Low molecular weight linear polyethylenimine-b-poly(ethylene glycol)-b-polyethylenimine triblock copolymers: Synthesis, characterization, and in vitro gene transfer properties. Biomacromolecules 2005, 6, 3440–3448. [Google Scholar] [CrossRef]
- Duchardt, F.; Fotin-Mleczek, M.; Schwarz, H.; Fischer, R.; Brock, R. A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic 2007, 8, 848–866. [Google Scholar] [CrossRef]
- Anderson, H.A.; Chen, Y.; Norkin, L.C. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell 1996, 7, 1825–1834. [Google Scholar] [CrossRef] [Green Version]
- Patino, T.; Soriano, J.; Barrios, L.; Ibanez, E.; Nogues, C. Surface modification of microparticles causes differential uptake responses in normal and tumoral human breast epithelial cells. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Passirani, C.; Barratt, G.; Devissaguet, J.P.; Labarre, D. Interactions of nanoparticles bearing heparin or dextran covalently bound to poly(methyl methacrylate) with the complement system. Life Sci. 1998, 62, 775–785. [Google Scholar] [CrossRef]
- Pohlert, T. The Pairwise Multiple Comparison of Mean Ranks Package (PMCMR). Available online: https://CRAN.R-project.org/package=PMCMR (accessed on 1 December 2018).
- Vonarbourg, A.; Passirani, C.; Saulnier, P.; Simard, P.; Leroux, J.C.; Benoit, J.P. Evaluation of pegylated lipid nanocapsules versus complement system activation and macrophage uptake. J. Biomed. Mater. Res. Part A 2006, 78, 620–628. [Google Scholar] [CrossRef]
- Koivusalo, M.; Welch, C.; Hayashi, H.; Scott, C.C.; Kim, M.; Alexander, T.; Touret, N.; Hahn, K.M.; Grinstein, S. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol. 2010, 188, 547–563. [Google Scholar] [CrossRef] [Green Version]
- Rodal, S.K.; Skretting, G.; Garred, O.; Vilhardt, F.; van Deurs, B.; Sandvig, K. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell 1999, 10, 961–974. [Google Scholar] [CrossRef]
- Grimmer, S.; van Deurs, B.; Sandvig, K. Membrane ruffling and macropinocytosis in A431 cells require cholesterol. J. Cell Sci. 2002, 115, 2953–2962. [Google Scholar] [PubMed]
- Gratton, S.E.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laine, A.L.; Gravier, J.; Henry, M.; Sancey, L.; Bejaud, J.; Pancani, E.; Wiber, M.; Texier, I.; Coll, J.L.; Benoit, J.P.; et al. Conventional versus stealth lipid nanoparticles: Formulation and in vivo fate prediction through FRET monitoring. J. Control. Release 2014, 188, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Velasco, D.; Rethore, G.; Newland, B.; Parra, J.; Elvira, C.; Pandit, A.; Rojo, L.; San Roman, J. Low polydispersity (N-ethyl pyrrolidine methacrylamide-co-1-vinylimidazole) linear oligomers for gene therapy applications. Eur. J. Pharm. Biopharm. 2012, 82, 465–474. [Google Scholar] [CrossRef]
- Tarley, C.R.T.; Corazza, M.Z.; Somera, B.F.; Segatelli, M.G. Preparation of new ion-selective cross-linked poly(vinylimidazole-co-ethylene glycol dimethacrylate) using a double-imprinting process for the preconcentration of Pb2+ ions. J. Colloid Interface Sci. 2015, 450, 254–263. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Sheth, V.R.; Pagel, M.D. Imaging in vivo extracellular pH with a single paramagnetic chemical exchange saturation transfer magnetic resonance imaging contrast agent. Mol. Imaging 2012, 11, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Boussif, O.; Lezoualc’h, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asayama, S.; Nishinohara, S.; Kawakami, H. Zinc-chelated poly(1-vinylimidazole) and a carbohydrate ligand polycation form DNA ternary complexes for gene delivery. Bioconjug. Chem. 2011, 22, 1864–1868. [Google Scholar] [CrossRef] [PubMed]
- Hakamatani, T.; Asayama, S.; Kawakami, H. Synthesis of alkylated poly(1-vinylimidazole) for a new pH-sensitive DNA carrier. Nucleic Acids Symp. Ser. 2008, 52, 677–678. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Resnier, P.; Guillot, A.; Pitard, B.; Benoit, J.P.; Passirani, C. siRNA LNCs—A novel platform of lipid nanocapsules for systemic siRNA administration. Eur. J. Pharm. Biopharm. 2012, 81, 448–452. [Google Scholar] [CrossRef] [Green Version]
- Topin-Ruiz, S.; Mellinger, A.; Lepeltier, E.; Bourreau, C.; Fouillet, J.; Riou, J.; Jaouen, G.; Martin, L.; Passirani, C.; Clere, N. p722 ferrocifen loaded lipid nanocapsules improve survival of murine xenografted-melanoma via a potentiation of apoptosis and an activation of CD8+ T lymphocytes. Int. J. Pharm. 2021, 593, 120111. [Google Scholar] [CrossRef]
- Kierstead, P.H.; Okochi, H.; Venditto, V.J.; Chuong, T.C.; Kivimae, S.; Frechet, J.M.J.; Szoka, F.C. The effect of polymer backbone chemistry on the induction of the accelerated blood clearance in polymer modified liposomes. J. Control. Release 2015, 213, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, T.; Maeda, T.; Sakamoto, H.; Takasaki, N.; Shigyo, M.; Ishida, T.; Kiwada, H.; Mizushima, Y.; Mizushima, T. Evasion of the accelerated blood clearance phenomenon by coating of nanoparticles with various hydrophilic polymers. Biomacromolecules 2010, 11, 2700–2706. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.; Vadon, M.; Rinner, B.; Novak, A.; Wintersteiger, R.; Frohlich, E. The role of nanoparticle size in hemocompatibility. Toxicology 2009, 258, 139–147. [Google Scholar] [CrossRef]
- Kuskov, A.N.; Kulikov, P.P.; Shtilman, M.I.; Rakitskii, V.N.; Tsatsakis, A.M. Amphiphilic poly-N-vynilpyrrolidone nanoparticles: Cytotoxicity and acute toxicity study. Food Chem. Toxicol. 2016, 96, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Gundel, D.; Allmeroth, M.; Reime, S.; Zentel, R.; Thews, O. Endocytotic uptake of HPMA-based polymers by different cancer cells: Impact of extracellular acidosis and hypoxia. Int. J. Nanomed. 2017, 12, 5571–5584. [Google Scholar] [CrossRef] [Green Version]
- Resnier, P.; Galopin, N.; Sibiril, Y.; Clavreul, A.; Cayon, J.; Briganti, A.; Legras, P.; Vessières, A.; Montier, T.; Jaouen, G.; et al. Efficient ferrocifen anticancer drug and Bcl-2 gene therapy using lipid nanocapsules on human melanoma xenograft in mouse. Pharmacol. Res. 2017, 126, 54–65. [Google Scholar] [CrossRef]
- Karim, R.; Lepeltier, E.; Esnault, L.; Pigeon, P.; Lemaire, L.; Lépinoux-Chambaud, C.; Clere, N.; Jaouen, G.; Eyer, J.; Piel, G.; et al. Enhanced and preferential internalization of lipid nanocapsules into human glioblastoma cells: Effect of a surface-functionalizing NFL peptide. Nanoscale 2018, 10, 13485–13501. [Google Scholar] [CrossRef] [PubMed]
- Resnier, P.; Lepeltier, E.; Emina, A.L.; Galopin, N.; Bejaud, J.; David, S.; Ballet, C.; Benvegnu, T.; Pecorari, F.; Chourpa, I.; et al. Model Affitin and PEG modifications onto siRNA lipid nanocapsules: Cell uptake and in vivo biodistribution improvements. RSC Adv. 2019, 9, 27264–27278. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Jacobs, T.M.; McCallen, J.D.; Moore, D.T.; Huckaby, J.T.; Edelstein, J.N.; Lai, S.K. Analysis of Pre-existing IgG and IgM Antibodies against Polyethylene Glycol (PEG) in the General Population. Anal. Chem. 2016, 88, 11804–11812. [Google Scholar] [CrossRef]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Zaguilan, R.; Seftor, E.A.; Seftor, R.E.; Chu, Y.W.; Gillies, R.J.; Hendrix, M.J. Acidic pH enhances the invasive behavior of human melanoma cells. Clin. Exp. Metastasis 1996, 14, 176–186. [Google Scholar] [CrossRef] [PubMed]
| Entry | Copolymer Composition | DP | Mn (g·mol−1) |
|---|---|---|---|
| P1 | C18H37-PNVP49 | 49 | 5900 |
| P2 | C18H37-P(NVP15-co-Vim5) | 20 | 2600 |
| P3 | C18H37-P(NVP22-co-Vim8) | 30 | 3600 |
| P4 | C18H37-P(NVP35-co-Vim10) | 45 | 5300 |
| P5 | C18H37-P(NVP21-co-Vim15) | 36 | 4200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pautu, V.; Lepeltier, E.; Mellinger, A.; Riou, J.; Debuigne, A.; Jérôme, C.; Clere, N.; Passirani, C. pH-Responsive Lipid Nanocapsules: A Promising Strategy for Improved Resistant Melanoma Cell Internalization. Cancers 2021, 13, 2028. https://doi.org/10.3390/cancers13092028
Pautu V, Lepeltier E, Mellinger A, Riou J, Debuigne A, Jérôme C, Clere N, Passirani C. pH-Responsive Lipid Nanocapsules: A Promising Strategy for Improved Resistant Melanoma Cell Internalization. Cancers. 2021; 13(9):2028. https://doi.org/10.3390/cancers13092028
Chicago/Turabian StylePautu, Vincent, Elise Lepeltier, Adélie Mellinger, Jérémie Riou, Antoine Debuigne, Christine Jérôme, Nicolas Clere, and Catherine Passirani. 2021. "pH-Responsive Lipid Nanocapsules: A Promising Strategy for Improved Resistant Melanoma Cell Internalization" Cancers 13, no. 9: 2028. https://doi.org/10.3390/cancers13092028
APA StylePautu, V., Lepeltier, E., Mellinger, A., Riou, J., Debuigne, A., Jérôme, C., Clere, N., & Passirani, C. (2021). pH-Responsive Lipid Nanocapsules: A Promising Strategy for Improved Resistant Melanoma Cell Internalization. Cancers, 13(9), 2028. https://doi.org/10.3390/cancers13092028







