Percutaneous Irreversible Electroporation for Treatment of Small Hepatocellular Carcinoma Invisible on Unenhanced CT: A Novel Combined Strategy with Prior Transarterial Tumor Marking
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
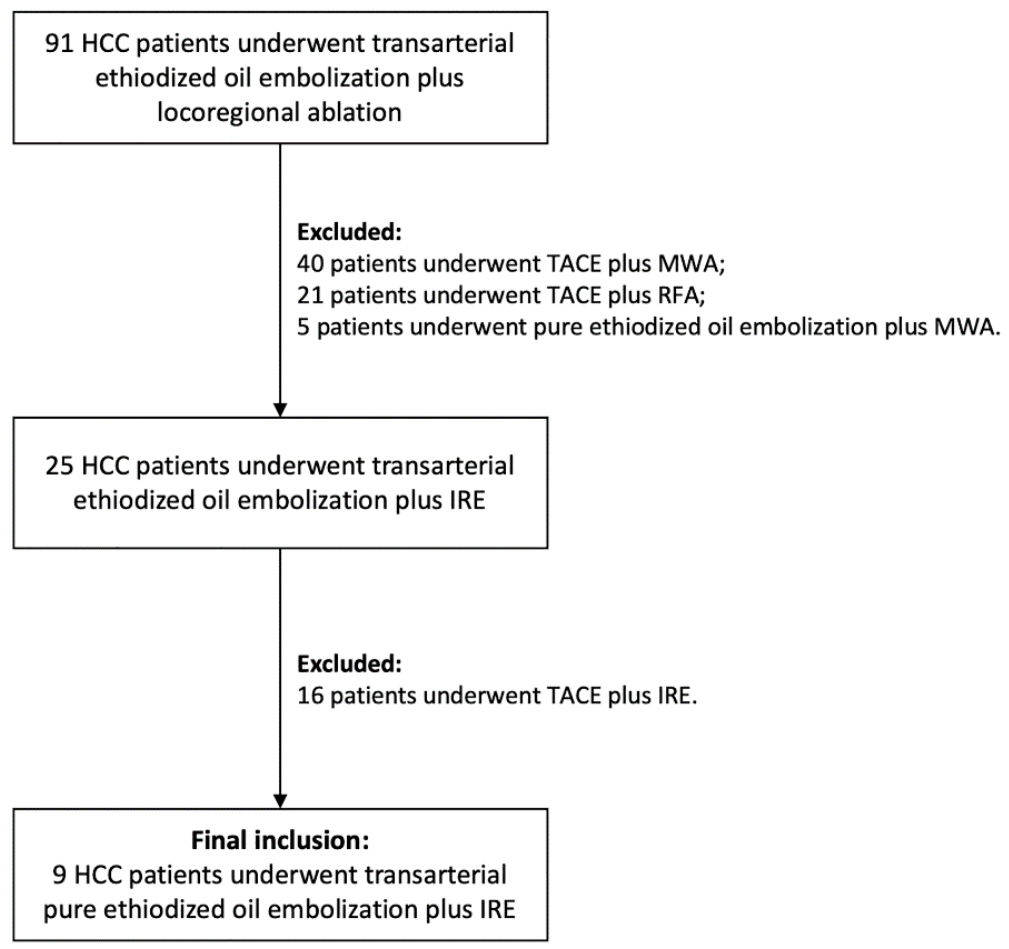
2.1. Treatment Strategy and Inclusion Criteria
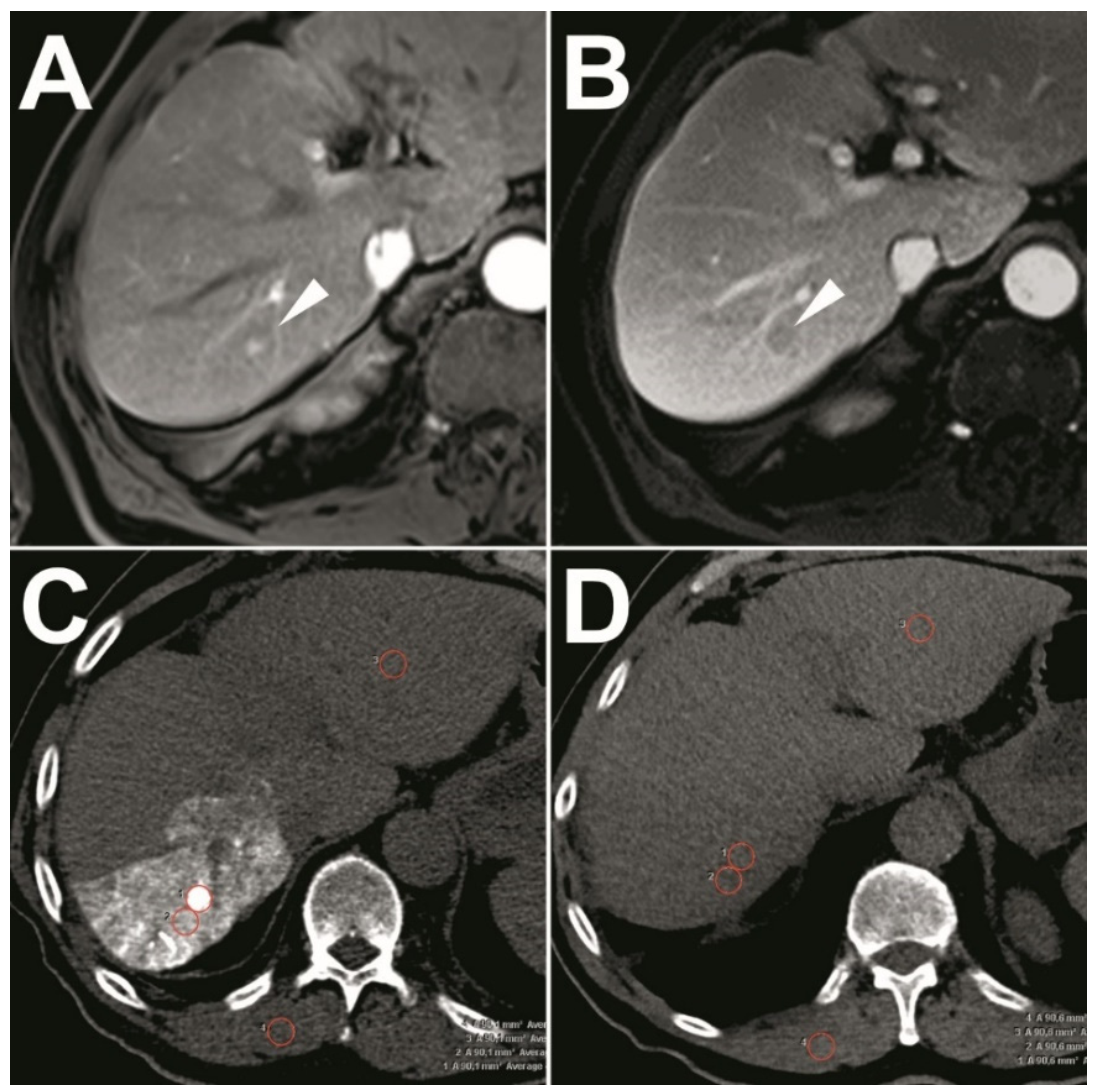
2.2. Pre-Interventional Imaging
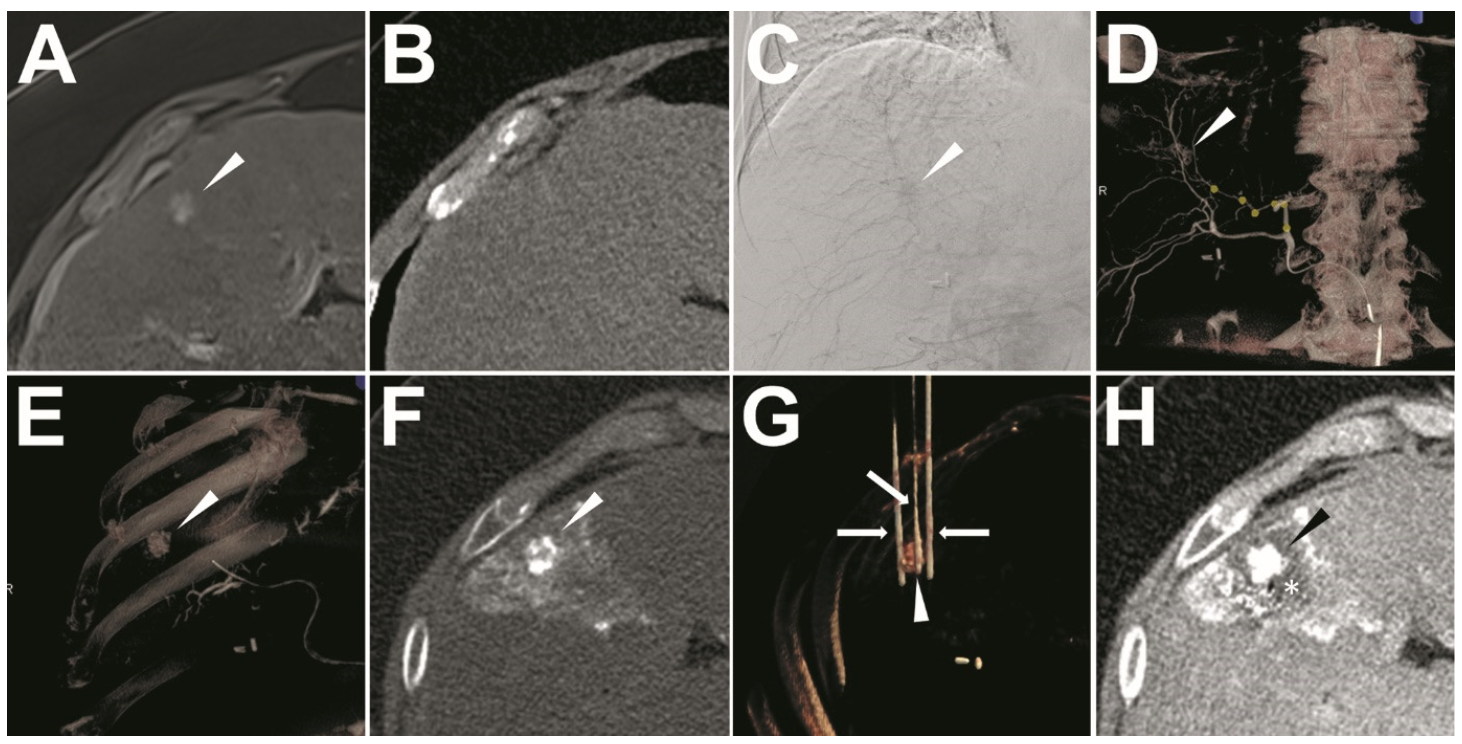
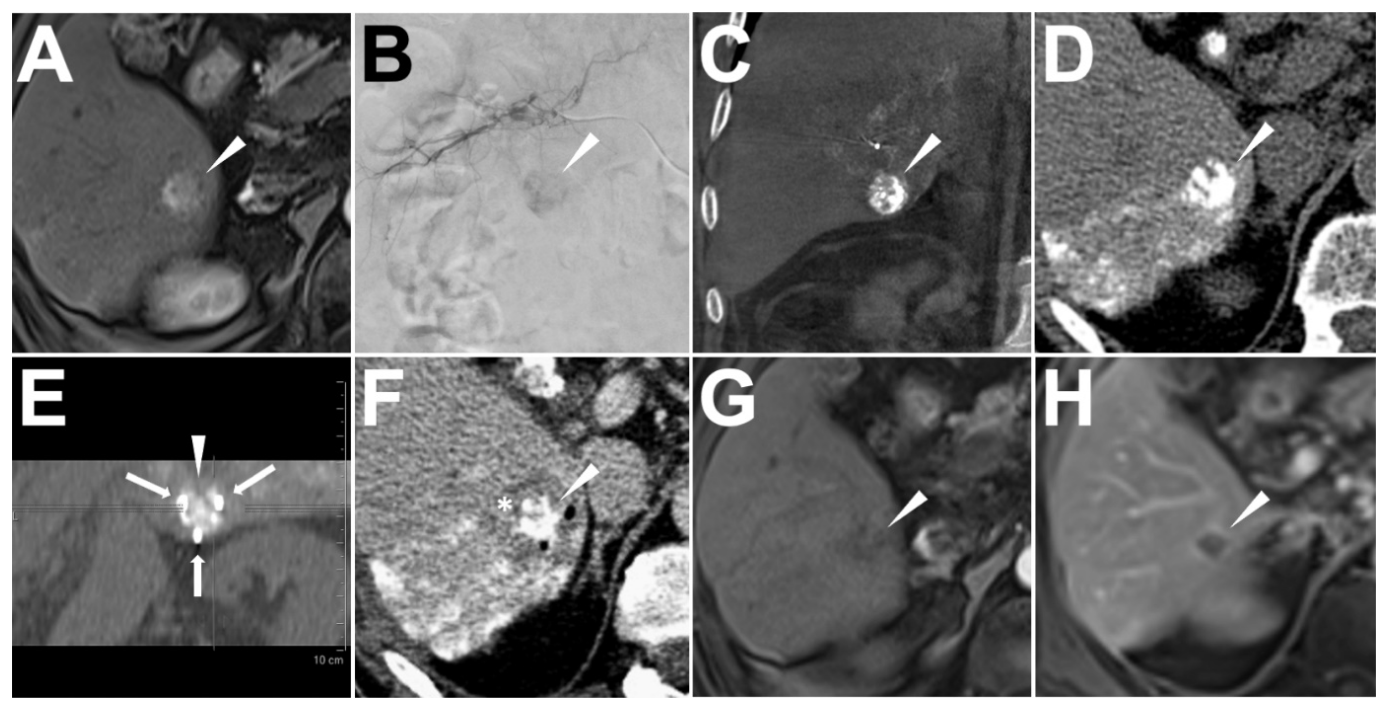
2.3. Ethiodized Oil Tumor Marking Procedure
2.4. IRE Treatment
2.5. Radiological Follow-Up
2.6. Study Endpoints
2.6.1. Success of Ethiodized Oil Tumor Marking
2.6.2. Quantitative Analysis of the Visualization of Target-HCCs
- SNR = CTtarget/SDnoise, in which CTtarget referred to the mean Hounsfield scales of the estimated tissue, and the SDnoise was the standard deviation for Hounsfield scales of the paraspinal muscles [28];
- CNR = ∣(CTHCC − CTliver)∣/SDnoise, in which CTHCC referred to the Hounsfield scale of the target-HCC, CTliver, and SDnoise referred to the mean and standard deviation of Hounsfield scale of the surrounding normal liver tissue [29].
2.6.3. Success of IRE
2.6.4. Adverse Events and Follow-Up
2.7. Statistics
3. Results
3.1. Patient Collective and Target-HCC Characteristics
3.2. Success of Ethiodized Oil Tumor Marking
3.3. Quantitative Analysis of the Visualization of Target-HCCs
3.4. Success of IRE
3.5. Adverse Events and Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| IRE | Irreversible electroporation |
| HCC | Hepatocellular carcinoma |
| SNR | Signal-to-noise ratio |
| CNR | Contrast-to-noise ratio |
| RFA | Radiofrequency ablation |
| TACE | Transarterial chemoembolization |
| MRI | Magnetic resonance imaging |
| LI-RADS 2018 | Liver Imaging Reporting and Data System 2018 |
| APHE | Arterial phase hyperenhancement |
| ΔA burst | Delta ampere in IRE burst |
| ΔA% burst | Delta percentage in IRE burst |
References
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Kobayashi, S.; Ye, X.; Meng, X. Comparison of Hepatic Resection and Radiofrequency Ablation for Small Hepatocellular Carcinoma: A Meta-Analysis of 16,103 Patients. Sci. Rep. 2015, 4, 7252. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Rhim, H. Thermal ablation for hepatocellular carcinoma: What’s new in 2019. Chin. Clin. Oncol. 2019, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.; Grand, D.; Charpentier, K.P. Irreversible electroporation of hepatocellular carcinoma: Patient selection and perspectives. J. Hepatocell. Carcinoma 2017, 4, 49–58. [Google Scholar] [CrossRef]
- Narayanan, G.; Froud, T.; Suthar, R.; Barbery, K. Irreversible Electroporation of Hepatic Malignancy. Semin. Interv. Radiol. 2013, 30, 067–073. [Google Scholar] [CrossRef]
- Vogel, J.A.; Van Veldhuisen, E.; Agnass, P.; Crezee, J.; Dijk, F.; Verheij, J.; Van Gulik, T.M.; Meijerink, M.R.; Vroomen, L.G.; Van Lienden, K.P.; et al. Time-Dependent Impact of Irreversible Electroporation on Pancreas, Liver, Blood Vessels and Nerves: A Systematic Review of Experimental Studies. PLoS ONE 2016, 11, e0166987. [Google Scholar] [CrossRef]
- Jiang, C.; Davalos, R.V.; Bischof, J.C. A Review of Basic to Clinical Studies of Irreversible Electroporation Therapy. IEEE Trans. Biomed. Eng. 2015, 62, 4–20. [Google Scholar] [CrossRef]
- Lee, E.W.; Chen, C.; Prieto, V.E.; Dry, S.M.; Loh, C.T.; Kee, S.T. Advanced Hepatic Ablation Technique for Creating Complete Cell Death: Irreversible Electroporation. Radiology 2010, 255, 426–433. [Google Scholar] [CrossRef]
- Ruarus, A.H.; Barabasch, A.; Catalano, O.; Leen, E.; Narayanan, G.; Nilsson, A.; Padia, S.A.; Wiggermann, P.; Scheffer, H.J.; Meijerink, M.R. Irreversible Electroporation for Hepatic Tumors: Protocol Standardization Using the Modified Delphi Technique. J. Vasc. Interv. Radiol. 2020, 31, 1765–1771.e15. [Google Scholar] [CrossRef]
- Wendler, J.J.; Fischbach, K.; Ricke, J.; Jürgens, J.; Fischbach, F.; Köllermann, J.; Porsch, M.; Baumunk, D.; Schostak, M.; Liehr, U.-B.; et al. Irreversible Electroporation (IRE): Standardization of Terminology and Reporting Criteria for Analysis and Comparison. Pol. J. Radiol. 2016, 81, 54–64. [Google Scholar] [CrossRef]
- Lee, M.W.; Rhim, H.; Cha, D.I.; Kim, Y.J.; Choi, D.; Lim, H.K. Percutaneous Radiofrequency Ablation of Hepatocellular Carcinoma: Fusion Imaging Guidance for Management of Lesions With Poor Conspicuity at Conventional Sonography. Am. J. Roentgenol. 2012, 198, 1438–1444. [Google Scholar] [CrossRef]
- Bartolozzi, C.; Lencioni, R.; Caramella, D.; Palla, A.; Bassi, A.M.; Di Candio, G. Small Hepatocellular Carcinoma. Acta Radiol. 1996, 37, 69–74. [Google Scholar] [CrossRef]
- Yumoto, Y.; Jinno, K.; Tokuyama, K.; Araki, Y.; Ishimitsu, T.; Maeda, H.; Konno, T.; Iwamoto, S.; Ohnishi, K.; Okuda, K. Hepatocellular carcinoma detected by iodized oil. Radiology 1985, 154, 19–24. [Google Scholar] [CrossRef]
- Schullian, P.; Johnston, E.; Laimer, G.; Putzer, D.; Eberle, G.; Westerlund, P.; Henninger, B.; Bale, R. Thermal ablation of CT ‘invisible’ liver tumors using MRI fusion: A case control study. Int. J. Hyperth. 2020, 37, 564–572. [Google Scholar] [CrossRef]
- Lee, M.W.; Kim, Y.J.; Park, S.W.; Hwang, J.H.; Jung, S.I.; Jeon, H.J.; Kwon, W.K. Percutaneous radiofrequency ablation of small hepatocellular carcinoma invisible on both ultrasonography and unenhanced CT: A preliminary study of combined treatment with transarterial chemoembolisation. Br. J. Radiol. 2009, 82, 908–915. [Google Scholar] [CrossRef]
- Lee, M.W.; Kim, Y.J.; Park, H.S.; Yu, N.C.; Jung, S.I.; Ko, S.Y.; Jeon, H.J. Targeted Sonography for Small Hepatocellular Carcinoma Discovered by CT or MRI: Factors Affecting Sonographic Detection. Am. J. Roentgenol. 2010, 194, W396–W400. [Google Scholar] [CrossRef] [PubMed]
- Vollherbst, D.F.; Gockner, T.; Do, T.; Holzer, K.; Mogler, C.; Flechsig, P.; Harms, A.; Schlett, C.L.; Pereira, P.L.; Richter, G.M.; et al. Computed tomography and histopathological findings after embolization with inherently radiopaque 40 μm-microspheres, standard 40μm-microspheres and iodized oil in a porcine liver model. PLoS ONE 2018, 13, e0198911. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Yoon, C.J.; Seong, N.J.; Jeong, S.-H.; Kim, J.-W. Comparison of Combined Therapy Using Conventional Chemoembolization and Radiofrequency Ablation Versus Conventional Chemoembolization for Ultrasound-Invisible Early-Stage Hepatocellular Carcinoma (Barcelona Clinic Liver Cancer Stage 0 or A). Korean J. Radiol. 2018, 19, 1130–1139. [Google Scholar] [CrossRef]
- Gandhi, S.; Iannitti, D.; Mayo-Smith, W.; Dupuy, D. Lipiodol-guided computed tomography for radiofrequency ablation of hepatocellular carcinoma. Clin. Radiol. 2006, 61, 888–891. [Google Scholar] [CrossRef]
- Chan, P.; McLean, C.; Chan, S.; Goh, G.S. The interaction between irreversible electroporation therapy (IRE) and embolization material using a validated vegetal model: An experimental study. Diagn. Interv. Radiol. 2019, 25, 304–309. [Google Scholar] [CrossRef]
- ACR, A.C.O.R. Liver Imaging Reporting and Data System Version 2018. Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS> (accessed on 31 October 2018).
- Mueller, C.; Waldburger, N.; Stampfl, U.; Kauczor, H.-U.; Schirmacher, P.; Sommer, C.M.; Longerich, T. Non-invasive diagnosis of hepatocellular carcinoma revisited. Gut 2017, 67, 991–993. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Bashir, M.R.; Corwin, M.T.; Cruite, I.; Dietrich, C.F.; Do, R.K.G.; Ehman, E.C.; Fowler, K.J.; Hussain, H.K.; Jha, R.C.; et al. Evidence Supporting LI-RADS Major Features for CT- and MR Imaging–based Diagnosis of Hepatocellular Carcinoma: A Systematic Review. Radiology 2018, 286, 29–48. [Google Scholar] [CrossRef]
- Deschamps, F.; Solomon, S.B.; Thornton, R.H.; Rao, P.; Hakime, A.; Kuoch, V.; De Baere, T. Computed Analysis of Three-Dimensional Cone-Beam Computed Tomography Angiography for Determination of Tumor-Feeding Vessels During Chemoembolization of Liver Tumor: A Pilot Study. Cardiovasc. Interv. Radiol. 2010, 33, 1235–1242. [Google Scholar] [CrossRef]
- Ahmed, M.; Solbiati, L.; Brace, C.L.; Breen, D.J.; Callstrom, M.R.; Charboneau, J.W.; Chen, M.-H.; Choi, B.I.; de Baère, T.; Dodd, G.D.; et al. Image-Guided Tumor Ablation: Standardization of Terminology and Reporting Criteria—A 10-Year Update. J. Vasc. Interv. Radiol. 2014, 25, 1691–1705.e4. [Google Scholar] [CrossRef]
- Sommer, C.; Pallwein-Prettner, L.; Vollherbst, D.; Seidel, R.; Rieder, C.; Radeleff, B.; Kauczor, H.; Wacker, F.; Richter, G.; Bücker, A.; et al. Transarterial embolization (TAE) as add-on to percutaneous radiofrequency ablation (RFA) for the treatment of renal tumors: Review of the literature, overview of state-of-the-art embolization materials and further perspective of advanced image-guided tumor ablation. Eur. J. Radiol. 2017, 86, 143–162. [Google Scholar] [CrossRef]
- Bannangkoon, K.; Hongsakul, K.; Tubtawee, T.; Piratvisuth, T. Safety margin of embolized area can reduce local recurrence of hepatocellular carcinoma after superselective transarterial chemoembolization. Clin. Mol. Hepatol. 2019, 25, 74–85. [Google Scholar] [CrossRef]
- Sommer, C.M.; Stampfl, U.; Bellemann, N.; Holzschuh, M.; Kueller, A.; Bluemmel, J.; Gehrig, T.; Shevchenko, M.; Kenngott, H.G.; Kauczor, H.U.; et al. Multimodal Visibility (Radiography, Computed Tomography, and Magnetic Resonance Imaging) of Microspheres for Transarterial Embolization Tested in Porcine Kidneys. Investig. Radiol. 2013, 48, 213–222. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, A.; Yang, J.; Zhang, Z.; Zhang, G. Assessment of Lipiodol Deposition and Residual Cancer for Hepatocellular Carcinoma After Transcatheter Arterial Chemoembolization via Iodine-Based Material Decomposition Images with Spectral Computed Tomography Imaging: A Preliminary Study. Iran. J. Radiol. 2015, 12, e26009. [Google Scholar] [CrossRef]
- Khalilzadeh, O.; Baerlocher, M.O.; Shyn, P.B.; Connolly, B.L.; Devane, A.M.; Morris, C.S.; Cohen, A.M.; Midia, M.; Thornton, R.H.; Gross, K.; et al. Proposal of a New Adverse Event Classification by the Society of Interventional Radiology Standards of Practice Committee. J. Vasc. Interv. Radiol. 2017, 28, 1432–1437.e3. [Google Scholar] [CrossRef]
- Vollherbst, D.; Bertheau, R.; Kauczor, H.-U.; Radeleff, B.A.; Pereira, P.L.; Sommer, C.-M. Treatment Failure after Image-Guided Percutaneous Radiofrequency Ablation (RFA) of Renal Tumors—A Systematic Review with Description of Type, Frequency, Risk Factors and Management. RöFo Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren 2016, 189, 219–227. [Google Scholar] [CrossRef]
- Mokry, T.; Bellemann, N.; Müller, D.; Bermejo, J.L.; Klaus, M.; Stampfl, U.; Radeleff, B.; Schemmer, P.; Kauczor, H.-U.; Sommer, C.-M. Accuracy of Estimation of Graft Size for Living-Related Liver Transplantation: First Results of a Semi-Automated Interactive Software for CT-Volumetry. PLoS ONE 2014, 9, e110201. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.-H.; Hwang, J.-I.; Cheng, Y.-C.; Wu, C.-Y.; Lee, S.-W.; Yang, S.-S.; Yeh, H.-Z.; Chang, C.-S.; Lee, T.-Y. Comparable Outcomes of Ultrasound versus Computed Tomography in the Guidance of Radiofrequency Ablation for Hepatocellular Carcinoma. PLoS ONE 2017, 12, e0169655. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Lee, M.W.; Rhim, H.; Cha, D.I.; Kang, T.W.; Min, J.H.; Song, K.D.; Choi, S.-Y.; Lim, H.K. Mistargeting after Fusion Imaging–Guided Percutaneous Radiofrequency Ablation of Hepatocellular Carcinomas. J. Vasc. Interv. Radiol. 2014, 25, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Isfort, P.; Rauen, P.; Na, H.-S.; Ito, N.; Von Stillfried, S.; Kuhl, C.; Bruners, P. Does Drug-Eluting Bead TACE Enhance the Local Effect of IRE? Imaging and Histopathological Evaluation in a Porcine Model. Cardiovasc. Interv. Radiol. 2019, 42, 880–885. [Google Scholar] [CrossRef]
- Takayasu, K.; Shima, Y.; Muramatsu, Y.; Moriyama, N.; Yamada, T.; Makuuchi, M.; Hasegawa, H.; Hirohashi, S. Hepatocellular carcinoma: Treatment with intraarterial iodized oil with and without chemotherapeutic agents. Radiology 1987, 163, 345–351. [Google Scholar] [CrossRef]
- Ben-David, E.; Ahmed, M.; Faroja, M.; Moussa, M.; Wandel, A.; Sosna, J.; Appelbaum, L.; Nissenbaum, I.; Goldberg, S.N. Irreversible Electroporation: Treatment Effect Is Susceptible to Local Environment and Tissue Properties. Radiology 2013, 269, 738–747. [Google Scholar] [CrossRef]
- Mathy, R.M.; Tinoush, P.; Da Florencia, R.D.; Braun, A.; Ghamarnejad, O.; Radeleff, B.; Kauczor, H.-U.; Chang, D.-H. Impact of needle positioning on ablation success of irreversible electroporation: A unicentric retrospective analysis. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Thomson, K.R.; Kavnoudias, H.; Neal, R.E. Introduction to Irreversible Electroporation—Principles and Techniques. Tech. Vasc. Interv. Radiol. 2015, 18, 128–134. [Google Scholar] [CrossRef]
- Sommer, C.M.; Fritz, S.; Vollherbst, D.; Zelzer, S.; Wachter, M.F.; Bellemann, N.; Gockner, T.; Mokry, T.; Schmitz, A.; Aulmann, S.; et al. CT-guided Irreversible Electroporation in an Acute Porcine Liver Model: Effect of Previous Transarterial Iodized Oil Tissue Marking on Technical Parameters, 3D Computed Tomographic Rendering of the Electroporation Zone, and Histopathology. Cardiovasc. Interv. Radiol. 2014, 38, 191–200. [Google Scholar] [CrossRef]
- Vollherbst, D.; Bertheau, R.C.; Fritz, S.; Mogler, C.; Kauczor, H.-U.; Ryschich, E.; Radeleff, B.A.; Pereira, P.L.; Sommer, C.M. Electrochemical Effects after Transarterial Chemoembolization in Combination with Percutaneous Irreversible Electroporation: Observations in an Acute Porcine Liver Model. J. Vasc. Interv. Radiol. 2016, 27, 913–921.e2. [Google Scholar] [CrossRef]
- Mafeld, S.; Wong, J.J.; Kibriya, N.; Stenberg, B.; Manas, D.; Bassett, P.; Aslam, T.; Evans, J.; Littler, P. Percutaneous Irreversible Electroporation (IRE) of Hepatic Malignancy: A Bi-institutional Analysis of Safety and Outcomes. Cardiovasc. Interv. Radiol. 2018, 42, 577–583. [Google Scholar] [CrossRef]
- Distelmaier, M.; Barabasch, A.; Heil, P.; Kraemer, N.A.; Isfort, P.; Keil, S.; Kuhl, C.K.; Bruners, P. Midterm Safety and Efficacy of Irreversible Electroporation of Malignant Liver Tumors Located Close to Major Portal or Hepatic Veins. Radiology 2017, 285, 1023–1031. [Google Scholar] [CrossRef]
- Kumar, N.; Gaba, R.C.; Knuttinen, M.G.; Omene, B.O.; Martinez, B.K.; Owens, C.A.; Bui, J.T. Tract Seeding Following Radiofrequency Ablation for Hepatocellular Carcinoma: Prevention, Detection, and Management. Semin. Interv. Radiol. 2011, 28, 187–192. [Google Scholar] [CrossRef]
- Sutter, O.; Calvo, J.; N’Kontchou, G.; Nault, J.-C.; Ourabia, R.; Nahon, P.; Ganne-Carrié, N.; Bourcier, V.; Zentar, N.; Bouhafs, F.; et al. Safety and Efficacy of Irreversible Electroporation for the Treatment of Hepatocellular Carcinoma Not Amenable to Thermal Ablation Techniques: A Retrospective Single-Center Case Series. Radiology 2017, 284, 877–886. [Google Scholar] [CrossRef]
- Qin, Z.; Jiang, J.; Long, G.; Lindgren, B.; Bischof, J.C. Irreversible Electroporation: An In Vivo Study with Dorsal Skin Fold Chamber. Ann. Biomed. Eng. 2012, 41, 619–629. [Google Scholar] [CrossRef]
| Patients No. | Etiology of Cirrhosis | Child-Pugh Grade | ECOG Grade | Prior HCC History | Prior HCC Resection History | Target-HCC No. | Location of Target-HCC (Segment) | HCC Size (mm) | APHE | Enhancing “Capsule” | Non-Peripheral “Washout” in Portal Phase | Threshold Growth * | LI-RADS Grades | Pathological Results of the Target Tumor |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | NASH | A | 0 | Yes | Yes | 1 | 7 | 15.5 | Yes | No | No | Yes | LR-5 | |
| 2 | Chronic hepatitis C | A | 0 | No | No | 2 | 8 | 13.5 | Yes | No | Yes | No | LR-5 | |
| 3 | 8 | 11.6 | Yes | No | Yes | Yes | LR-5 | |||||||
| 3 | Ethytotoxic cirrhosis | A | 0 | Yes | Yes | 4 | 5 | 11.2 | Yes | No | Yes | Yes | LR-5 | |
| 4 | Ethytotoxic cirrhosis | A | 0 | No | No | 5 | 4a | 15.4 | Yes | No | Yes | Yes | LR-5 | |
| 5 | PSC | A | 0 | Yes | Yes | 6 | 5 | 15.7 | Yes | No | Yes | Yes | LR-5 | |
| 7 | 8 | 18.7 | Yes | No | Yes | Yes | LR-5 | |||||||
| 6 | Ethytotoxic cirrhosis | A | 0 | No | No | 8 | 4a | 18.8 | Yes | No | No | No | LR-4 | Moderately- differentiated HCC |
| 7 | Chronic hepatitis C and B | A | 0 | Yes | Yes | 9 | 6 | 11.5 | Yes | No | Yes | Yes | LR-5 | |
| 8 | Chronic hepatitis B | A | 0 | No | No | 10 | 6 | 17.8 | Yes | No | No | Yes | LR-5 | |
| 9 | Chronic hepatitis C | A | 0 | No | No | 11 | 8 | 11.1 | Yes | No | Yes | No prior examination | LR-5 |
| CT Measurement Parameters | Pre-Marking CT | Post-Marking CT | p-Value * |
|---|---|---|---|
| Area of ROIs (mm2) | 147.4 ± 90.0 (60.0–332.7) | 148.6 ± 97.2 (49.1–331.8) | 0.445 |
| Hounsfield scale of the target-HCCs (HU) | 50.6 ± 11.2 (34.8–65.9) | 206.4 ± 68.0 (113.4–350.5) | 0.001 |
| Hounsfield scale of the surrounding normal liver tissue (HU) | 56.9 ± 9.0 (40.5–67.1) | 94.4 ± 22.6 (68.4–133.0) | 0.005 |
| Hounsfield scale of the peripheral normal liver tissue (HU) | 59.4 ± 8.5 (43.7–66.6) | 58.2 ± 9.1 (45.2–69.7) | 0.130 |
| SNR of the target-HCCs | 3.36 ± 1.79 (1.92–7.48) | 11.07 ± 4.23 (4.02–19.52) | 0.006 |
| SNR of the surrounding normal liver tissue | 3.74 ± 1.72 (2.66–7.63) | 5.09 ± 1.81 (2.79–8.29) | 0.087 |
| SNR of the peripheral normal liver tissue | 3.74 ± 1.59 (2.58–7.10) | 3.13 ± 0.98 (1.87–5.04) | 0.258 |
| CNR of the target-HCCs | 0.43 ± 0.28 (0.11–0.88) | 4.32 ± 3.31 (1.44–13.01) | 0.023 |
| Target-HCC No. | Operating Time between First Electrode Insertion and Last Electrode Removal (min) | Application Time of the IRE Pulse Delivery (min) | Index Tumor Size (Depth/ Width/ Length) (mm) | Number of Electrodes | Electrode Configuration | Active Tip Length (cm) | Average Inter-Electrode Space (cm) | Number of Electrode Pairs | Pulse Length (μs) | Number of IRE Burst | Average Pulse Number per IRE Burst | Total Pulse Number | Average Voltage Setting (V/cm) | Average Current (A) | Energy (J) | Average ΔA Burst (Minimum-Maximum) (A) | Average ΔA% burst (Minimum-Maximum) (%) | IRE Zone (Depth/Width/Length) (mm) | Technical Success of IRE | Treatment Failure (Endpoint-1 and -2) * | Period until Endpoint-2 * (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | 9 | 16.5/15.7/ 15.1 | 3 | Triangle | 2 | 1.5 | 3 | 90 | 3 | 90 | 270 | 1589 | 25.4 | 358.2 | 4.5 (3.1–5.3) | 20.0 (15.3–23) | 32.7/30.3/31.0 | Success | ||
| 2 | 34 | 14 | 13.7/14.5/ 13.9 | 3 | Triangle | 2 | 1.4 | 3 | 90 | 4 | 90 | 360 | 2000 | 30.1 | 506 | 5.3 (3.2–6.4) | 21.1 (11.4–25.5) | 31.6/29.7/30.1 | Success | ||
| 3 | 57 | 10 | 12.9/12.3/ 11.5 | 3 | Triangle | 2 | 1.4 | 3 | 90 | 3 | 90 | 270 | 1817 | 29.9 | 457.1 | 4.2 (2.3–5.4) | 17.6 (10.5–22.6) | 32.8/30.1/31.2 | Success | ||
| 4 | 33 | 17 | 12.5/11.9/ 11.7 | 3 | Triangle | 2 | 1 | 3 | 90 | 3 | 90 | 270 | 2347 | 26.6 | 380.8 | 5.4 (3.1–6.6) | 25.0 (15.2–33.8) | 32.1/25.2/26.9 | Success | ||
| 5 | 29 | 11 | 16.7/15.7/ 16.1 | 3 | Triangle | 2 | 1.5 | 3 | 90 | 3 | 90 | 270 | 1448 | 24.6 | 322.7 | 2.9 (1.9–3.6) | 14.2 (9.2–17.5) | 33.5/30.5/31.5 | Success | Local tumor progression | 11 # |
| 6 | 30 | 10 | 16.5/17.1/ 16.2 | 3 | Triangle | 2 | 1.6 | 3 | 90 | 3 | 90 | 270 | 1875 | 31.1 | 552.2 | 6.7 (5.9–7.8) | 27.9 (24.5–32.4) | 31.5/32.7/30.6 | Success | ||
| 7 | 74 | 32 | 19.9/18.7/ 18.2 | 4 | Square | 2.5 | 1.8 | 6 | 90 | 11 | 90 | 990 | 1580 | 30.1 | 522.6 | 7.0 (2.8–11) | 30.4 (11.8–52.9) | 37.6/35.5/35.8 | Success | Local tumor progression | 14 # |
| 8 | 61 | 28 | 19.2/19.7/ 18.8 | 4 | Square | 2.5 | 1.8 | 6 | 90 | 7 | 90 | 630 | 1525 | 34.7 | 547.4 | 5.5 (1.6–10.1) | 22.2 (5.5–50.8) | 36.2/37.8/35.3 | Success | ||
| 9 | 28 | 8 | 10.1/0.91/ 0.87 | 2 | Line | 2 | 1.5 | 1 | 90 | 2 | 90 | 180 | 2000 | 26.7 | 481.3 | 4.4 (4.2–4.5) | 18.7 (17–20.5) | 29.5/22.9/23.9 | Success | ||
| 10 | 57 | 15 | 18.8/18.1/ 18.5 | 3 | Triangle | 2 | 1.5 | 3 | 90 | 3 | 90 | 270 | 2006 | 43.6 | 756.5 | 10.6 (9.1–13.5) | 32.2 (27.6–40.9) | 33.4/34.5/32.7 | Success | ||
| 11 | 37 | 6 | 10.3/0.95/ 0.91 | 2 | Line | 2 | 1.5 | 1 | 90 | 2 | 90 | 180 | 2000 | 28.7 | 406.4 | 7.7 (3.8–11.5) | 33.0 (17–48.9) | 29.9/23.1/23.5 | Success | Local tumor progression | 17 # |
| Average | 46 ± 17 | 15 ± 8 | 3 ± 1 | 2.0 ± 0.2 | 1.5 ± 0.2 | 3 ± 2 | 90 ± 0 | 4 ± 3 | 90 ± 0 | 360 ± 241 | 1835 ± 273 | 30.1 ± 5.3 | 481.0 ± 120.0 | 5.8 ± 2.1 | 23.8 ± 6.3 | ||||||
| (28–74) | (6–32) | (2–4) | (2.0–2.5) | (1.0–1.8) | (1–6) | (90–90) | (2–13) | (90–90) | (180–990) | (1448–2347) | (24.6–43.6) | (322.7–756.5) | (2.9–10.6) | (14.2–33.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, F.; Do, T.D.; Vollherbst, D.F.; Pereira, P.L.; Richter, G.M.; Faerber, M.; Weiss, K.H.; Mehrabi, A.; Kauczor, H.U.; Sommer, C.M. Percutaneous Irreversible Electroporation for Treatment of Small Hepatocellular Carcinoma Invisible on Unenhanced CT: A Novel Combined Strategy with Prior Transarterial Tumor Marking. Cancers 2021, 13, 2021. https://doi.org/10.3390/cancers13092021
Pan F, Do TD, Vollherbst DF, Pereira PL, Richter GM, Faerber M, Weiss KH, Mehrabi A, Kauczor HU, Sommer CM. Percutaneous Irreversible Electroporation for Treatment of Small Hepatocellular Carcinoma Invisible on Unenhanced CT: A Novel Combined Strategy with Prior Transarterial Tumor Marking. Cancers. 2021; 13(9):2021. https://doi.org/10.3390/cancers13092021
Chicago/Turabian StylePan, Feng, Thuy D. Do, Dominik F. Vollherbst, Philippe L. Pereira, Götz M. Richter, Michael Faerber, Karl H. Weiss, Arianeb Mehrabi, Hans U. Kauczor, and Christof M. Sommer. 2021. "Percutaneous Irreversible Electroporation for Treatment of Small Hepatocellular Carcinoma Invisible on Unenhanced CT: A Novel Combined Strategy with Prior Transarterial Tumor Marking" Cancers 13, no. 9: 2021. https://doi.org/10.3390/cancers13092021
APA StylePan, F., Do, T. D., Vollherbst, D. F., Pereira, P. L., Richter, G. M., Faerber, M., Weiss, K. H., Mehrabi, A., Kauczor, H. U., & Sommer, C. M. (2021). Percutaneous Irreversible Electroporation for Treatment of Small Hepatocellular Carcinoma Invisible on Unenhanced CT: A Novel Combined Strategy with Prior Transarterial Tumor Marking. Cancers, 13(9), 2021. https://doi.org/10.3390/cancers13092021






