Age- and Intravenous Methotrexate-Associated Leukoencephalopathy and Its Neurological Impact in Pediatric Patients with Lymphoblastic Leukemia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Acquisition
2.3. Data Analyses
3. Results
4. Discussion
4.1. Prevalence and Severity of Lesions
4.2. Risk Factors for Leukoencephalopathy/Neurotoxicity
4.3. Limitations
4.4. Future Directions: Mechanisms and Long-Term Outcomes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A.; IICC-3 Contributors. International incidence of childhood cancer, 2001–2010: A population-based registry study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef]
- Olivier-Gougenheim, L.; Arfeuille, C.; Suciu, S.; Sirvent, N.; Plat, G.; Ferster, A.; De Moerloose, B.; Domenech, C.; Uyttebroeck, A.; Rohrlich, P.; et al. Pediatric randomized trial EORTC CLG 58951: Outcome for adolescent population with acute lymphoblastic leukemia. Hematol. Oncol. 2020, 38, 763–772. [Google Scholar] [CrossRef]
- Duffner, P.K.; Armstrong, F.D.; Chen, L.; Helton, K.J.; Brecher, M.L.; Bell, B.; Chauvenet, A.R. Neurocognitive and Neuroradiologic Central Nervous System Late Effects in Children Treated on Pediatric Oncology Group (POG) P9605 (Standard Risk) and P9201 (Lesser Risk) Acute Lymphoblastic Leukemia Protocols (ACCL0131). J. Pediatr. Hematol. 2014, 36, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.T.; Krull, K.R. Neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia treated on contemporary treatment protocols: A systematic review. Neurosci. Biobehav. Rev. 2015, 53, 108–120. [Google Scholar] [CrossRef]
- Cheung, Y.T.; Sabin, N.D.; Reddick, W.E.; Bhojwani, D.; Liu, W.; Brinkman, T.M.; Glass, J.O.; Hwang, S.N.; Srivastava, D.; Pui, C.-H.; et al. Leukoencephalopathy and long-term neurobehavioural, neurocognitive, and brain imaging outcomes in survivors of childhood acute lymphoblastic leukaemia treated with chemotherapy: A longitudinal analysis. Lancet Haematol. 2016, 3, e456–e466. [Google Scholar] [CrossRef]
- Goldsby, R.E.; Liu, Q.; Nathan, P.C.; Bowers, D.C.; Yeaton-Massey, A.; Raber, S.H.; Hill, D.; Armstrong, G.T.; Yasui, Y.; Zeltzer, L.; et al. Late-Occurring Neurologic Sequelae in Adult Survivors of Childhood Acute Lymphoblastic Leukemia: A Report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2010, 28, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Iuvone, L.; Mariotti, P.; Colosimo, C.; Guzzetta, F.; Ruggiero, A.; Riccardi, R. Long-term cognitive outcome, brain computed tomography scan, and magnetic resonance imaging in children cured for acute lymphoblastic leukemia. Cancer 2002, 95, 2562–2570. [Google Scholar] [CrossRef]
- Anastasopoulou, S.; Eriksson, M.A.; Heyman, M.; Wang, C.; Niinimäki, R.; Mikkel, S.; Vaitkevičienė, G.E.; Johannsdottir, I.M.; Myrberg, I.H.; Jonsson, O.G.; et al. Posterior reversible encephalopathy syndrome in children with acute lymphoblastic leukemia: Clinical characteristics, risk factors, course, and outcome of disease. Pediatr. Blood Cancer 2019, 66, e27594. [Google Scholar] [CrossRef]
- Reddick, W.E.; Shan, Z.Y.; Glass, J.O.; Helton, S.; Xiong, X.; Wu, S.; Bonner, M.J.; Howard, S.C.; Christensen, R.; Khan, R.B.; et al. Smaller white-matter volumes are associated with larger deficits in attention and learning among long-term survivors of acute lymphoblastic leukemia. Cancer 2006, 106, 941–949. [Google Scholar] [CrossRef]
- Billiet, T.; Elens, I.; Sleurs, C.; Uyttebroeck, A.; D’Hooge, R.; Lemiere, J.; Deprez, S. Brain Connectivity and Cognitive Flexibility in Nonirradiated Adult Survivors of Childhood Leukemia. J. Natl. Cancer Inst. 2018, 110, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Carey, M.; Haut, M.; Reminger, S.; Hutter, J.; Theilmann, R.; Kaemingk, K. Reduced Frontal White Matter Volume in Long-Term Childhood Leukemia Survivors: A Voxel-Based Morphometry Study. Am. J. Neuroradiol. 2008, 29, 792–797. [Google Scholar] [CrossRef]
- Morris, E.B.; Laningham, F.H.; Sandlund, J.T.; Khan, R.B. Posterior reversible encephalopathy syndrome in children with cancer. Pediatr. Blood Cancer 2007, 48, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Bhojwani, D.; Sabin, N.D.; Pei, D.; Yang, J.J.; Khan, R.B.; Panetta, J.C.; Krull, K.R.; Inaba, H.; Rubnitz, J.E.; Metzger, M.L.; et al. Methotrexate-Induced Neurotoxicity and Leukoencephalopathy in Childhood Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2014, 32, 949–959. [Google Scholar] [CrossRef]
- Partap, S.; Russo, S.; Esfahani, B.; Yeom, K.; Mazewski, C.; Embry, L.; Wheeler, G.; Ullrich, N.J.; Bowers, D.C. A Review of Chronic Leukoencephalopathy among Survivors of Childhood Cancer. Pediatr. Neurol. 2019, 101, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Schroyen, G.; Meylaers, M.; Deprez, S.; Blommaert, J.; Smeets, A.; Jacobs, S.; Sunaert, S.; Sleurs, C.; Uyttebroeck, A. Prevalence of leukoencephalopathy and its potential cognitive sequelae in cancer patients. J. Chemother. 2020, 32, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Pryweller, J.R.; Glass, J.O.; Sabin, N.D.; Laningham, F.H.; Li, Y.; Jacola, L.M.; Conklin, H.M.; Reddick, W.E. Characterization of Leukoencephalopathy and Association with Later Neurocognitive Performance in Pediatric Acute Lymphoblastic Leukemia. Investig. Radiol. 2021, 56, 117–126. [Google Scholar] [CrossRef]
- Krull, K.R.; Sabin, N.D.; Reddick, W.E.; Zhu, L.; Armstrong, G.T.; Green, D.M.; Arevalo, A.R.; Krasin, M.J.; Srivastava, D.K.; Robison, L.L.; et al. Neurocognitive Function and CNS Integrity in Adult Survivors of Childhood Hodgkin Lymphoma. J. Clin. Oncol. 2012, 30, 3618–3624. [Google Scholar] [CrossRef] [PubMed]
- Nassar, S.L.; Conklin, H.M.; Zhou, Y.; Ashford, J.M.; Reddick, W.E.; Glass, J.O.; Laningham, F.H.; Jeha, S.; Cheng, C.; Pui, C.-H. Neurocognitive outcomes among children who experienced seizures during treatment for acute lymphoblastic leukemia. Pediatr. Blood Cancer 2017, 64, e26436. [Google Scholar] [CrossRef]
- Krull, K.R.; Cheung, Y.T.; Liu, W.; Fellah, S.; Reddick, W.E.; Brinkman, T.M.; Kimberg, C.; Ogg, R.; Srivastava, D.; Pui, C.-H.; et al. Chemotherapy Pharmacodynamics and Neuroimaging and Neurocognitive Outcomes in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2016, 34, 2644–2653. [Google Scholar] [CrossRef]
- Simmonds, D.J.; Hallquist, M.N.; Asato, M.; Luna, B. Developmental stages and sex differences of white matter and behavioral development through adolescence: A longitudinal diffusion tensor imaging (DTI) study. NeuroImage 2014, 92, 356–368. [Google Scholar] [CrossRef]
- Buizer, A.I.; De Sonneville, L.M.; Heuvel-Eibrink, M.M.V.D.; Veerman, A.J. Chemotherapy and attentional dysfunction in survivors of childhood acute lymphoblastic leukemia: Effect of treatment intensity. Pediatr. Blood Cancer 2005, 45, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Atra, A.; Pinkerton, C.; Bouffet, E.; Norton, A.; Hobson, R.; Imeson, J.; Gerrard, M. Acute neurotoxicity in children with advanced stage B-non-Hodgkin’s lymphoma and B-acute lymphoblastic leukaemia treated with the United Kingdom children cancer study group 9002/9003 protocols. Eur. J. Cancer 2004, 40, 1346–1350. [Google Scholar] [CrossRef]
- Tsujimoto, S.-I.; Yanagimachi, M.; Tanoshima, R.; Urayama, K.Y.; Tanaka, F.; Aida, N.; Goto, H.; Ito, S. Influence ofADORA2Agene polymorphism on leukoencephalopathy risk in MTX-treated pediatric patients affected by hematological malignancies. Pediatr. Blood Cancer 2016, 63, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Reddick, W.E.; Glass, J.O.; Helton, K.J.; Langston, J.W.; Xiong, X.; Wu, S.; Pui, C.-H. Prevalence of leukoencephalopathy in children treated for acute lymphoblastic leukemia with high-dose methotrexate. Am. J. Neuroradiol. 2005, 26, 1263–1269. [Google Scholar]
- Sabin, N.; Cheung, Y.; Reddick, W.; Bhojwani, D.; Liu, W.; Glass, J.; Brinkman, T.; Hwang, S.; Srivastava, D.; Pui, C.-H.; et al. The Impact of Persistent Leukoencephalopathy on Brain White Matter Microstructure in Long-Term Survivors of Acute Lymphoblastic Leukemia Treated with Chemotherapy Only. Am. J. Neuroradiol. 2018, 39, 1919–1925. [Google Scholar] [CrossRef]
- Cole, P.D.; Beckwith, K.A.; Vijayanathan, V.; Roychowdhury, S.; Smith, A.K.; Kamen, B.A. Folate Homeostasis in Cerebrospinal Fluid During Therapy for Acute Lymphoblastic Leukemia. Pediatr. Neurol. 2009, 40, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Reddick, W.E.; Glass, J.O.; Helton, K.J.; Langston, J.W.; Li, C.-S.; Pui, C.-H. A quantitative MR imaging assessment of leukoencephalopathy in children treated for acute lymphoblastic leukemia without irradiation. Am. J. Neuroradiol. 2005, 26, 2371–2377. [Google Scholar] [PubMed]
- De Moerloose, B.; Suciu, S.; Bertrand, Y.; Mazingue, F.; Robert, A.; Uyttebroeck, A.; Yakouben, K.; Ferster, A.; Margueritte, G.; Lutz, P.; et al. Improved outcome with pulses of vincristine and corticosteroids in continuation therapy of children with average risk acute lymphoblastic leukemia (ALL) and lymphoblastic non-Hodgkin lymphoma (NHL): Report of the EORTC randomized phase 3 trial 58951. Blood 2010, 116, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Mondelaers, V.V.; Suciu, S.; De Moerloose, B.M.J.B.; Ferster, A.; Mazingue, F.F.; Plat, G.G.; Yakouben, K.; Uyttebroeck, A.; Lutz, P.; Costa, V.V.; et al. Prolonged versus standard native E. coli asparaginase therapy in childhood acute lymphoblastic leukemia and non-Hodgkin lymphoma: Final results of the EORTC-CLG randomized phase III trial 58951. Haematologica 2017, 102, 1727–1738. [Google Scholar] [CrossRef]
- Tustison, N.J.; Avants, B.B.; Cook, P.A.; Zheng, Y.; Egan, A.; Yushkevich, P.A.; Gee, J.C. N4ITK: Improved N3 Bias Correction. IEEE Trans. Med. Imaging 2010, 29, 1310–1320. [Google Scholar] [CrossRef]
- Isensee, F.; Schell, M.; Pflueger, I.; Brugnara, G.; Bonekamp, D.; Neuberger, U.; Wick, A.; Schlemmer, H.; Heiland, S.; Wick, W.; et al. Automated brain extraction of multisequence MRI using artificial neural networks. Hum. Brain Mapp. 2019, 40, 4952–4964. [Google Scholar] [CrossRef] [PubMed]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerma&, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef]
- Kamiya-Matsuoka, C.; Paker, A.M.; Chi, L.; Youssef, A.; Tummala, S.; Loghin, M.E. Posterior reversible encephalopathy syndrome in cancer patients: A single institution retrospective study. J. Neuro-Oncol. 2016, 128, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Chen, J.; Tan, X.; Ye, X.-Y.; Ma, M.-S.; Huang, J.-P.; Zou, L.-P. Posterior reversible encephalopathy syndrome in patients with hematologic tumor confers worse outcome. World J. Pediatr. 2015, 11, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Kingma, A.; Van Dommelen, R.I.; Mooyaart, E.L.; Wilmink, J.T.; Deelman, B.G.; Kamps, W.A. Slight cognitive impairment and magnetic resonance imaging abnormalities but normal school levels in children treated for acute lymphoblastic leukemia with chemotherapy only. J. Pediatr. 2001, 139, 413–420. [Google Scholar] [CrossRef]
- Brown, R.T.; Madan-Swain, A.; Walco, G.A.; Cherrick, I.; Ievers, C.E.; Conte, M.P.M.; Vega, R.; Bell, B.; Lauer, S.J. Cognitive and Academic Late Effects Among Children Previously Treated for Acute Lymphocytic Leukemia Receiving Chemotherapy as CNS Prophylaxis. J. Pediatr. Psychol. 1998, 23, 333–340. [Google Scholar] [CrossRef][Green Version]
- Langer, T.; Martus, P.; Ottensmeier, H.; Hertzberg, H.; Meier, W. CNS late-effects after ALL therapy in childhood. Part III: Neuropsychological performance in long-term survivors of childhood ALL: Impairments of concentration, attention, and memory. Med. Pediatr. Oncol. 2002, 38, 320–328. [Google Scholar] [CrossRef]
- Von Der Weid, N.; Mosimann, I.; Hirt, A.; Wacker, P.; Beck, M.N.; Imbach, P.; Caflisch, U.; Niggli, F.; Feldges, A.; Wagner, H. Intellectual outcome in children and adolescents with acute lymphoblastic leukaemia treated with chemotherapy alone: Age- and sex-related differences. Eur. J. Cancer 2003, 39, 359–365. [Google Scholar] [CrossRef]
- Lebel, C.; Walker, L.; Leemans, A.; Phillips, L.; Beaulieu, C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage 2008, 40, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Yakovlev, P.; Lecours, A.-R. The Myelogenetic Cycles of Regional Maturation of the Brain: Regional Development of the Brain in Early Life; Blackwell Sci. Publ.: Oxford, UK, 1967; pp. 3–70. [Google Scholar]
- Hertzberg, H.; Huk, W.J.; Ueberall, M.A.; Langer, T.; Meier, W.; Dopfer, R.; Skalej, M.; Lackner, H.; Bode, U.; Janssen, G.; et al. CNS late effects after ALL therapy in childhood. Part I: Neuroradiological findings in long-term survivors of childhood ALL—an evaluation of the interferences between morphology and neuropsychological performance. Med. Pediatr. Oncol. 1997, 28, 387–400. [Google Scholar] [CrossRef]
- Matsumoto, K.; Takahashi, S.; Sato, A.; Imaizumi, M.; Higano, S.; Sakamoto, K.; Asakawa, H.; Tada, K. Leukoencephalopathy in childhood hematopoietic neoplasm caused by moderate-dose methotrexate and prophylactic cranial radiotherapy—An MR analysis. Int. J. Radiat. Oncol. 1995, 32, 913–918. [Google Scholar] [CrossRef]
- Buizer, A.I.; De Sonneville, L.M.J.; Veerman, A.J.P. Effects of chemotherapy on neurocognitive function in children with acute lymphoblastic leukemia: A critical review of the literature. Pediatr. Blood Cancer 2009, 52, 447–454. [Google Scholar] [CrossRef]
- Sleurs, C.; Lemiere, J.; Vercruysse, T.; Nolf, N.; Van Calster, B.; Deprez, S.; Renard, M.; Vandecruys, E.; Benoit, Y.; Uyttebroeck, A. Intellectual development of childhood ALL patients: A multicenter longitudinal study. Psychooncology 2016, 26, 508–514. [Google Scholar] [CrossRef]
- Conklin, H.M.; Krull, K.R.; Reddick, W.E.; Pei, D.; Cheng, C.; Pui, C.H. Cognitive Outcomes Following Contemporary Treatment Without Cranial Irradiation for Childhood Acute Lymphoblastic Leukemia. J. Natl. Cancer Inst. 2012, 104, 1386–1395. [Google Scholar] [CrossRef]
- Froklage, F.E.; Reijneveld, J.C.; Heimans, J.J. Central neurotoxicity in cancer chemotherapy: Pharmacogenetic insights. Pharmacogenomics 2011, 12, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Sleurs, C.; Lemiere, J.; Radwan, A.; Verly, M.; Elens, I.; Renard, M.; Jacobs, S.; Sunaert, S.; Deprez, S.; Uyttebroeck, A. Long-term leukoencephalopathy and neurocognitive functioning in childhood sarcoma patients treated with high-dose intravenous chemotherapy. Pediatr. Blood Cancer 2019, 66, e27893. [Google Scholar] [CrossRef]
- Brugnoletti, F.; Morris, E.B.; Laningham, F.H.; Patay, Z.; Pauley, J.L.; Pui, C.-H.; Jeha, S.; Inaba, H. Recurrent intrathecal methotrexate induced neurotoxicity in an adolescent with acute lymphoblastic leukemia: Serial clinical and radiologic findings. Pediatr. Blood Cancer 2008, 52, 293–295. [Google Scholar] [CrossRef]
- Edelmann, M.N.; Ogg, R.J.; Scoggins, M.A.; Brinkman, T.M.; Sabin, N.D.; Pui, C.-H.; Srivastava, D.K.; Robison, L.L.; Hudson, M.M.; Krull, K.R. Dexamethasone exposure and memory function in adult survivors of childhood acute lymphoblastic leukemia: A report from the SJLIFE cohort. Pediatr. Blood Cancer 2013, 60, 1778–1784. [Google Scholar] [CrossRef] [PubMed]
- Phillips, N.S.; Cheung, Y.T.; Glass, J.O.; Scoggins, M.A.; Liu, W.; Ogg, R.J.; Mulrooney, D.A.; Pui, C.; Robison, L.L.; Reddick, W.E.; et al. Neuroanatomical abnormalities related to dexamethasone exposure in survivors of childhood acute lymphoblastic leukemia. Pediatr. Blood Cancer 2020, 67, e27968. [Google Scholar] [CrossRef]
- Schirmer, M.D.; Dalca, A.V.; Sridharan, R.; Giese, A.-K.; Donahue, K.L.; Nardin, M.J.; Mocking, S.J.; McIntosh, E.C.; Frid, P.; Wasselius, J.; et al. White matter hyperintensity quantification in large-scale clinical acute ischemic stroke cohorts—The MRI-GENIE study. NeuroImage Clin. 2019, 23, 101884. [Google Scholar] [CrossRef]
- Edelmann, M.N.; Krull, K.R.; Liu, W.; Glass, J.O.; Ji, Q.; Ogg, R.J.; Sabin, N.D.; Srivastava, D.K.; Robison, L.L.; Hudson, M.M.; et al. Diffusion tensor imaging and neurocognition in survivors of childhood acute lymphoblastic leukaemia. Brain 2014, 137, 2973–2983. [Google Scholar] [CrossRef]
- Hearps, S.; Seal, M.; Anderson, V.; McCarthy, M.; Connellan, M.; Downie, P.; De Luca, C. The relationship between cognitive and neuroimaging outcomes in children treated for acute lymphoblastic leukemia with chemotherapy only: A systematic review. Pediatr. Blood Cancer 2016, 64, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Fonov, V.; Evans, A.C.; Botteron, K.; Almli, C.R.; McKinstry, R.C.; Collins, D.L. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage 2011, 54, 313–327. [Google Scholar] [CrossRef]
- Caron, J.E.; Krull, K.R.; Hockenberry, M.; Jain, N.; Kaemingk, K.; Moore, I.M. Oxidative stress and executive function in children receiving chemotherapy for acute lymphoblastic leukemia. Pediatr. Blood Cancer 2009, 53, 551–556. [Google Scholar] [CrossRef]
- Elens, I.; Deprez, S.; Danckaerts, M.; Bijttebier, P.; Labarque, V.; Uyttebroeck, A.; Van Gool, S.; D’Hooge, R.; Lemiere, J. Neurocognitive Sequelae in Adult Childhood Leukemia Survivors Related to Levels of Phosphorylated Tau. J. Natl. Cancer Inst. 2017, 109, 1–4. [Google Scholar] [CrossRef]
- Krawczuk-Rybak, M.; Grabowska, A.; Protas, P.; Muszynska-Roslan, K.; Holownia, A.; Braszko, J. Intellectual functioning of childhood leukemia survivors—relation to Tau protein—a marker of white matter injury. Adv. Med. Sci. 2012, 57, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Protas, P.T.; Holownia, A.; Grabowska, A.; Wielgat, P.; Braszko, J.J.; Muszynska-Roslan, K.; Krawczuk-Rybak, M. Negative correlation between cerebrospinal fluid tau protein and cognitive functioning in children with acute lymphoblastic leukemia. Pediatr. Blood Cancer 2009, 53, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.T.; Khan, R.B.; Liu, W.; Brinkman, T.M.; Edelmann, M.N.; Reddick, W.E.; Pei, D.; Panoskaltsis-Mortari, A.; Srivastava, D.; Cheng, C.; et al. Association of Cerebrospinal Fluid Biomarkers of Central Nervous System Injury with Neurocognitive and Brain Imaging Outcomes in Children Receiving Chemotherapy for Acute Lymphoblastic Leukemia. JAMA Oncol. 2018, 4, e180089. [Google Scholar] [CrossRef]
- Mazur, B.; Mertas, A.; Sońta-Jakimczyk, D.; Szczepański, T.; Janik-Moszant, A. Concentration of IL-2, IL-6, IL-8, IL-10 and TNF-alpha in children with acute lymphoblastic leukemia after cessation of chemotherapy. Hematol. Oncol. 2004, 22, 27–34. [Google Scholar] [CrossRef]
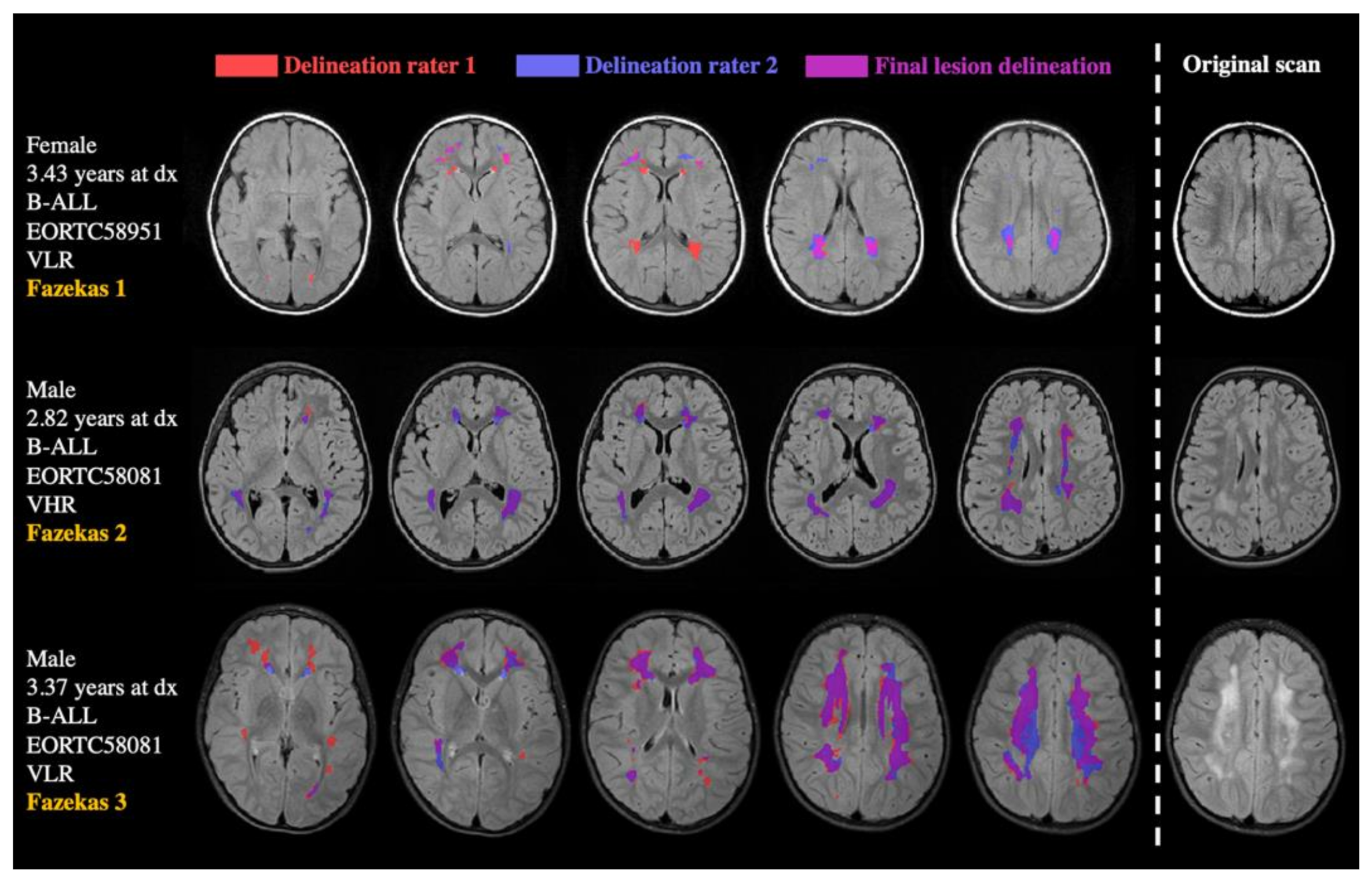
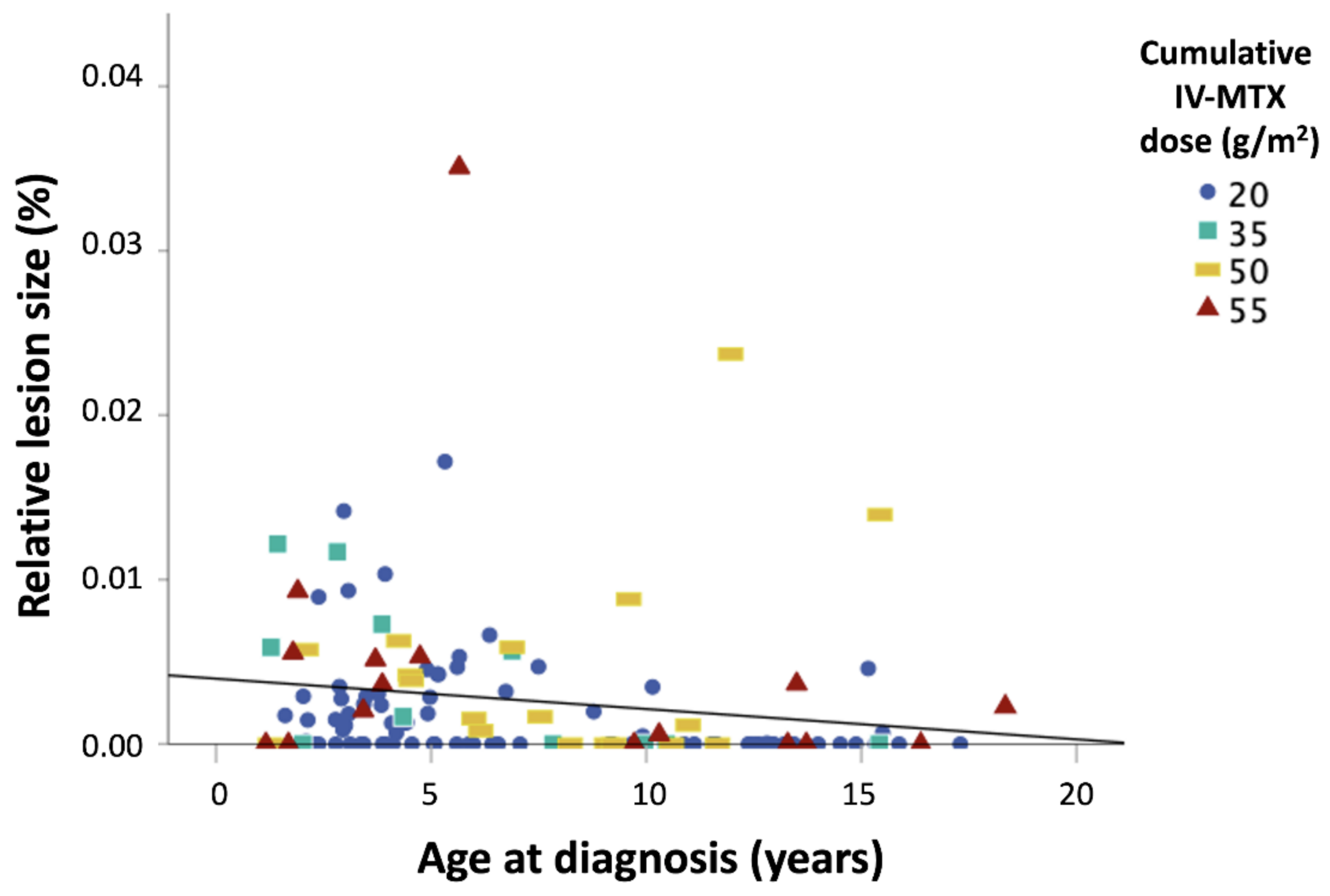
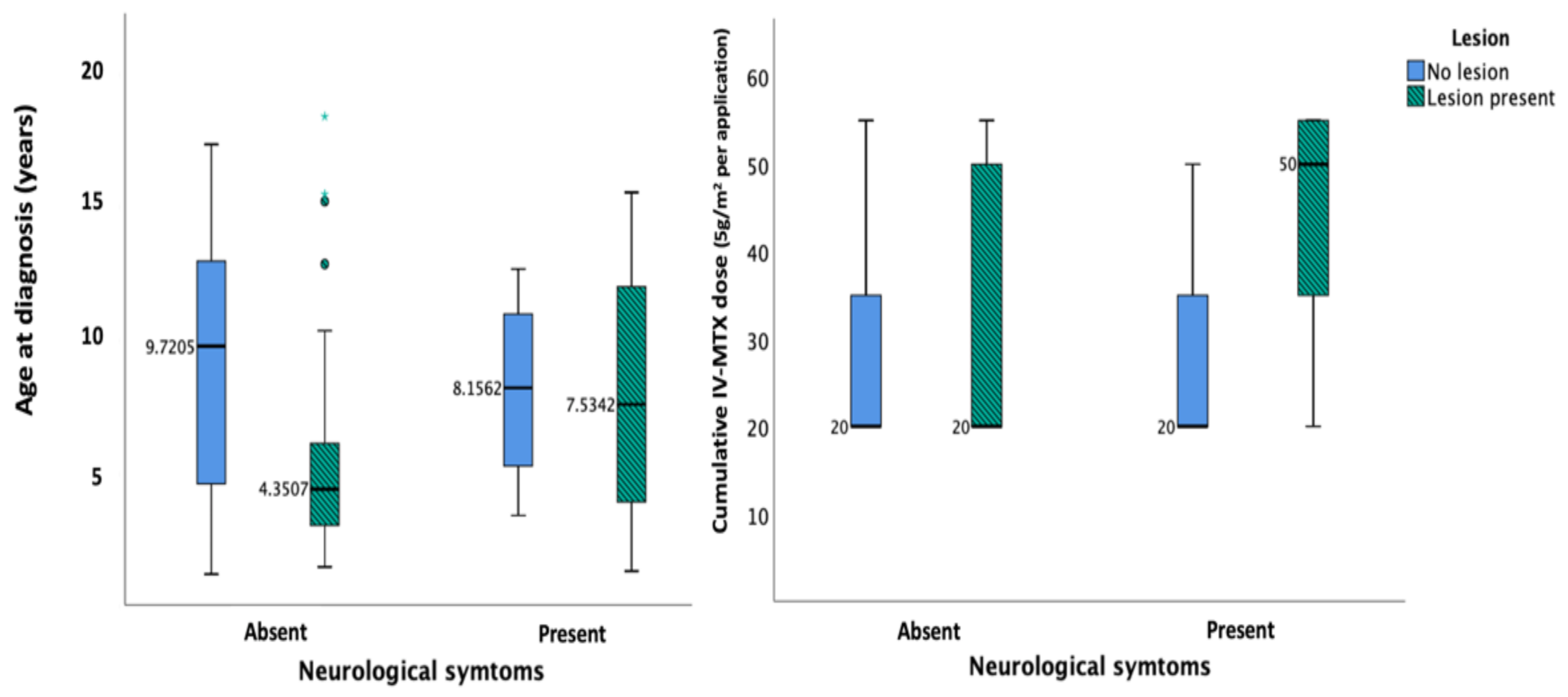
| Number of Patients | |
|---|---|
| Total sample | 129 |
| Gender | |
| Female (n) (%) | 59 (46%) |
| Male (n) (%) | 70 (54%) |
| Age at diagnosis (years) | |
| Median | 5.65 |
| Mean | 7.18 |
| Range | 1.16–18.35 |
| Treatment risk group A | |
| Low (n) | 20 |
| Standard/high | |
| AR1 (n) | 65 |
| AR2 (n) | 28 |
| VHR (n) | 16 |
| Neurological symptoms B (n) | 13 |
| CNS invasion (n) | 10 |
| Variable | β1 Coefficient | SE | p Value | Odds-Ratio | Chi-Square, p (Model) |
|---|---|---|---|---|---|
| Age (years) | −0.194 | 0.048 | <0.001 *** | 0.824 | χ2 = 24.753 |
| Gender | −0.306 | 0.393 | 0.436 | 0.736 | p < 0.001 *** |
| Intrathecal MTX | −0.072 | 0.083 | 0.388 | 0.931 | |
| Intravenous MTX | 0.211 | 0.084 | 0.012 * | 1.235 | |
| CNS invasion | −0.927 | 0.789 | 0.241 | 0.396 |
| Variable | β1 Coefficient | t Value | p Value | R2, F, p (model) |
|---|---|---|---|---|
| Age (years) | −0.207 | −1.679 | 0.098 | R2 = 0.119, F = 1.678, p = 0.153 |
| Gender | 0.132 | 1.092 | 0.279 | |
| Intrathecal MTX | −0.021 | −0.162 | 0.872 | |
| Intravenous MTX | 0.308 | 2.194 | 0.032 * | |
| CNS invasion | −0.019 | −0.152 | 0.880 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rijmenams, I.; Moechars, D.; Uyttebroeck, A.; Radwan, A.; Blommaert, J.; Deprez, S.; Sunaert, S.; Segers, H.; Gillebert, C.R.; Lemiere, J.; et al. Age- and Intravenous Methotrexate-Associated Leukoencephalopathy and Its Neurological Impact in Pediatric Patients with Lymphoblastic Leukemia. Cancers 2021, 13, 1939. https://doi.org/10.3390/cancers13081939
Rijmenams I, Moechars D, Uyttebroeck A, Radwan A, Blommaert J, Deprez S, Sunaert S, Segers H, Gillebert CR, Lemiere J, et al. Age- and Intravenous Methotrexate-Associated Leukoencephalopathy and Its Neurological Impact in Pediatric Patients with Lymphoblastic Leukemia. Cancers. 2021; 13(8):1939. https://doi.org/10.3390/cancers13081939
Chicago/Turabian StyleRijmenams, Ilona, Daan Moechars, Anne Uyttebroeck, Ahmed Radwan, Jeroen Blommaert, Sabine Deprez, Stefan Sunaert, Heidi Segers, Céline R. Gillebert, Jurgen Lemiere, and et al. 2021. "Age- and Intravenous Methotrexate-Associated Leukoencephalopathy and Its Neurological Impact in Pediatric Patients with Lymphoblastic Leukemia" Cancers 13, no. 8: 1939. https://doi.org/10.3390/cancers13081939
APA StyleRijmenams, I., Moechars, D., Uyttebroeck, A., Radwan, A., Blommaert, J., Deprez, S., Sunaert, S., Segers, H., Gillebert, C. R., Lemiere, J., & Sleurs, C. (2021). Age- and Intravenous Methotrexate-Associated Leukoencephalopathy and Its Neurological Impact in Pediatric Patients with Lymphoblastic Leukemia. Cancers, 13(8), 1939. https://doi.org/10.3390/cancers13081939







