Prospective Comparison of 18-FDG PET/CT and Whole-Body MRI with Diffusion-Weighted Imaging in the Evaluation of Treatment Response of Multiple Myeloma Patients Eligible for Autologous Stem Cell Transplant
Abstract
Simple Summary
Abstract
1. Introduction
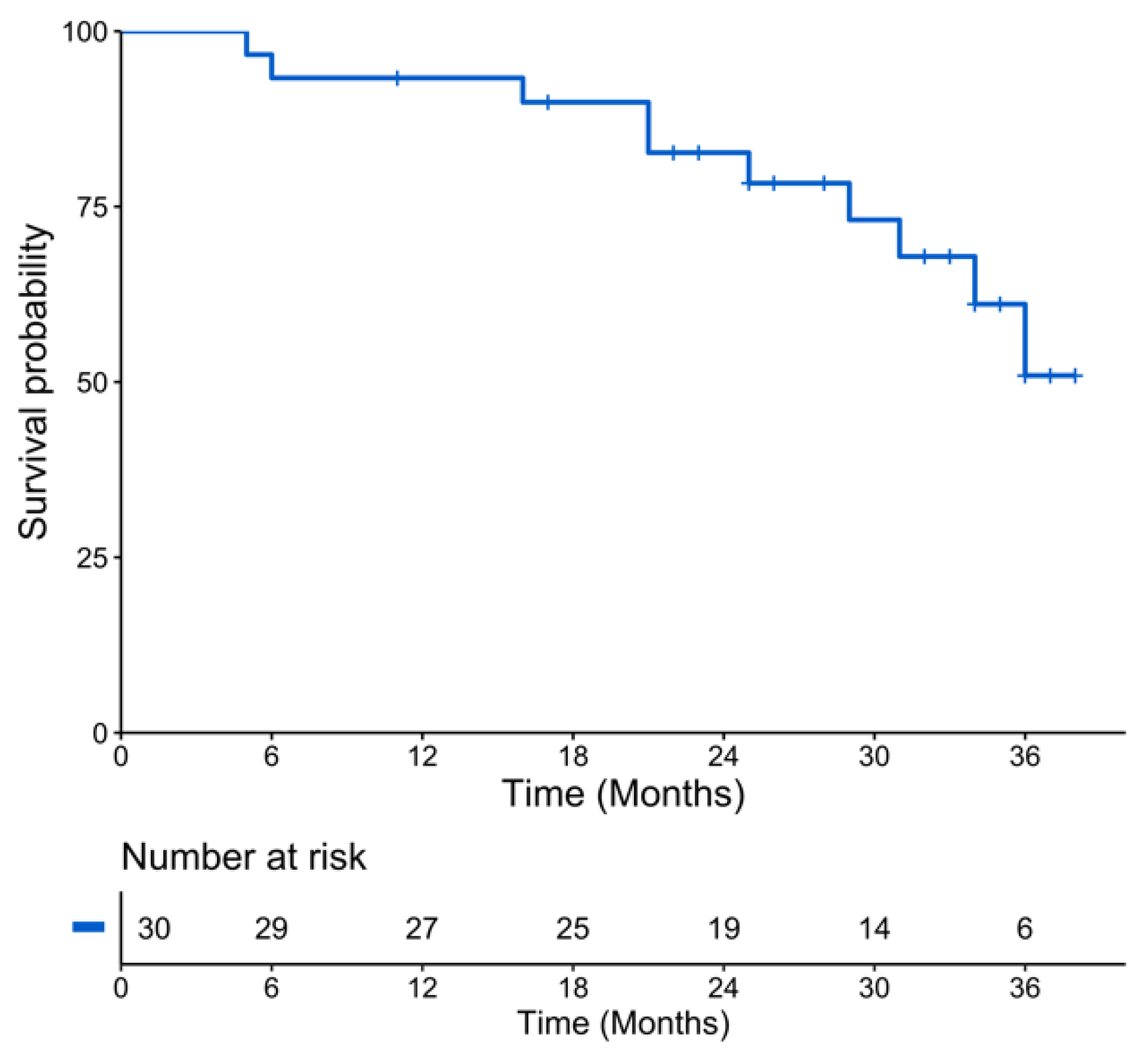
2. Results
2.1. Patients Characteristics
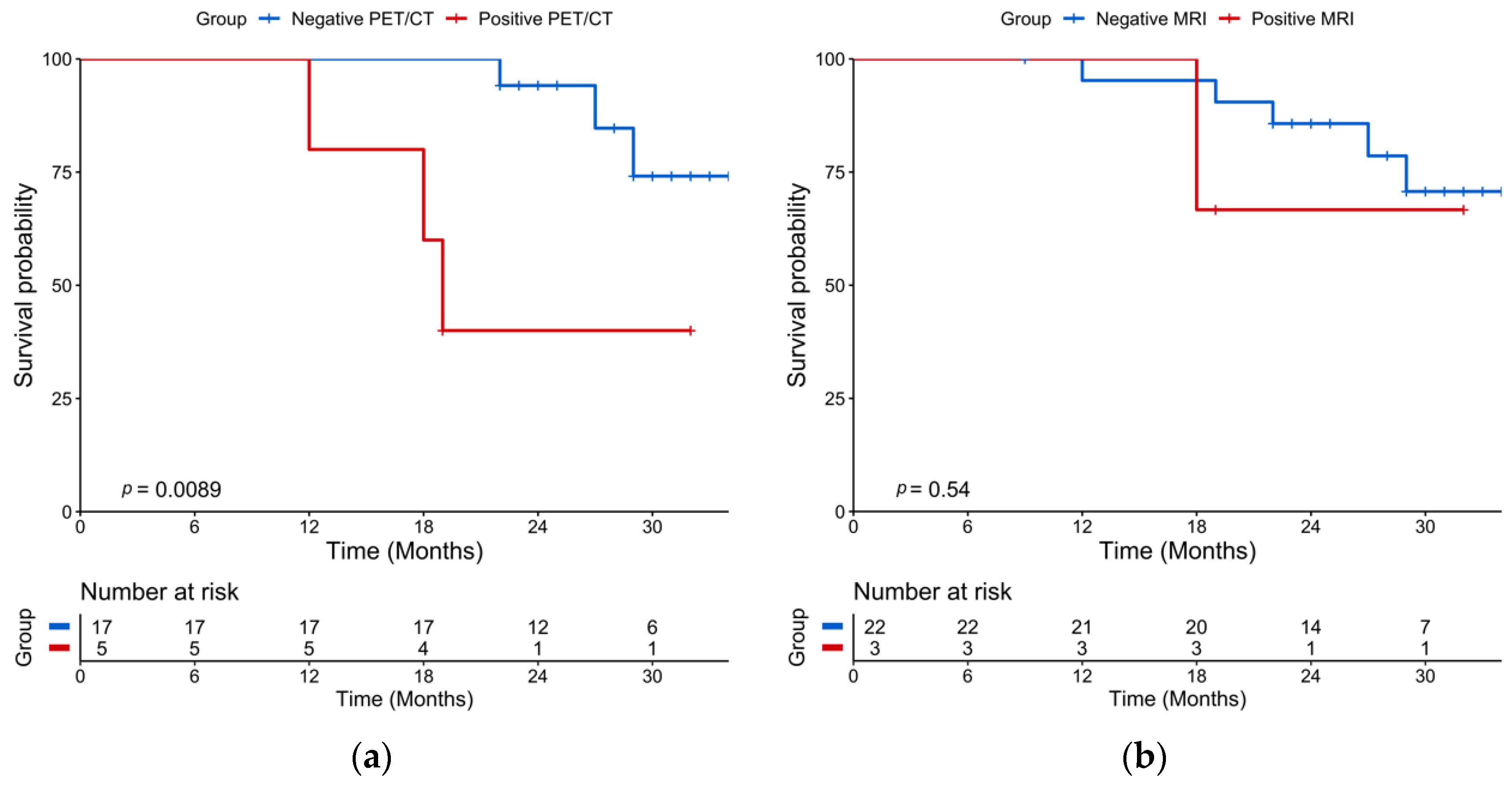
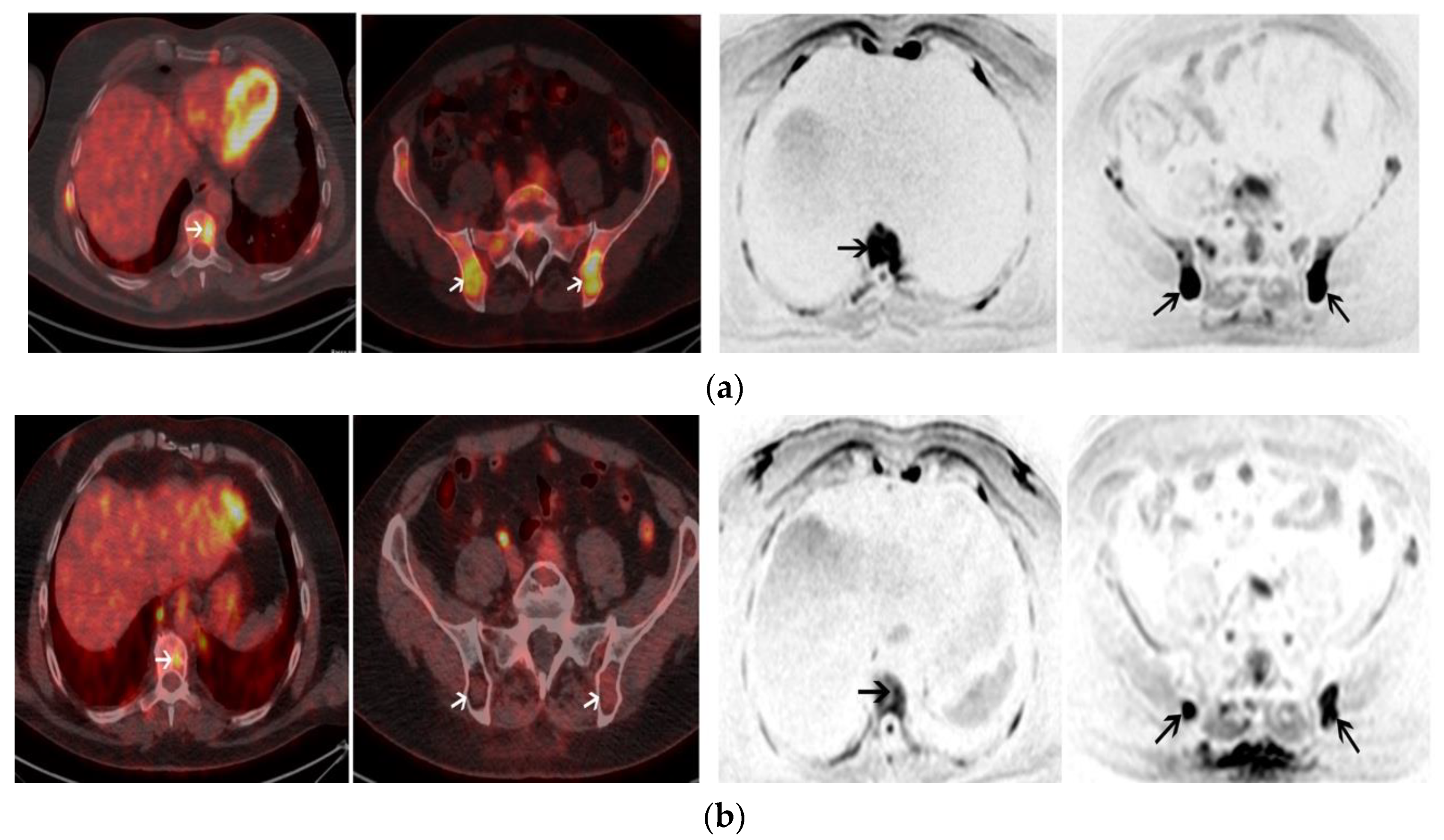
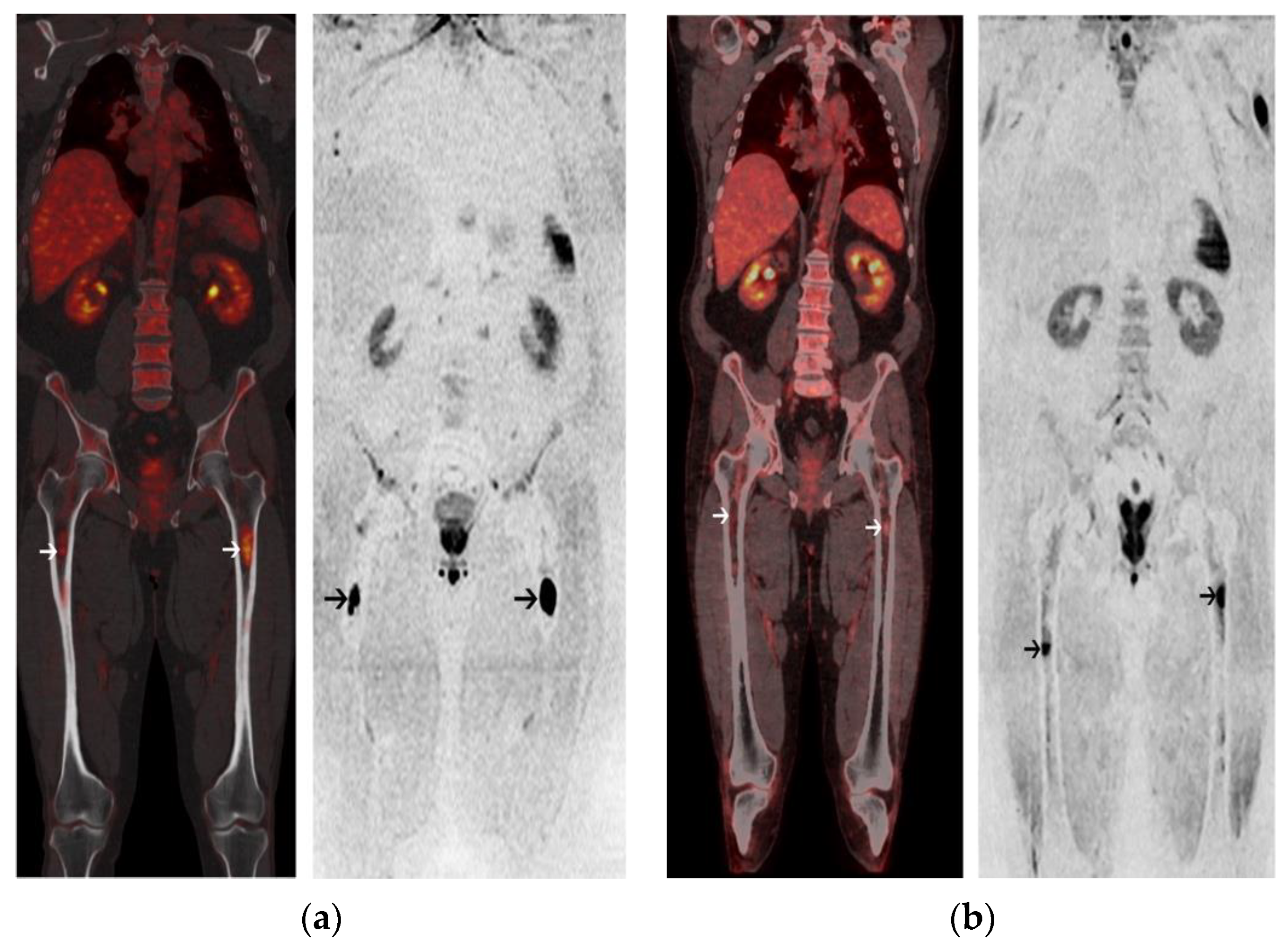
2.2. Post-Induction Chemotherapy Imaging
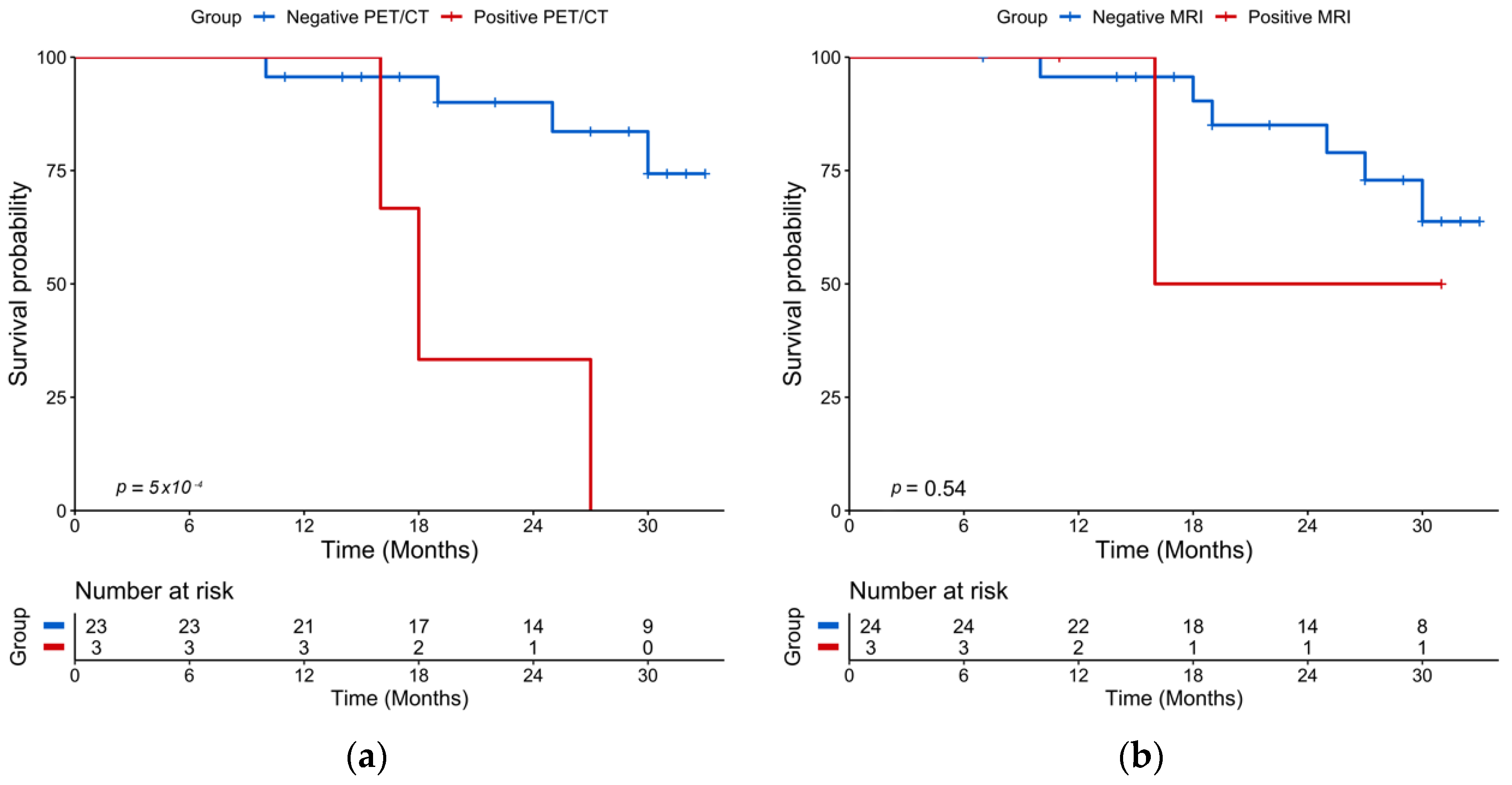
2.3. Post-ASCT Imaging
2.4. Baseline Imaging
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. End Points
4.3. 18-FDG PET/CT
4.4. Whole-Body MRI with Diffusion-Weighted Imaging
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Regelink, J.C.; Minnema, M.C.; Terpos, E.; Kamphuis, M.H.; Raijmakers, P.G.; Pieters-van den Bos, I.C.; Heggelman, B.G.; Nievelstein, R.J.; Otten, R.H.; van Lammeren-Venema, D.; et al. Comparison of modern and conventional imaging techniques in establishing multiple myeloma-related bone disease: A systematic review. Br. J. Hematol. 2013, 162, 50–61. [Google Scholar] [CrossRef]
- Hillengass, J.; Moulopoulos, L.A.; Delorme, S.; Koutoulidis, V.; Mosebach, J.; Hielscher, T.; Drake, M.; Rajkumar, S.V.; Oestergaard, B.; Abildgaard, N.; et al. Whole-body computed tomography versus conventional skeletal survey in patients with multiple myeloma: A study of the International Myeloma Working Group. Blood Cancer J. 2017, 7, e599. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Zamagni, E.; Patriarca, F.; Nanni, C.; Zannetti, B.; Englaro, E.; Pezzi, A.; Tacchetti, P.; Buttignol, S.; Perrone, G.; Brioli, A.; et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood 2011, 118, 5989–5995. [Google Scholar] [CrossRef] [PubMed]
- Usmani, S.Z.; Mitchell, A.; Waheed, S.; Crowley, J.; Hoering, A.; Petty, N.; Brown, T.; Bartel, T.; Anaissie, E.; van Rhee, F.; et al. Prognostic implications of serial 18-fluoro-deoxyglucose emission tomography in multiple myeloma treated with total therapy 3. Blood 2013, 121, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Attal, M.; Caillot, D.; Macro, M.; Karlin, L.; Garderet, L.; Facon, T.; Benboubker, L.; Escoffre-Barbe, M.; Stoppa, A.M.; et al. Prospective Evaluation of Magnetic Resonance Imaging and [(18)F] Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography at Diagnosis and Before Maintenance Therapy in Symptomatic Patients with Multiple Myeloma Included in the IFM/DFCI 2009 Trial: Results of the IMAJEM Study. J. Clin. Oncol. 2017, 35, 2911–2918. [Google Scholar] [CrossRef]
- Spinnato, P.; Bazzocchi, A.; Brioli, A.; Nanni, C.; Zamagni, E.; Albisinni, U.; Cavo, M.; Fanti, S.; Battista, G.; Salizzoni, E. Contrast enhanced MRI and (1)(8)F-FDG PET-CT in the assessment of multiple myeloma: A comparison of results in different phases of the disease. Eur. J. Radiol. 2012, 81, 4013–4018. [Google Scholar] [CrossRef]
- Derlin, T.; Peldschus, K.; Munster, S.; Bannas, P.; Herrmann, J.; Stubig, T.; Habermann, C.R.; Adam, G.; Kroger, N.; Weber, C. Comparative diagnostic performance of (1)(8)F-FDG PET/CT versus whole-body MRI for determination of remission status in multiple myeloma after stem cell transplantation. Eur. Radiol. 2013, 23, 570–578. [Google Scholar] [CrossRef]
- Messiou, C.; Giles, S.; Collins, D.J.; West, S.; Davies, F.E.; Morgan, G.J.; Desouza, N.M. Assessing response of myeloma bone disease with diffusion-weighted MRI. Br. J. Radiol. 2012, 85, e1198–e1203. [Google Scholar] [CrossRef]
- Giles, S.L.; Messiou, C.; Collins, D.J.; Morgan, V.A.; Simpkin, C.J.; West, S.; Davies, F.E.; Morgan, G.J.; de Souza, N.M. Whole-body diffusion-weighted MR imaging for assessment of treatment response in myeloma. Radiology 2014, 271, 785–794. [Google Scholar] [CrossRef]
- Lacognata, C.; Crimi, F.; Guolo, A.; Varin, C.; De March, E.; Vio, S.; Ponzoni, A.; Barila, G.; Lico, A.; Branca, A.; et al. Diffusion-weighted whole-body MRI for evaluation of early response in multiple myeloma. Clin. Radiol. 2017, 72, 850–857. [Google Scholar] [CrossRef]
- Messiou, C.; Hillengass, J.; Delorme, S.; Lecouvet, F.E.; Moulopoulos, L.A.; Collins, D.J.; Blackledge, M.D.; Abildgaard, N.; Ostergaard, B.; Schlemmer, H.P.; et al. Guidelines for Acquisition, Interpretation, and Reporting of Whole-Body MRI in Myeloma: Myeloma Response Assessment and Diagnosis System (MY-RADS). Radiology 2019, 291, 5–13. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2020, 95, 548–567. [Google Scholar] [CrossRef]
- Caldarella, C.; Isgro, M.A.; Treglia, I.; Treglia, G. Is fluorine-18-fluorodeoxyglucose positron emission tomography useful in monitoring the response to treatment in patients with multiple myeloma? Int. J. Hematol. 2012, 96, 685–691. [Google Scholar] [CrossRef]
- Rasche, L.; Alapat, D.; Kumar, M.; Gershner, G.; McDonald, J.; Wardell, C.P.; Samant, R.; Van Hemert, R.; Epstein, J.; Williams, A.F.; et al. Combination of flow cytometry and functional imaging for monitoring of residual disease in myeloma. Leukemia 2019, 33, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Zamagni, E.; Nanni, C.; Dozza, L.; Carlier, T.; Bailly, C.; Tacchetti, P.; Versari, A.; Chauvie, S.; Gallamini, A.; Gamberi, B.; et al. Standardization of (18)F-FDG-PET/CT According to Deauville Criteria for Metabolic Complete Response Definition in Newly Diagnosed Multiple Myeloma. J. Clin. Oncol. 2020, 39, 116–125. [Google Scholar] [CrossRef]
- Latifoltojar, A.; Hall-Craggs, M.; Bainbridge, A.; Rabin, N.; Popat, R.; Rismani, A.; D’Sa, S.; Dikaios, N.; Sokolska, M.; Antonelli, M.; et al. Whole-body MRI quantitative biomarkers are associated significantly with treatment response in patients with newly diagnosed symptomatic multiple myeloma following bortezomib induction. Eur. Radiol. 2017, 27, 5325–5336. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, X.; Fu, Z.; Dai, H.; Yao, F.; Liu, D.; Deng, S.; Hu, C. Whole-body diffusion-weighted MRI for evaluation of response in multiple myeloma patients following bortezomib-based therapy: A large single-center cohort study. Eur. J. Radiol. 2019, 120, 108695. [Google Scholar] [CrossRef]
- Mesguich, C.; Zanotti-Fregonara, P.; Hindie, E. New Perspectives Offered by Nuclear Medicine for the Imaging and Therapy of Multiple Myeloma. Theranostics 2016, 6, 287–290. [Google Scholar] [CrossRef]
- Mesguich, C.; Hulin, C.; Lascaux, A.; Bordenave, L.; Marit, G.; Hindie, E. Choline PET/CT in Multiple Myeloma. Cancers 2020, 12, 1394. [Google Scholar] [CrossRef]
- Jamet, B.; Bailly, C.; Carlier, T.; Touzeau, C.; Nanni, C.; Zamagni, E.; Barre, L.; Michaud, A.V.; Cherel, M.; Moreau, P.; et al. Interest of Pet Imaging in Multiple Myeloma. Front. Med. 2019, 6, 69. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Heuck, C.; Mitchell, A.; Szymonifka, J.; Nair, B.; Hoering, A.; Alsayed, Y.; Waheed, S.; Haider, S.; Restrepo, A.; et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over-represented in high-risk disease even in the era of novel agents. Haematologica 2012, 97, 1761–1767. [Google Scholar] [CrossRef]
- Matsue, K.; Kobayashi, H.; Matsue, Y.; Abe, Y.; Narita, K.; Kitadate, A.; Takeuchi, M. Prognostic significance of bone marrow abnormalities in the appendicular skeleton of patients with multiple myeloma. Blood Adv. 2018, 2, 1032–1039. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Blade, J.; Mateos, M.V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Mesguich, C.; Hulin, C.; Latrabe, V.; Lascaux, A.; Bordenave, L.; Hindie, E.; Marit, G. Prospective comparison of 18-FDG PET/CT and whole-body diffusion-weighted MRI in the assessment of multiple myeloma. Ann. Hematol. 2020, 99, 2869–2880. [Google Scholar] [CrossRef]
- Zamagni, E.; Nanni, C.; Gay, F.; Pezzi, A.; Patriarca, F.; Bello, M.; Rambaldi, I.; Tacchetti, P.; Hillengass, J.; Gamberi, B.; et al. 18F-FDG PET/CT focal, but not osteolytic, lesions predict the progression of smoldering myeloma to active disease. Leukemia 2016, 30, 417–422. [Google Scholar] [CrossRef]
- Mesguich, C.; Fardanesh, R.; Tanenbaum, L.; Chari, A.; Jagannath, S.; Kostakoglu, L. State of the art imaging of multiple myeloma: Comparative review of FDG PET/CT imaging in various clinical settings. Eur. J. Radiol. 2014, 83, 2203–2223. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Value N = 30 | Range or % |
|---|---|---|
| Median age, years (range) | 56 | 34–66 |
| Male | 17 | 56 |
| Monoclonal protein isotype | ||
| IgG | 12 | 40 |
| IgA | 5 | 17 |
| IgD | 1 | 3 |
| Light chain | 12 | 40 |
| kappa | 10 | 33 |
| lambda | 2 | 7 |
| Low hemoglobin (≤10 g/dL) | 7 | 63 |
| Low platelets (<150 G/L) | 3 | 10 |
| High calcium (>12 mg/dL) | 4 | 13 |
| High LDH (>250 UI/L) | 13 | 43 |
| Altered renal function (creatinine ≤ 2 mg/dL) | 4 | 13 |
| Beta-2 microglobulin | ||
| 3.5–5.5 mg/L | 7 | 23 |
| >5.5 mg/L | 5 | 17 |
| Median bone marrow plasmocytosis, % | 28 | 0–87 |
| Median serum free light chain (mg/L) | 343 | 3.2–17,600 |
| High-risk cytogenetic: t (4;14) or del17p | 2 | 7 |
| ISS 1 | 17 | 57 |
| ISS 2 | 8 | 27 |
| ISS 3 | 5 | 17 |
| Induction chemotherapy | ||
| VRD | 12 | 40 |
| VTD | 6 | 20 |
| VTD-Dara | 6 | 20 |
| VRD-Dara | 4 | 13 |
| IRD | 1 | 3 |
| IRD-Dara | 1 | 3 |
| Variable | Hazard Ratio | ||
|---|---|---|---|
| Estimate | 95% CI | p | |
| Age | 0.99 | 0.92–1.09 | 0.96 |
| Male sex | 2.82 | 0.68–11.8 | 0.16 |
| Low hemoglobin (≤10 g/dL) | 2.37 | 0.61–9.21 | 0.21 |
| Low platelets (<150 G/L) | 1.55 | 0.15–13.2 | 0.59 |
| Altered renal function (creatinine ≤ 2mg/dL) | 4.10 | 0.74–22.6 | 0.11 |
| High calcium (>12 mg/dL) | 1.81 | 0.20–16.3 | 0.60 |
| High LDH (>250 UI/L) | 0.85 | 0.21–3.37 | 0.82 |
| Low albumin (<35 g/L) | 2.50 | 0.26–24.5 | 0.43 |
| High CRP (>5 mg/L) | 6.30 | 1.26–31,6 | 0.03 |
| Cytogenetic t (4;14); del17p | 12.84 | 2.06–80.1 | 0.006 |
| ISS 2 to 3 | 6.00 | 1.23–29.1 | 0.03 |
| FL SUVmax > 4.2 on baseline PET/CT | 2.47 | 0.31–19.6 | 0.39 |
| FL SUVmax > 6.1 on baseline PET/CT | 6.08 | 1.29–28.8 | 0.02 |
| FL ADC on baseline WB-DW-MRI | 1.00 | 0.99–1.00 | 0.58 |
| >3FL on baseline PET/CT | 2.95 | 0.33–0.63 | 0.17 |
| >11FL on baseline PET/CT | 5.06 | 1.28–20.0 | 0.02 |
| >7FL on baseline MRI | 4.38 | 0.23–0.87 | 0.07 |
| Inferior limb involvement on baseline PET/CT | 3.74 | 1.03–13.6 | 0.05 |
| >4 skeletal areas involved on baseline PET/CT | 5.46 | 1.49–20.0 | 0.01 |
| >4 skeletal areas involved on baseline MRI | 3.98 | 1.10–14.5 | 0.04 |
| Extramedullary disease on baseline PET/CT | 7.00 | 1.34–36.5 | 0.02 |
| Diffuse disease on baseline PET/CT | 1.16 | 0.33–4.12 | 0.82 |
| Diffuse disease on baseline MRI | 3.07 | 0.64–7.84 | 0.21 |
| Response ≥ VGPR after induction chemotherapy | 1.11 | 0.26–4.73 | 0.89 |
| Response ≥ VGPR after ASCT | 1.25 | 0.15–10.9 | 0.84 |
| Positive PET/CT after induction chemotherapy | 6.79 | 1.30–35.5 | 0.02 |
| FL SUVmax > 3.95 on post-induction PET/CT | 8.30 | 1.16–42.5 | 0.01 |
| Positive PET/CT after ASCT | 10.15 | 2.00–51.4 | 0.005 |
| FL SUVmax > 3.2 on post-ASCT PET/CT | 11.32 | 2.06–62.4 | 0.005 |
| Positive WB-DW-MRI after induction chemotherapy | 2.12 | 0.24–18.4 | 0.50 |
| FL ADC on post-induction WB-DW-MRI | 1.00 | 0.99–1.00 | 0.69 |
| Positive WB-DW-MRI after ASCT | 1.89 | 0.22–15.8 | 0.56 |
| FL ADC on post-ASCT WB-DW-MRI | 1.00 | 0.99–1.00 | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesguich, C.; Latrabe, V.; Hulin, C.; Lascaux, A.; Bordenave, L.; Hindié, E.; Marit, G. Prospective Comparison of 18-FDG PET/CT and Whole-Body MRI with Diffusion-Weighted Imaging in the Evaluation of Treatment Response of Multiple Myeloma Patients Eligible for Autologous Stem Cell Transplant. Cancers 2021, 13, 1938. https://doi.org/10.3390/cancers13081938
Mesguich C, Latrabe V, Hulin C, Lascaux A, Bordenave L, Hindié E, Marit G. Prospective Comparison of 18-FDG PET/CT and Whole-Body MRI with Diffusion-Weighted Imaging in the Evaluation of Treatment Response of Multiple Myeloma Patients Eligible for Autologous Stem Cell Transplant. Cancers. 2021; 13(8):1938. https://doi.org/10.3390/cancers13081938
Chicago/Turabian StyleMesguich, Charles, Valérie Latrabe, Cyrille Hulin, Axelle Lascaux, Laurence Bordenave, Elif Hindié, and Gerald Marit. 2021. "Prospective Comparison of 18-FDG PET/CT and Whole-Body MRI with Diffusion-Weighted Imaging in the Evaluation of Treatment Response of Multiple Myeloma Patients Eligible for Autologous Stem Cell Transplant" Cancers 13, no. 8: 1938. https://doi.org/10.3390/cancers13081938
APA StyleMesguich, C., Latrabe, V., Hulin, C., Lascaux, A., Bordenave, L., Hindié, E., & Marit, G. (2021). Prospective Comparison of 18-FDG PET/CT and Whole-Body MRI with Diffusion-Weighted Imaging in the Evaluation of Treatment Response of Multiple Myeloma Patients Eligible for Autologous Stem Cell Transplant. Cancers, 13(8), 1938. https://doi.org/10.3390/cancers13081938







