Multicenter Analysis of Treatment Outcomes for Systemic Therapy in Well Differentiated Grade 3 Neuroendocrine Tumors (NET G3)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics, Survival, and Follow-Up
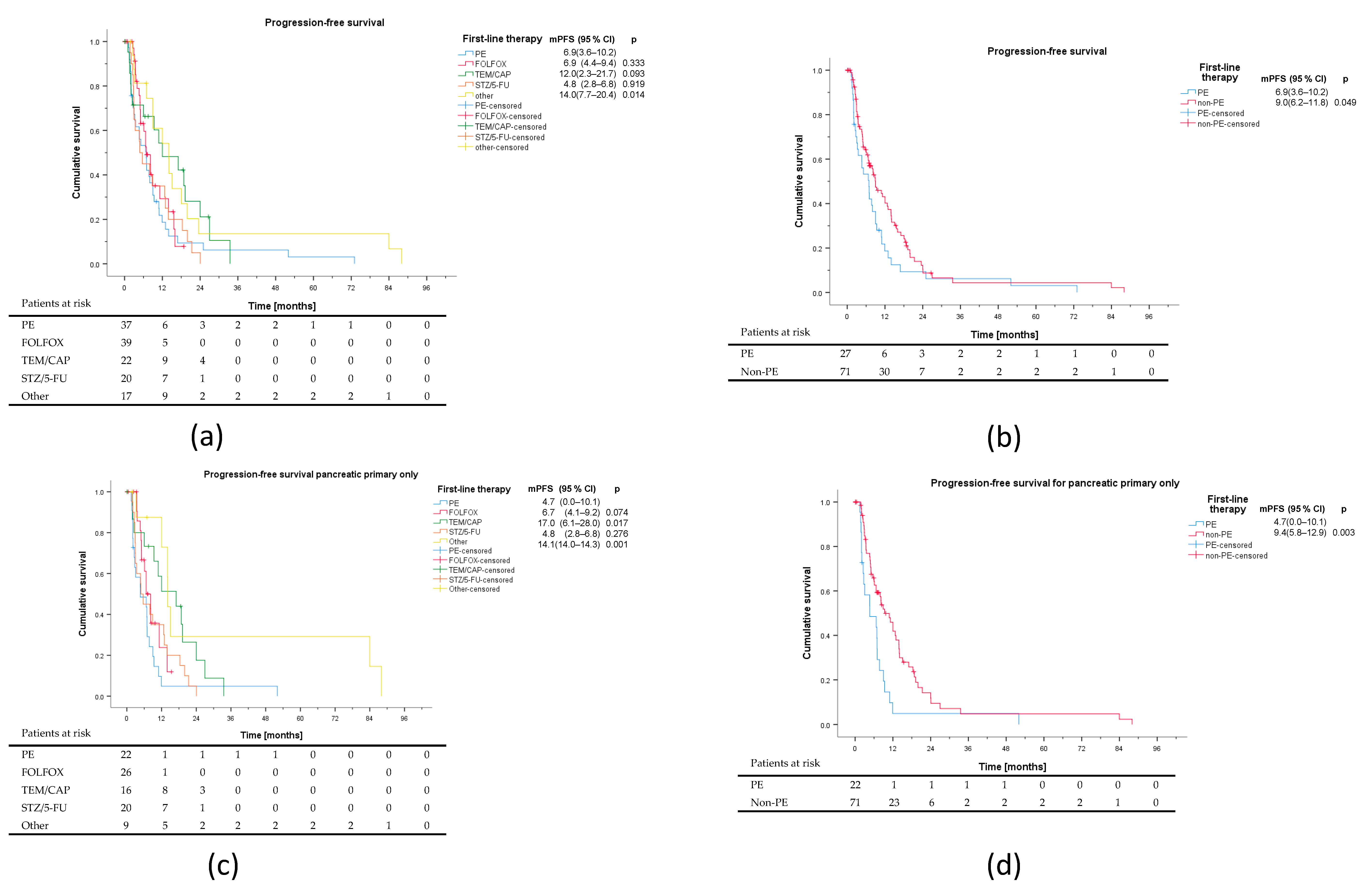
3.2. First-Line Therapy
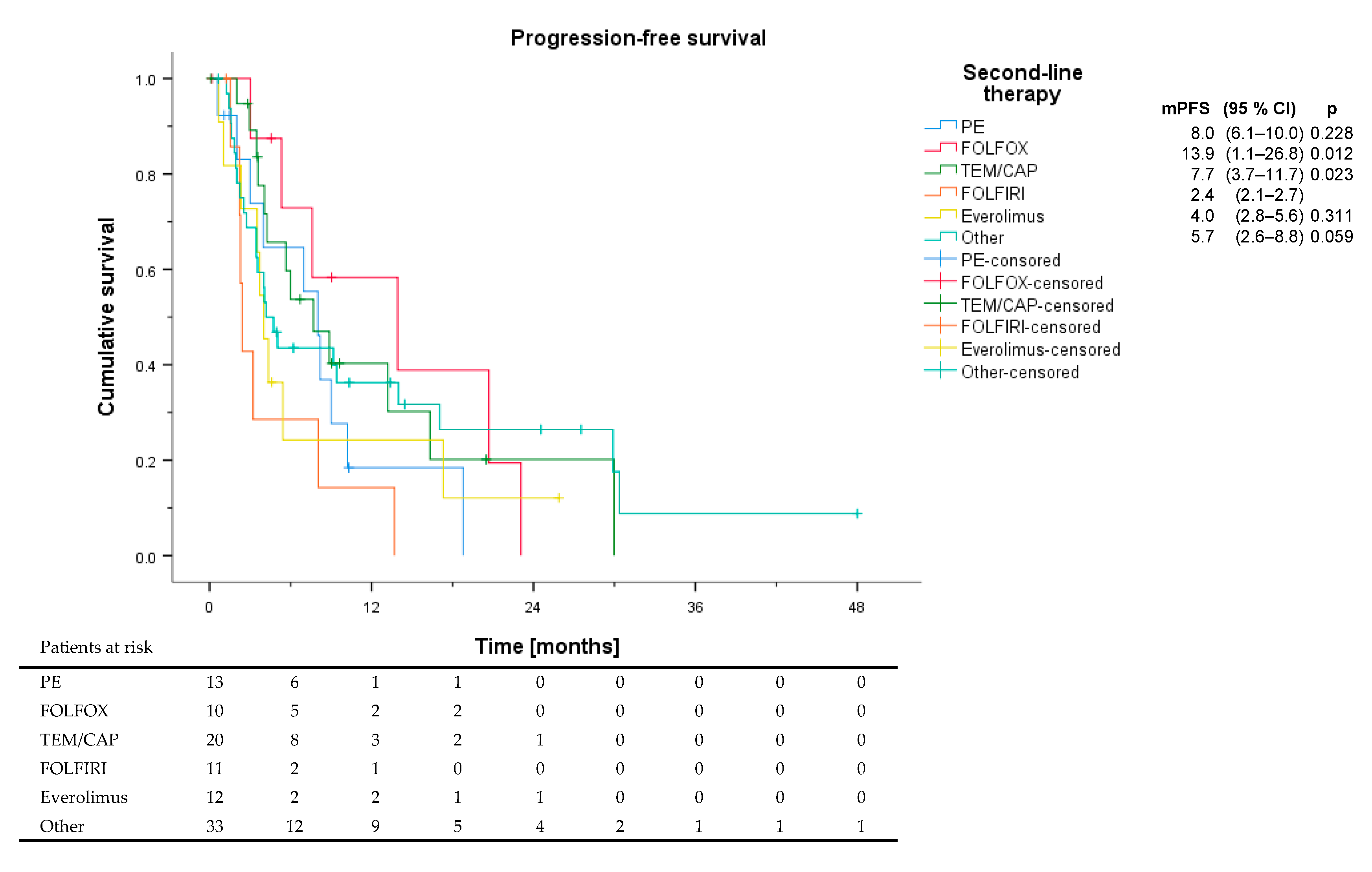
3.3. Second-Line Therapy and Beyond
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. WHO Classification of Tumours of the Digestive System, 4th ed.; IARC Press: Lyon, France, 2010. [Google Scholar]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs, 4th ed.; IARC Press: Lyon, France, 2017. [Google Scholar]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours. Digestive System Tumours, 5th ed.; IARC Press: Lyon, France, 2019. [Google Scholar]
- Sorbye, H.; Welin, S.; Langer, S.W.; Vestermark, L.W.; Holt, N.; Osterlund, P.; Dueland, S.; Hofsli, E.; Guren, M.G.; Ohrling, K.; et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann. Oncol. 2013, 24, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Elvebakken, H.; Perren, A.; Scoazec, J.Y.; Tang, L.H.; Federspiel, B.; Klimstra, D.S.; Vestermark, L.W.; Ali, A.S.; Zlobec, I.; Myklebust, T.A.; et al. A consensus developed morphological re-evaluation of 196 high-grade gastroenteropancreatic neuroendocrine neoplasms and its clinical correlations. Neuroendocrinology 2020. [Google Scholar] [CrossRef]
- Heetfeld, M.; Chougnet, C.N.; Olsen, I.H.; Rinke, A.; Borbath, I.; Crespo, G.; Barriuso, J.; Pavel, M.; O’Toole, D.; Walter, T.; et al. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr. Relat. Cancer 2015, 22, 657–664. [Google Scholar] [CrossRef]
- Velayoudom-Cephise, F.L.; Duvillard, P.; Foucan, L.; Hadoux, J.; Chougnet, C.N.; Leboulleux, S.; Malka, D.; Guigay, J.; Goere, D.; Debaere, T.; et al. Are G3 ENETS neuroendocrine neoplasms heterogeneous? Endocr. Relat. Cancer 2013, 20, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Deutsche Gesellschaft fur Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS); Netzwerk Neuroendokrine Tumoren e.V.; Bundesorganisation Selbsthilfe NeuroEndokrine Tumoren e.V. (NET-sgh).; Deutsche Gesellschaft fur Hamatologie und Medizinische Onkologie, e.V. (DGHO), und Arbeitsgemeinschaft Internistische Onkologie (AIO) der Deutschen Krebsgesellschaft e.V.; Deutsche Gesellschaft fur Allgemein-und Viszeralchirurgie-und Viszeralchirurgie e.V. (DGAV); Deutsche Gesellschaft fur Chirurgie (DGCH); Deutsche Gesellschaft fur Endoskopie und Bildgebende Verfahren (DGEBV); Deutsche Gesellschaft fur Nuklearmedizin e.V. (DGNM); Deutsche Gesellschaft fur Innere Medizin (DGIM); Deutsche Gesellschaft fur Endokrinologie (DGE); et al. Practice guideline neuroendocrine tumors—AWMF-Reg. 021-27. Z Gastroenterol. 2018, 56, 583–681. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Sorbye, H.; Baudin, E.; Raymond, E.; Wiedenmann, B.; Niederle, B.; Sedlackova, E.; Toumpanakis, C.; Anlauf, M.; Cwikla, J.B.; et al. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology 2016, 103, 186–194. [Google Scholar] [CrossRef]
- Pavel, M.; Oberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Hadoux, J.; Malka, D.; Planchard, D.; Scoazec, J.Y.; Caramella, C.; Guigay, J.; Boige, V.; Leboulleux, S.; Burtin, P.; Berdelou, A.; et al. Post-first-line FOLFOX chemotherapy for grade 3 neuroendocrine carcinoma. Endocr. Relat. Cancer 2015, 22, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Welin, S.; Sorbye, H.; Sebjornsen, S.; Knappskog, S.; Busch, C.; Oberg, K. Clinical effect of temozolomide-based chemotherapy in poorly differentiated endocrine carcinoma after progression on first-line chemotherapy. Cancer 2011, 117, 4617–4622. [Google Scholar] [CrossRef]
- Konukiewitz, B.; Schlitter, A.M.; Jesinghaus, M.; Pfister, D.; Steiger, K.; Segler, A.; Agaimy, A.; Sipos, B.; Zamboni, G.; Weichert, W.; et al. Somatostatin receptor expression related to TP53 and RB1 alterations in pancreatic and extrapancreatic neuroendocrine neoplasms with a Ki67-index above 20. Mod. Pathol. 2017, 30, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Yachida, S.; Vakiani, E.; White, C.M.; Zhong, Y.; Saunders, T.; Morgan, R.; de Wilde, R.F.; Maitra, A.; Hicks, J.; Demarzo, A.M.; et al. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am. J. Surg. Pathol. 2012, 36, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Merola, E.; Dal Buono, A.; Denecke, T.; Arsenic, R.; Pape, U.F.; Jann, H.; Wiedenmann, B.; Pavel, M.E. Efficacy and Toxicity of 5-Fluorouracil-Oxaliplatin in Gastroenteropancreatic Neuroendocrine Neoplasms. Pancreas 2020, 49, 912–917. [Google Scholar] [CrossRef]
- Spada, F.; Antonuzzo, L.; Marconcini, R.; Radice, D.; Antonuzzo, A.; Ricci, S.; Di Costanzo, F.; Fontana, A.; Gelsomino, F.; Luppi, G.; et al. Oxaliplatin-Based Chemotherapy in Advanced Neuroendocrine Tumors: Clinical Outcomes and Preliminary Correlation with Biological Factors. Neuroendocrinology 2016, 103, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.R.; Fine, R.L.; Choi, J.; Nasir, A.; Coppola, D.; Chen, D.T.; Helm, J.; Kvols, L. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011, 117, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Kunz, P.L.; Catalano, P.J.; Nimeiri, H.; Fisher, G.A.; Longacre, T.A.; Suarez, C.J.; Yao, J.C.; Kulke, M.H.; Hendifar, A.E.; Shanks, J.C.; et al. A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: A trial of the ECOG-ACRIN Cancer Research Group (E2211). J. Clin. Oncol. 2018, 36, 4004. [Google Scholar] [CrossRef]
- De Mestier, L.; Walter, T.; Evrard, C.; de Boissieu, P.; Hentic, O.; Cros, J.; Tougeron, D.; Lombard-Bohas, C.; Rebours, V.; Hammel, P.; et al. Temozolomide Alone or Combined with Capecitabine for the Treatment of Advanced Pancreatic Neuroendocrine Tumor. Neuroendocrinology 2020, 110, 83–91. [Google Scholar] [CrossRef]
- Chatzellis, E.; Angelousi, A.; Daskalakis, K.; Tsoli, M.; Alexandraki, K.I.; Wachula, E.; Meirovitz, A.; Maimon, O.; Grozinsky-Glasberg, S.; Gross, D.; et al. Activity and Safety of Standard and Prolonged Capecitabine/Temozolomide Administration in Patients with Advanced Neuroendocrine Neoplasms. Neuroendocrinology 2019, 109, 333–345. [Google Scholar] [CrossRef]
- Moertel, C.G.; Hanley, J.A.; Johnson, L.A. Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N. Engl. J. Med. 1980, 303, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Moertel, C.G.; Lefkopoulo, M.; Lipsitz, S.; Hahn, R.G.; Klaassen, D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N. Engl. J. Med. 1992, 326, 519–523. [Google Scholar] [CrossRef]
- Hentic, O.; Hammel, P.; Couvelard, A.; Rebours, V.; Zappa, M.; Palazzo, M.; Maire, F.; Goujon, G.; Gillet, A.; Levy, P.; et al. FOLFIRI regimen: An effective second-line chemotherapy after failure of etoposide-platinum combination in patients with neuroendocrine carcinomas grade 3. Endocr. Relat. Cancer 2012, 19, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.; et al. Everolimus for advanced pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef]
- Okuyama, H.; Ikeda, M.; Okusaka, T.; Furukawa, M.; Ohkawa, S.; Hosokawa, A.; Kojima, Y.; Hara, H.; Murohisa, G.; Shioji, K.; et al. A Phase II Trial of Everolimus in Patients with Advanced Pancreatic Neuroendocrine Carcinoma Refractory or Intolerant to Platinum-Containing Chemotherapy (NECTOR Trial). Neuroendocrinology 2020, 110, 988–993. [Google Scholar] [CrossRef]
- Panzuto, F.; Rinzivillo, M.; Spada, F.; Antonuzzo, L.; Ibrahim, T.; Campana, D.; Fazio, N.; Delle Fave, G. Everolimus in Pancreatic Neuroendocrine Carcinomas G3. Pancreas 2017, 46, 302–305. [Google Scholar] [CrossRef]
- Raymond, E.; Dahan, L.; Raoul, J.L.; Bang, Y.J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Pellat, A.; Dreyer, C.; Couffignal, C.; Walter, T.; Lombard-Bohas, C.; Niccoli, P.; Seitz, J.F.; Hentic, O.; Andre, T.; Coriat, R.; et al. Clinical and Biomarker Evaluations of Sunitinib in Patients with Grade 3 Digestive Neuroendocrine Neoplasms. Neuroendocrinology 2018, 107, 24–31. [Google Scholar] [CrossRef]
- Thang, S.P.; Lung, M.S.; Kong, G.; Hofman, M.S.; Callahan, J.; Michael, M.; Hicks, R.J. Peptide receptor radionuclide therapy (PRRT) in European Neuroendocrine Tumour Society (ENETS) grade 3 (G3) neuroendocrine neoplasia (NEN)—A single-institution retrospective analysis. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, E.A.; Fazio, N.; Granberg, D.; Grozinsky-Glasberg, S.; Ahmadzadehfar, H.; Grana, C.M.; Zandee, W.T.; Cwikla, J.; Walter, M.A.; Oturai, P.S.; et al. Peptide receptor radionuclide therapy in gastroenteropancreatic NEN G3: A multicenter cohort study. Endocr. Relat. Cancer 2019, 26, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, S.; Severi, S.; Ianniello, A.; Sansovini, M.; Ambrosetti, A.; Bongiovanni, A.; Scarpi, E.; Di Mauro, F.; Rossi, A.; Matteucci, F.; et al. Investigation of receptor radionuclide therapy with (177)Lu-DOTATATE in patients with GEP-NEN and a high Ki-67 proliferation index. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kulkarni, H.R.; Singh, A.; Niepsch, K.; Muller, D.; Baum, R.P. Peptide Receptor Radionuclide Therapy in Grade 3 Neuroendocrine Neoplasms: Safety and Survival Analysis in 69 Patients. J. Nucl. Med. 2019, 60, 377–385. [Google Scholar] [CrossRef]
- Vijayvergia, N.; Dasari, A.; Deng, M.; Litwin, S.; Al-Toubah, T.; Alpaugh, R.K.; Dotan, E.; Hall, M.J.; Ross, N.M.; Runyen, M.M.; et al. Pembrolizumab monotherapy in patients with previously treated metastatic high-grade neuroendocrine neoplasms: Joint analysis of two prospective, non-randomised trials. Br. J. Cancer 2020, 122, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Othus, M.; Chae, Y.K.; Giles, F.J.; Hansel, D.E.; Singh, P.P.; Fontaine, A.; Shah, M.H.; Kasi, A.; Baghdadi, T.A.; et al. A Phase II Basket Trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2020, 26, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Klein, O.; Kee, D.; Markman, B.; Michael, M.; Underhill, C.; Carlino, M.S.; Jackett, L.; Lum, C.; Scott, C.; Nagrial, A.; et al. Immunotherapy of Ipilimumab and Nivolumab in Patients with Advanced Neuroendocrine Tumors: A Subgroup Analysis of the CA209-538 Clinical Trial for Rare Cancers. Clin. Cancer Res. 2020, 26, 4454–4459. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Number of Patients (n = 142) | % | ||
|---|---|---|---|---|
| Age (years) | Median (Range) | 57 (14–81) | ||
| Sex | Male | 79 | 55.6 | |
| Female | 63 | 44.4 | ||
| Stage | I | 2 | 1.4 | |
| II | 7 | 4.9 | ||
| III | 26 | 18.3 | ||
| IV | 107 | 75.4 | ||
| Ki67 (%) | Median (Range) | 30 (21–70) | ||
| Primary | Pancreas | 99 | 69.7 | |
| Small intestine | 13 | 9.2 | ||
| Stomach/esophagus | 8 | 5.6 | ||
| Colorectal | 3 | 2.1 | ||
| Other | 3 | 2.1 | ||
| Unknown | 16 | 11.3 | ||
| SSTR Imaging | Positive | 64 | 45.1 | |
| Negative | 19 | 13.4 | ||
| Prior NET G1/G2 | 23 | 16.2 | ||
| Functional activity | 16 | 11.3 | ||
| Metastatic sites | Median (Range) | 2 (0–4) | ||
| Liver | 123 | 86.6 | ||
| Lymph nodes | 70 | 49.3 | ||
| Bone | 34 | 23.9 | ||
| Lung | 14 | 9.9 | ||
| Peritoneum | 19 | 13.4 | ||
| Brain | 4 | 2.8 | ||
| Pleura | 2 | 1.4 | ||
| Other | 14 | 9.9 | ||
| Liver only | 41 | 28.9 | ||
| LDH | Median [U/mL] (Range) | 249.0 (110.0–1647.0) | ||
| >ULN | 16 | 11.3 | ||
| ≤ULN | 87 | 72.5 | ||
| CgA | Median [ng/mL] (Range) | 256.6 (5.0–220,900.0) | ||
| >ULN | 101 | 71.1 | ||
| ≤ULN | 20 | 14.1 | ||
| NSE | Median [ng/mL] (Range) | 29.05 (5.2–346.9) | ||
| >ULN | 96 | 67.6 | ||
| ≤ULN | 12 | 8.5 |
| PE | (%) | FOLFOX | (%) | TEM/CAP | (%) | STZ/5-FU | (%) | Other | (%) | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CR | 1 | 2.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 5.6 | |
| PR | 12 | 32.4 | 22 | 56.4 | 6 | 27.3 | 9 | 45.0 | 2 | 11.1 | |
| SD | 11 | 29.7 | 10 | 25.6 | 9 | 40.9 | 5 | 25.0 | 8 | 44.4 | |
| PD | 12 | 32.4 | 4 | 10.3 | 6 | 27.3 | 6 | 30.0 | 5 | 27.8 | |
| NE | 1 | 2.7 | 3 | 7.7 | 1 | 4.5 | 0 | 0.0 | 2 | 11.1 | |
| ORR | 13 | 35.1 | 22 | 56.4 | 6 | 27.3 | 9 | 45.0 | 3 | 16.7 | 0.032 |
| p * | 0.063 | ref | 0.028 | 0.406 | 0.005 | ||||||
| DCR | 24 | 64.9 | 32 | 82.1 | 15 | 68.2 | 14 | 70.0 | 11 | 61.1 | 0.420 |
| Characteristics | PE (n = 37) | FOLFOX (n = 32) | TEM/CAP (n = 29) | STZ/5-FU (n = 20) | Other (n = 18) | p |
|---|---|---|---|---|---|---|
| Sex, male | 19 (51.4%) | 23 (59.0%) | 14 (63.6%) | 11 (55.0%) | 9 (50.0%) | 0.869 |
| Age | 59 (25–79) | 62 (21–77) | 60 (29–80) | 50 (32–70) | 51 (14–81) | 0.045 |
| Ki67 | 40 (21–70) | 30 (22–70) | 25 (21–50) | 25 (20–60) | 30 (21–70) | <0.001 |
| p * | ref | 0.728 | 0.004 | <0.001 | 0.002 | |
| p * | 0.728 | ref | 0.002 | <0.001 | 0.019 | |
| Primary | 0.044 | |||||
| Pancreas | 22 (59.5%) | 26 (66.7%) | 16 (72.7%) | 20 (100.0%) | 10 (55.6%) | |
| Stomach/esophagus | 3 (8.1%) | 4 (10.3%) | 0 (0.0%) | 0 (0.0%) | 1 (5.6%) | |
| Small intestine | 1 (2.7%) | 5 (12.8%) | 2 (9.1%) | 0 (0.0%) | 5 (27.8%) | |
| Colorectal | 2 (5.4%) | 1 (2.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Other | 2 (5.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (5.6%) | |
| Unknown | 7 (18.9%) | 3 (7.7%) | 4 (18.2%) | 0 (0.0%) | 1 (5.6%) | |
| SSTR positive | 10 (62.5%) | 15 (71.4%) | 15 (78.9%) | 12 (80.0%) | 11 (100.0%) | 0.228 |
| Functional activity | 1 (2.7%) | 5 (12.8%) | 1 (4.5%) | 5 (25.0%) | 4 (22.2%) | |
| Prior NET G1/G2 | 2 (5.4%) | 12 (30.8%) | 4 (18.2%) | 2 (10.0%) | 3 (16.7%) | 0.049 |
| Metastatic sites | 1 (1–4) | 2 (1–5) | 2 (1–5) | 2 (1–4) | 3 (1–5) | 0.018 |
| p * | 0.001 | 0.026 | 0.014 | 0.125 | ref | |
| Liver only | 16 (44.4%) | 9 (23.7%) | 6 (27.3%) | 8 (40.0%) | 1 (5.9%) | 0.040 |
| LDH | ||||||
| Median [U/mL] (Range) | 253.0 (3.9–495.0) | 258.0 (168.0–1647.0) | 247.0 (179.0–403.0) | 200.5 (110.0–292.0) | 231.0 (144.0–420.0) | 0.192 |
| >ULN | 3 (10.0%) | 9 (27.3%) | 2 (12.5%) | 0 (0.0%) | 1 (6.7%) | 0.201 |
| ≤ULN | 27 (90.0%) | 24 (72.7%) | 14 (87.5%) | 4 (100.0%) | 14 (93.3%) | |
| CgA | ||||||
| Median [ng/mL] (Range) | 233.5 (16.3–220,900.0) | 494.9 (5.0–84,240.0) | 294.8 (60.3–42,260.0) | 256.2 (37.4–1973.0) | 293.1 (27.4–5867.0) | 0.704 |
| >ULN | 28 (84.8%) | 29 (80.6%) | 16 (84.2%) | 14 (93.3%) | 11 (73.3%) | 0.665 |
| ≤ULN | 5 (15.2%) | 7 (19.4%) | 3 (15.8%) | 1 (6.7%) | 4 (26.7%) | |
| NSE | ||||||
| Median [ng/mL] (Range) | 29.1 (5.2–205.7) | 36.7 (16.1–346.9) | 31.2 (8.0–105.7) | 29.2 (13.1–92.0) | 24.5 (15.6–51.8) | 0.166 |
| >ULN | 26 (86.7%) | 33 (97.1%) | 16 (88.9%) | 6 (75.0%) | 12 (80.0%) | 0.280 |
| ≤ULN | 4 (13.3%) | 1 (2.9%) | 2 (11.1%) | 2 (25.0%) | 3 (20.0%) |
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Sex | 1.262 (0.860–1.851) | 0.234 | 1.214 (0.655–2.250) | 0.537 |
| Age * | 1.001 (0.987–1.015) | 0.893 | ||
| Primary pancreatic | 0.987 (0.656–1.483) | 0.948 | ||
| Ki67 * | 1.007 (0.991–1.022) | 0.392 | ||
| SSTR positive | 0.681 (0.380–1.220) | 0.194 | 0.728 (0.350–1.514) | 0.395 |
| Prior NET G1/G2 | 0.977 (0.588–1.624) | 0.928 | ||
| Metastatic sites * | 0.969 (0.807–1.163) | 0.735 | ||
| Liver only | 0.996 (0.656–1.512) | 0.984 | ||
| LDH * | 1.000 (0.999–1.002) | 0.498 | ||
| LDH elevated | 1.005 (0.510–1.980) | 0.988 | ||
| CgA * | 1.000 (1.000–1.000) | 0.328 | ||
| CgA elevated | 1.214 (0.696–2.118) | 0.493 | ||
| NSE * | 1.003 (0.999–1.006) | 0.112 | 1.003 (0.998–1.008) | 0.189 |
| NSE elevated | 1.118 (0.588–2.124) | 0.734 | ||
| Non-PE vs. PE | 0.666 (0.443–1.000) | 0.049 | 0.773 (0.390–1.530) | 0.459 |
| PE | % | FOLFOX | % | TEM/CAP | % | FOLFIRI | % | Everolimus | % | Other | % | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CR | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| PR | 6 | 46.2 | 4 | 40.0 | 4 | 20.0 | 1 | 9.1 | 2 | 16.7 | 6 | 18.2 |
| SD | 3 | 23.1 | 4 | 40.0 | 9 | 45.0 | 2 | 18.2 | 3 | 25.0 | 9 | 27.3 |
| PD | 4 | 30.8 | 1 | 10.0 | 4 | 20.0 | 5 | 45.5 | 6 | 50.0 | 15 | 45.5 |
| NE | 0 | 0.0 | 1 | 10.0 | 3 | 15.0 | 3 | 27.3 | 1 | 8.3 | 3 | 9.1 |
| ORR | 6 | 46.2 | 4 | 40.0 | 4 | 20.0 | 1 | 9.1 | 2 | 16.7 | 6 | 18.2 |
| DCR | 9 | 69.2 | 8 | 80.0 | 13 | 65.0 | 3 | 27.3 | 5 | 41.7 | 15 | 45.5 |
| PFS (months) | 8.0 | 13.9 | 7.7 | 2.4 | 4.0 | 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apostolidis, L.; Dal Buono, A.; Merola, E.; Jann, H.; Jäger, D.; Wiedenmann, B.; Winkler, E.C.; Pavel, M. Multicenter Analysis of Treatment Outcomes for Systemic Therapy in Well Differentiated Grade 3 Neuroendocrine Tumors (NET G3). Cancers 2021, 13, 1936. https://doi.org/10.3390/cancers13081936
Apostolidis L, Dal Buono A, Merola E, Jann H, Jäger D, Wiedenmann B, Winkler EC, Pavel M. Multicenter Analysis of Treatment Outcomes for Systemic Therapy in Well Differentiated Grade 3 Neuroendocrine Tumors (NET G3). Cancers. 2021; 13(8):1936. https://doi.org/10.3390/cancers13081936
Chicago/Turabian StyleApostolidis, Leonidas, Arianna Dal Buono, Elettra Merola, Henning Jann, Dirk Jäger, Bertram Wiedenmann, Eva Caroline Winkler, and Marianne Pavel. 2021. "Multicenter Analysis of Treatment Outcomes for Systemic Therapy in Well Differentiated Grade 3 Neuroendocrine Tumors (NET G3)" Cancers 13, no. 8: 1936. https://doi.org/10.3390/cancers13081936
APA StyleApostolidis, L., Dal Buono, A., Merola, E., Jann, H., Jäger, D., Wiedenmann, B., Winkler, E. C., & Pavel, M. (2021). Multicenter Analysis of Treatment Outcomes for Systemic Therapy in Well Differentiated Grade 3 Neuroendocrine Tumors (NET G3). Cancers, 13(8), 1936. https://doi.org/10.3390/cancers13081936






