Automated Non-Coplanar VMAT for Dose Escalation in Recurrent Head and Neck Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Automated Non-Coplanar VMAT Planning
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brockstein, B.; Haraf, D.J.; Rademaker, A.W.; Kies, M.S.; Stenson, K.M.; Rosen, F.; Mittal, B.B.; Pelzer, H.; Fung, B.B.; Witt, M.-E.; et al. Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: A 9-year, 337-patient, multi-institutional experience. Ann. Oncol. 2004, 15, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Jesse, R.H.; Sugarbaker, E.V. Squamous cell carcinoma of the oropharynx: Why we fail. Am. J. Surg. 1976, 132, 435–438. [Google Scholar] [CrossRef]
- Kim, Y.S. Reirradiation of head and neck cancer in the era of intensity-modulated radiotherapy: Patient selection, practical aspects, and current evidence. Radiat. Oncol. J. 2017, 35, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.Y.; Wei, W.I.; Lam, L.K.; Yuen, A.P.W. Salvage of recurrent head and neck squamous cell carcinoma after primary curative surgery. Head Neck 2003, 25, 953–959. [Google Scholar] [CrossRef] [PubMed]
- de Crevoisier, R.; Bourhis, J.; Domenge, C.; Wibault, P.; Koscielny, S.; Lusinchi, A.; Mamelle, G.; Janot, F.; Julieron, M.; Leridant, A.M.; et al. Full-dose reirradiation for unresectable head and neck carcinoma: Experience at the Gustave-Roussy Institute in a series of 169 patients. J. Clin. Oncol. 1998, 16, 3556–3562. [Google Scholar] [CrossRef]
- Haraf, D.J.; Weichselbaum, R.R.; Vokes, E.E. Re-irradiation with concomitant chemotherapy of unresectable recurrent head and neck cancer: A potentially curable disease. Ann. Oncol. 1996, 7, 913–918. [Google Scholar] [CrossRef]
- Baliga, S.; Kabarriti, R.; Ohri, N.; Haynes–Lewis, H.; Yaparpalvi, R.; Kalnicki, S.; Garg, M.K. Stereotactic body radiotherapy for recurrent head and neck cancer: A critical review. Head Neck 2016, 39, 595–601. [Google Scholar] [CrossRef]
- Rwigema, J.M.; Nguyen, D.; Heron, D.E.; Chen, A.M.; Lee, P.; Wang, P.; Vargo, J.A.; Low, D.A.; Huq, M.S.; Tenn, S.; et al. 4pi noncoplanar stereotactic body radiation therapy for head-and-neck cancer: Potential to improve tumor control and late toxicity. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 401–409. [Google Scholar] [CrossRef]
- Podgorsak, E.B.; Pace, G.B.; Olivier, A.; Pla, M.; Souhami, L. Radiosurgery with high energy photon beams: A comparison among techniques. Int. J. Radiat. Oncol. 1989, 16, 857–865. [Google Scholar] [CrossRef]
- Dong, P.; Lee, P.; Ruan, D.; Long, T.; Romeijn, E.; Yang, Y.; Low, D.; Kupelian, P.; Sheng, K. 4π Non-Coplanar Liver SBRT: A Novel Delivery Technique. Int. J. Radiat. Oncol. 2013, 85, 1360–1366. [Google Scholar] [CrossRef]
- Woods, K.; Nguyen, D.; Tran, A.; Yu, V.Y.; Cao, M.; Niu, T.; Lee, P.; Sheng, K. Viability of Non-Coplanar VMAT for Liver SBRT as Compared to Coplanar VMAT and Beam Orientation Optimized 4π IMRT. Adv. Radiat. Oncol. 2016, 1, 67–75. [Google Scholar] [CrossRef]
- Dong, P.; Lee, P.; Ruan, D.; Long, T.; Romeijn, E.; Low, D.A.; Kupelian, P.; Abraham, J.; Yang, Y.; Sheng, K. 4π Noncoplanar Stereotactic Body Radiation Therapy for Centrally Located or Larger Lung Tumors. Int. J. Radiat. Oncol. 2013, 86, 407–413. [Google Scholar] [CrossRef]
- Dong, P.; Nguyen, D.; Ruan, D.; King, C.; Long, T.; Romeijn, E.; Low, D.A.; Kupelian, P.; Steinberg, M.; Yang, Y.; et al. Feasibility of prostate robotic radiation therapy on conventional C-arm linacs. Pract. Radiat. Oncol. 2014, 4, 254–260. [Google Scholar] [CrossRef]
- Tran, A.; Zhang, J.; Woods, K.; Yu, V.; Nguyen, D.; Gustafson, G.; Rosen, L.; Sheng, K. Treatment planning comparison of IMPT, VMAT and 4π radiotherapy for prostate cases. Radiat. Oncol. 2017, 12, 10. [Google Scholar] [CrossRef]
- Murzin, V.L.; Woods, K.; Moiseenko, V.; Karunamuni, R.; Tringale, K.R.; Seibert, T.M.; Connor, M.J.; Simpson, D.R.; Sheng, K.; Hattangadi-Gluth, J.A. 4π plan optimization for cortical-sparing brain radiotherapy. Radiother. Oncol. 2018, 127, 128–135. [Google Scholar] [CrossRef]
- Nguyen, D.; Rwigema, J.-C.M.; Yu, V.Y.; Kaprealian, T.; Kupelian, P.; Selch, M.; Lee, P.; Low, D.A.; Sheng, K. Feasibility of extreme dose escalation for glioblastoma multiforme using 4π radiotherapy. Radiat. Oncol. 2014, 9, 239. [Google Scholar] [CrossRef]
- Woods, K.; Lee, P.; Kaprealian, T.; Yang, I.; Sheng, K. Cochlea-sparing acoustic neuroma treatment with 4π radiation therapy. Adv. Radiat. Oncol. 2018, 3, 100–107. [Google Scholar] [CrossRef]
- Lyu, Q.; Yu, V.Y.; Ruan, D.; Neph, R.; O’Connor, D.; Sheng, K. A novel optimization framework for VMAT with dynamic gantry couch rotation. Phys. Med. Biol. 2018, 63, 125013. [Google Scholar] [CrossRef]
- Yu, V.Y.; Landers, A.; Woods, K.; Nguyen, D.; Cao, M.; Du, D.; Chin, R.K.; Sheng, K.; Kaprealian, T.B. A Prospective 4π Radiation Therapy Clinical Study in Recurrent High-Grade Glioma Patients. Int. J. Radiat. Oncol. 2018, 101, 144–151. [Google Scholar] [CrossRef]
- Nicosia, L.; Figlia, V.; Mazzola, R.; Napoli, G.; Giaj-Levra, N.; Ricchetti, F.; Rigo, M.; Lunardi, G.; Tomasini, D.; Bonù, M.L.; et al. Repeated stereotactic radiosurgery (SRS) using a non-coplanar mono-isocenter (HyperArc™) technique versus upfront whole-brain radiotherapy (WBRT): A matched-pair analysis. Clin. Exp. Metastasis 2020, 37, 77–83. [Google Scholar] [CrossRef]
- Ohira, S.; Ueda, Y.; Akino, Y.; Hashimoto, M.; Masaoka, A.; Hirata, T.; Miyazaki, M.; Koizumi, M.; Teshima, T. HyperArc VMAT planning for single and multiple brain metastases stereotactic radiosurgery: A new treatment planning approach. Radiat. Oncol. 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Ruggieri, R.; Naccarato, S.; Mazzola, R.; Ricchetti, F.; Corradini, S.; Fiorentino, A.; Alongi, F. Linac-based VMAT radiosurgery for multiple brain lesions: Comparison between a conventional multi-isocenter approach and a new dedicated mono-isocenter technique. Radiat. Oncol. 2018, 13, 38. [Google Scholar] [CrossRef]
- Alongi, F.; Fiorentino, A.; Gregucci, F.; Corradini, S.; Giaj-Levra, N.; Romano, L.; Rigo, M.; Ricchetti, F.; Beltramello, A.; Lunardi, G.; et al. First experience and clinical results using a new non-coplanar mono-isocenter technique (HyperArc™) for Linac-based VMAT radiosurgery in brain metastases. J. Cancer Res. Clin. Oncol. 2019, 145, 193–200. [Google Scholar] [CrossRef]
- Vergalasova, I.; Liu, H.; Alonso-Basanta, M.; Dong, L.; Li, J.; Nie, K.; Shi, W.; Teo, B.-K.K.; Yu, Y.; Yue, N.J.; et al. Multi-Institutional Dosimetric Evaluation of Modern Day Stereotactic Radiosurgery (SRS) Treatment Options for Multiple Brain Metastases. Front. Oncol. 2019, 9, 483. [Google Scholar] [CrossRef]
- Ohira, S.; Sagawa, T.; Ueda, Y.; Inui, S.; Masaoka, A.; Akino, Y.; Mizuno, H.; Miyazaki, M.; Koizumi, M.; Teshima, T. Effect of collimator angle on HyperArc stereotactic radiosurgery planning for single and multiple brain metastases. Med. Dosim. 2020, 45, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Gay, H.A.; Niemierko, A. A free program for calculating EUD-based NTCP and TCP in external beam radiotherapy. Phys. Med. 2007, 23, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Niemierko, A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med. Phys. 1997, 24, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Mohan, R.; Niemierko, A.; Schmidt-Ullrich, R. Optimization of intensity-modulated radiotherapy plans based on the equivalent uniform dose. Int. J. Radiat. Oncol. 2002, 52, 224–235. [Google Scholar] [CrossRef]
- Stuschke, M.; Thames, H.D. Fractionation sensitivities and dose-control relations of head and neck carcinomas: Analysis of the randomized hyperfractionation trials. Radiother. Oncol. 1999, 51, 113–121. [Google Scholar] [CrossRef]
- Mesbahi, A.; Rasuli, N.; Nasiri, B.; Mohammadzadeh, M. Radiobiological Model-Based Comparison of Three-Dimensional Conformal and Intensity-Modulated Radiation Therapy Plans for Nasopharyngeal Carcinoma. Iran. J. Med Phys. 2017, 14, 190–196. [Google Scholar]
- Yamamoto, M.; Serizawa, T.; Shuto, T.; Akabane, A.; Higuchi, Y.; Kawagishi, J.; Yamanaka, K.; Sato, Y.; Jokura, H.; Yomo, S.; et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014, 15, 387–395. [Google Scholar] [CrossRef]
- Ho, H.-W.; Lee, S.P.; Lin, H.-M.; Chen, H.-Y.; Huang, C.-C.; Wang, S.-C.; Yang, C.-C.; Lin, Y.-W. Dosimetric comparison between RapidArc and HyperArc techniques in salvage stereotactic body radiation therapy for recurrent nasopharyngeal carcinoma. Radiat. Oncol. 2020, 15, 1–11. [Google Scholar] [CrossRef]
- Sagawa, T.; Ohira, S.; Ueda, Y.; Akino, Y.; Mizuno, H.; Matsumoto, M.; Miyazaki, M.; Koizumi, M.; Teshima, T. Dosimetric effect of rotational setup errors in stereotactic radiosurgery with HyperArc for single and multiple brain metastases. J. Appl. Clin. Med. Phys. 2019, 20, 84–91. [Google Scholar] [CrossRef]
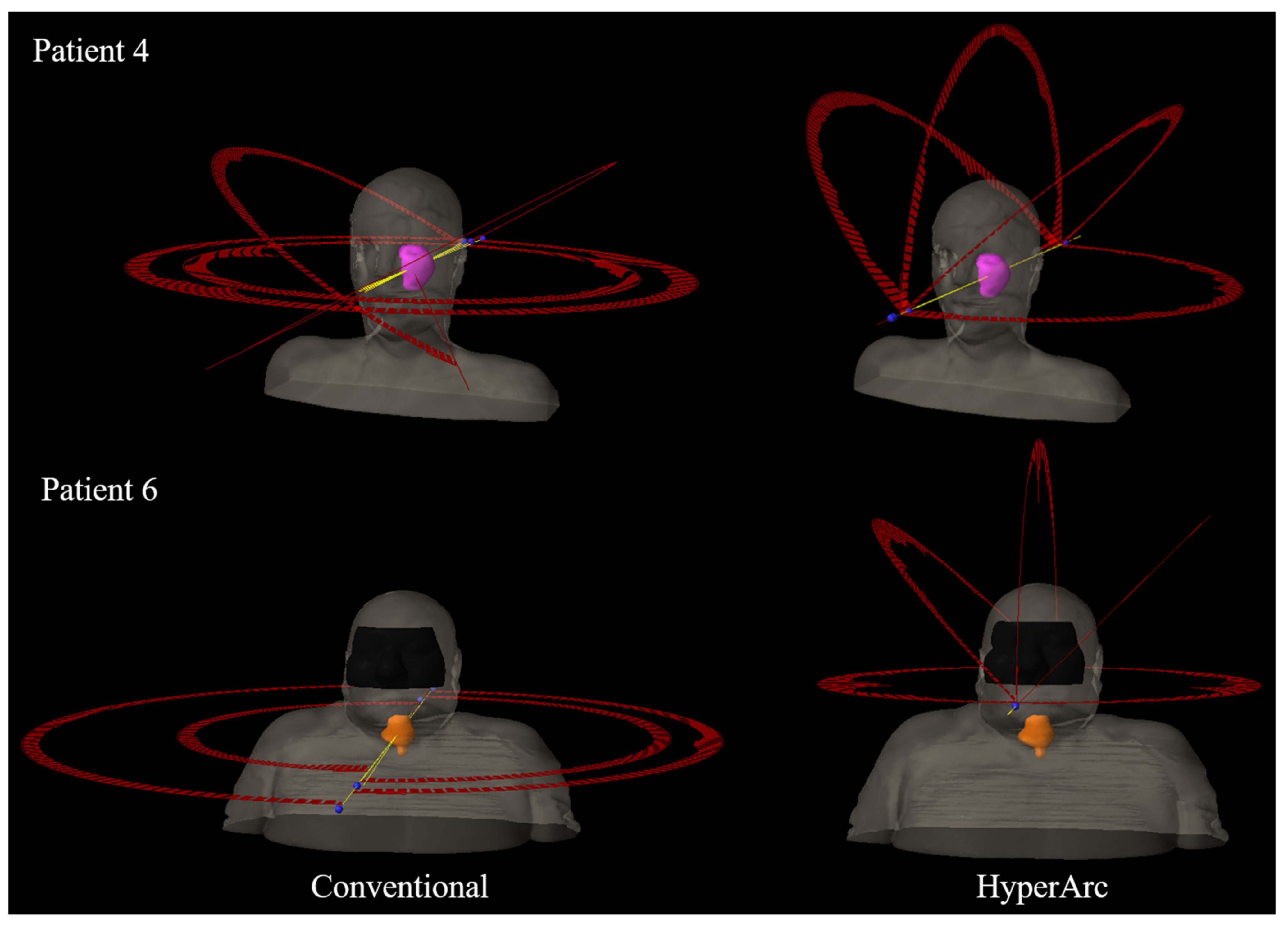
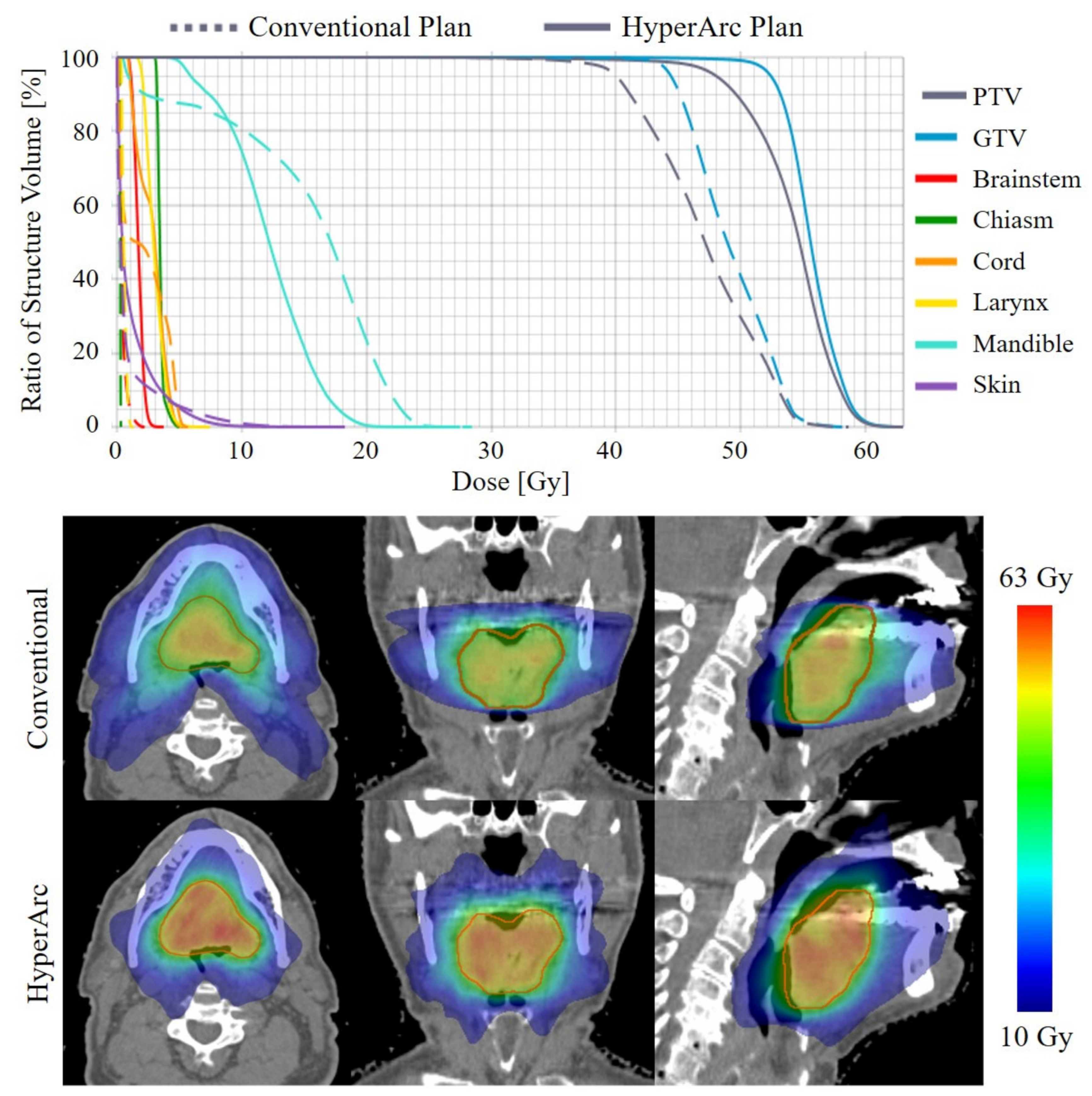
| Patient | Site | Histology * | PTV Volume (cm3) | Conventional Plan (arcs) | |
|---|---|---|---|---|---|
| Coplanar | Non-Coplanar | ||||
| 1 | Hypopharynx | SCC | 49.5 | 2 half | 0 |
| 2 | Hypopharynx | SCC | 34.2 | 4 partial | 0 |
| 3 | Submandibular gland | PTC | 15.1 | 4 partial | 0 |
| 4 | Maxillary sinus | Mucosal melanoma | 80.6 | 2 full | 2 partial |
| 5 | Oral cavity | ACC | 74.0 | 4 half | 0 |
| 6 | Larynx | SCC | 52.2 | 4 half | 0 |
| 7 | Left neck | SCC | 60.7 | 2 partial | 0 |
| 8 | Left neck | SCC | 56.0 | 4 half | 0 |
| 9 | Right neck | SCC | 77.1 | 2 partial | 0 |
| 10 | Cavernous sinus | ACC | 9.2 | 2 partial | 3 partial |
| 11 | Hypopharynx | SCC | 7.3 | 2 partial | 0 |
| 12 | Right neck | Undifferentiated carcinoma | 4.9 | 2 half | 0 |
| 13 | Right neck | SCC | 38.8 | 2 half | 0 |
| 14 | Right neck | SCC | 43.8 | 2 half | 0 |
| 15 | Supraclavicular nodes | SCC | 13.8 | 0 | 2 half |
| 16 | Base of tongue | SCC | 9.5 | 2 full | 0 |
| 17 | Temporalis | Salivary ductal carcinoma | 44.5 | 2 half | 0 |
| 18 | Hypopharynx | SCC | 5.4 | 0 | 2 half |
| 19 | Pharynx | Leiomyosarcoma | 17.1 | 4 half | 0 |
| 20 | Tongue | SCC | 9.5 | 2 full | 0 |
| Structure | Effect | α/β | a | γ50 | TCD50/TD50 |
|---|---|---|---|---|---|
| Target * | Long term tumor control | 10.5 | −8.0 | 3.2 | 66.8 |
| Brainstem † | Necrosis | 3.0 | 7.0 | 3.0 | 65.0 |
| Cord † | Necrosis | 3.0 | 7.4 | 4.0 | 66.5 |
| Larynx † | Edema | 3.8 | 12.5 | 4.0 | 70.0 |
| Mandible † | Limited joint function | 3.0 | 14.0 | 4.0 | 72.0 |
| Conventional | HyperArc | Difference (HA–Conv) | |
| Total MU | 2237 ± 640 | 3183 ± 688 | 947 ± 873 * |
| Delivery Time (min, n = 10) | 2.3 ± 0.6 | 4.3 ± 0.5 | 2.0 ± 1.0 * |
| R50% | 4.1 ± 2.6 | 3.6 ± 1.7 | −0.5 ± 1.6 |
| GI | 3.7 ± 1.4 | 3.0 ± 0.9 | −0.7 ± 0.7 * |
| GM (mm) | 9.8 ± 2.1 | 8.2 ± 1.7 | −1.6 ± 1.3 * |
| Mean Dose (Gy) | |||
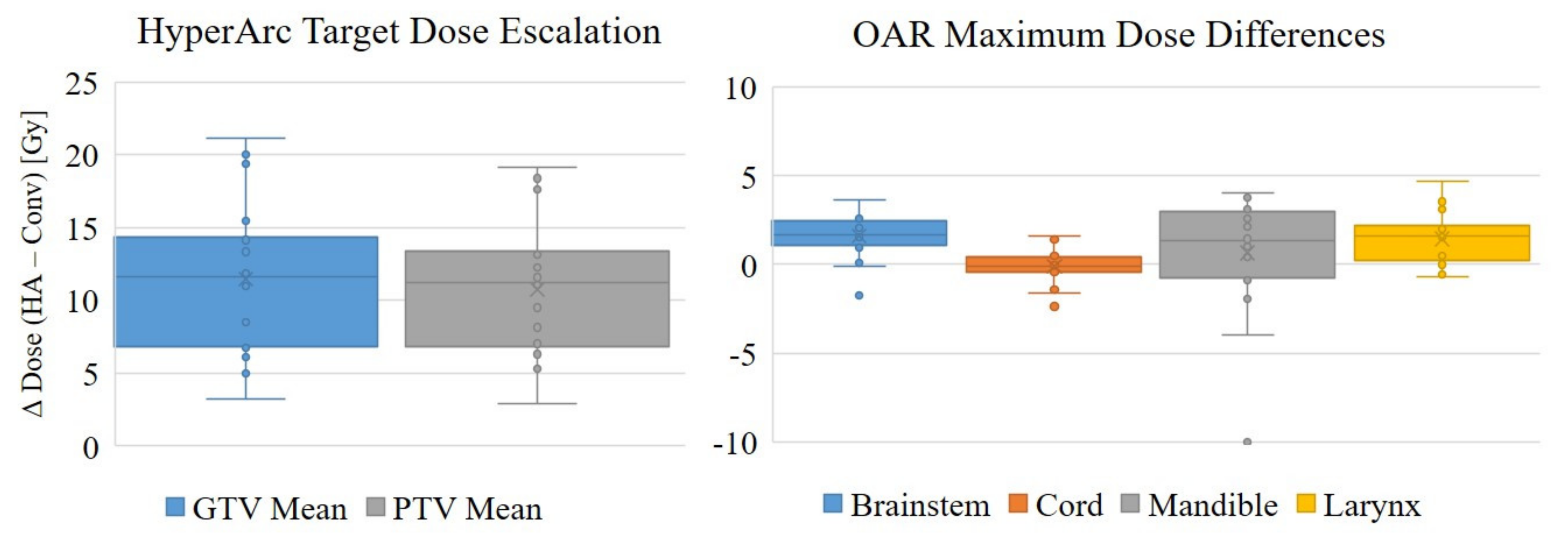
| PTV | 43.2 ± 3.4 | 53.9 ± 5.9 | 10.8 ± 4.4 * |
| GTV | 44.4 ± 3.7 | 55.9 ± 6.5 | 11.5 ± 5.1 * |
| Maximum Dose (Gy) | |||
| Brainstem | 2.5 ± 5.8 | 4.1 ± 5.1 | 1.6 ± 1.2 * |
| Spinal Cord | 5.6 ± 3.1 | 5.5 ± 3.3 | −0.1 ± 1.0 |
| Mandible | 10.4 ± 11.7 | 11.0 ± 8.5 | 0.6 ± 3.3 |
| Larynx | 14.9 ± 18.9 | 16.4 ± 19.1 | 1.5 ± 1.5 * |
| Skin | 25.5 ± 11.4 | 25.8 ± 11.9 | 0.3 ± 2.7 |
| Right Optic Nerve | 1.3 ± 3.2 | 2.7 ± 3.1 | 1.4 ± 1.8 * |
| Left Optic Nerve | 1.5 ± 4.2 | 2.3 ± 3.2 | 0.8 ± 1.8 * |
| Chiasm | 1.1 ± 2.7 | 2.3 ± 3.0 | 1.2 ± 1.3 * |
| TCP (%) | NTCP (%) | |||||
|---|---|---|---|---|---|---|
| GTV | PTV | Brainstem | Cord | Mandible | Larynx | |
| Conventional | 43.8 ± 31.1 | 28.6 ± 23.5 | 0 | 0 | 4.4 ± 19.5 | 15.6 ± 31.2 |
| HyperArc | 61.4 ± 42.5 | 51.5 ± 40.8 | 0 | 0 | 0.1 ± 0.4 | 17.5 ± 33.0 |
| Difference (HA–Conv) | 17.6 ± 15.0 * | 22.9 ± 20.6 * | 0 | 0 | −4.3 ± 19.1 | 1.9 ± 5.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woods, K.; Chin, R.K.; Cook, K.A.; Sheng, K.; Kishan, A.U.; Hegde, J.V.; Tenn, S.; Steinberg, M.L.; Cao, M. Automated Non-Coplanar VMAT for Dose Escalation in Recurrent Head and Neck Cancer Patients. Cancers 2021, 13, 1910. https://doi.org/10.3390/cancers13081910
Woods K, Chin RK, Cook KA, Sheng K, Kishan AU, Hegde JV, Tenn S, Steinberg ML, Cao M. Automated Non-Coplanar VMAT for Dose Escalation in Recurrent Head and Neck Cancer Patients. Cancers. 2021; 13(8):1910. https://doi.org/10.3390/cancers13081910
Chicago/Turabian StyleWoods, Kaley, Robert K. Chin, Kiri A. Cook, Ke Sheng, Amar U. Kishan, John V. Hegde, Stephen Tenn, Michael L. Steinberg, and Minsong Cao. 2021. "Automated Non-Coplanar VMAT for Dose Escalation in Recurrent Head and Neck Cancer Patients" Cancers 13, no. 8: 1910. https://doi.org/10.3390/cancers13081910
APA StyleWoods, K., Chin, R. K., Cook, K. A., Sheng, K., Kishan, A. U., Hegde, J. V., Tenn, S., Steinberg, M. L., & Cao, M. (2021). Automated Non-Coplanar VMAT for Dose Escalation in Recurrent Head and Neck Cancer Patients. Cancers, 13(8), 1910. https://doi.org/10.3390/cancers13081910








