Morphological and Molecular Characterization of Proliferative Inflammatory Atrophy in Canine Prostatic Samples
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Animal Demographic Data
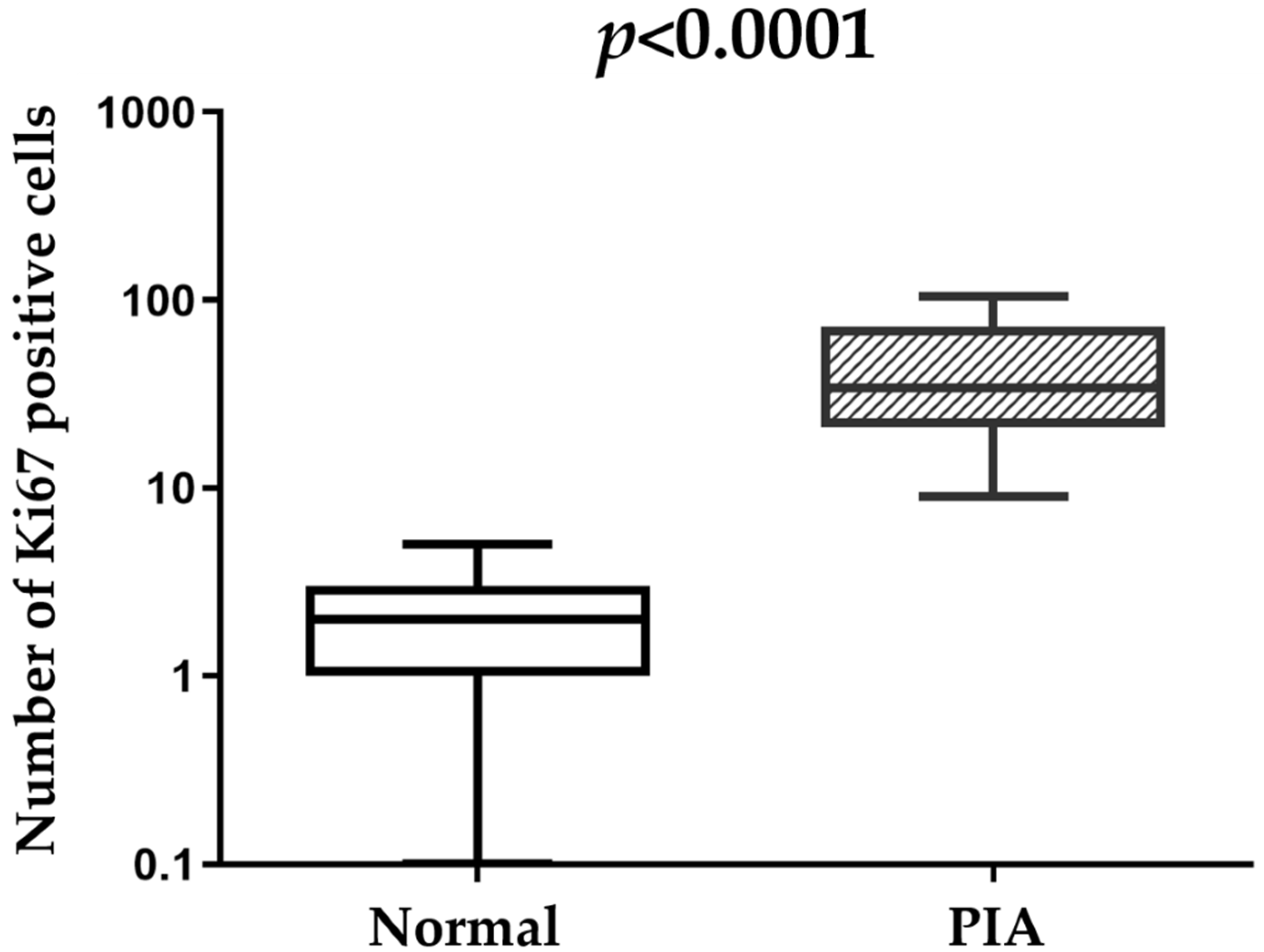
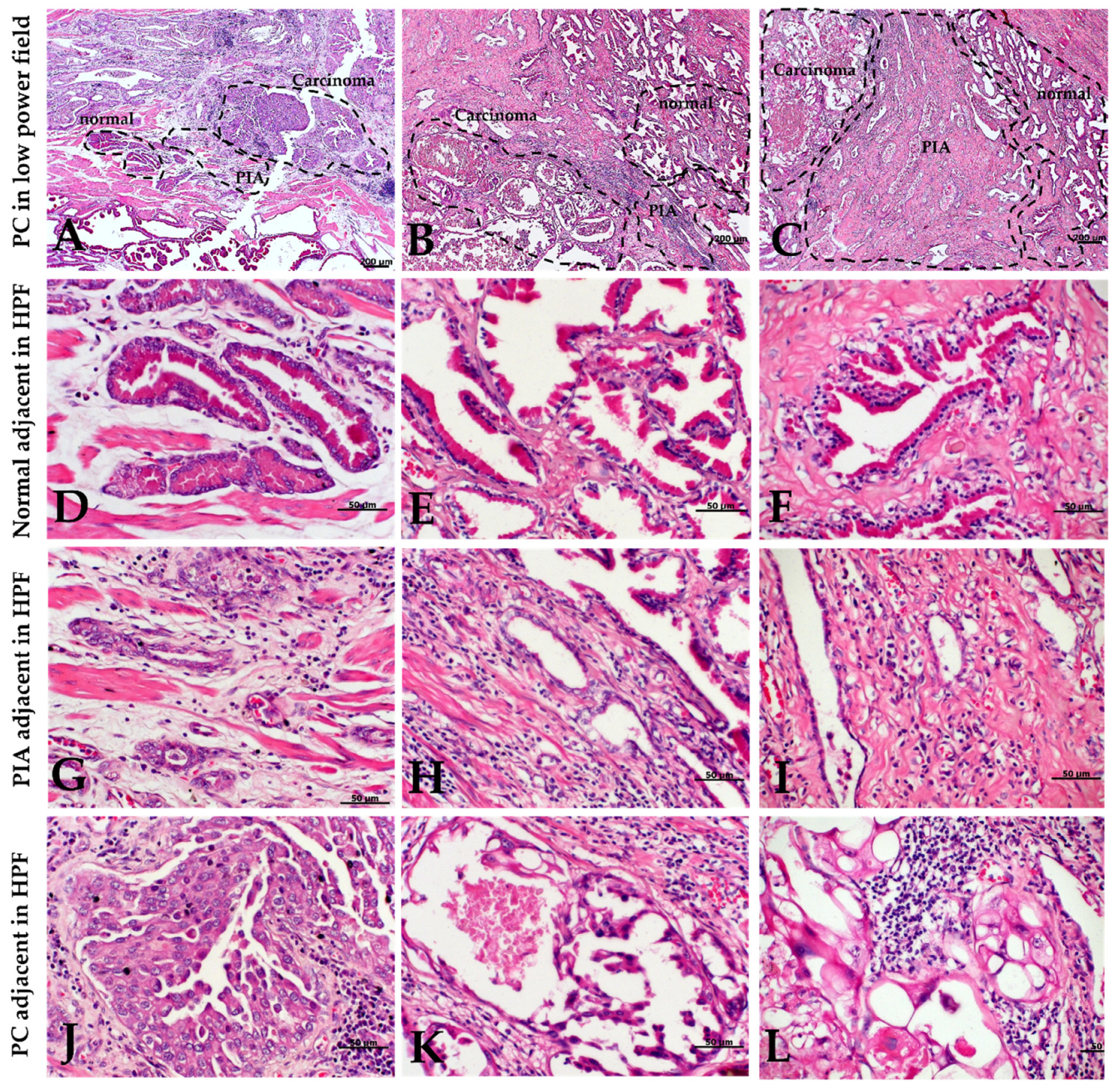
2.2. Morphology and Immunophenotype of PIA
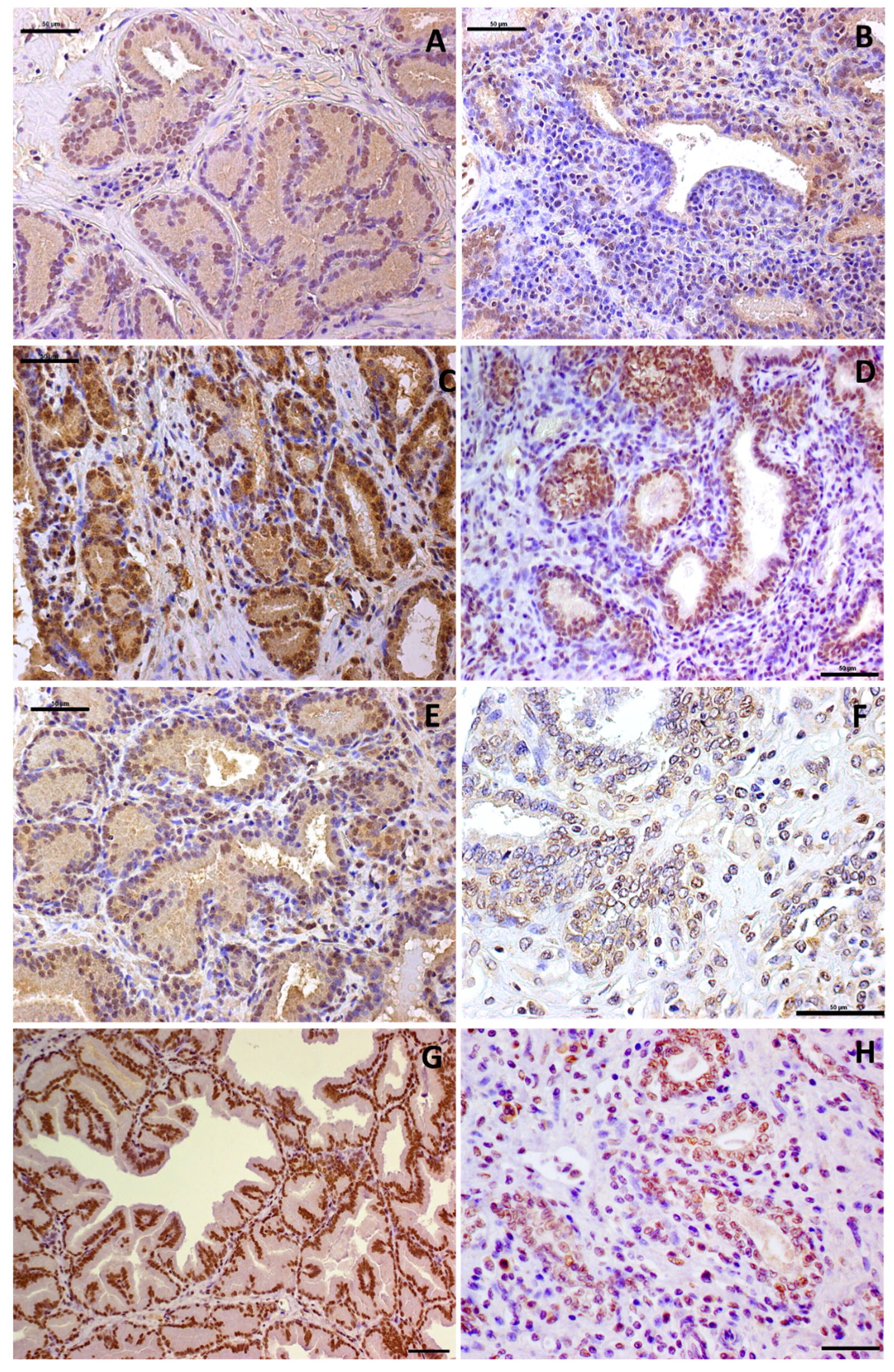
2.3. Immunohistochemical Features
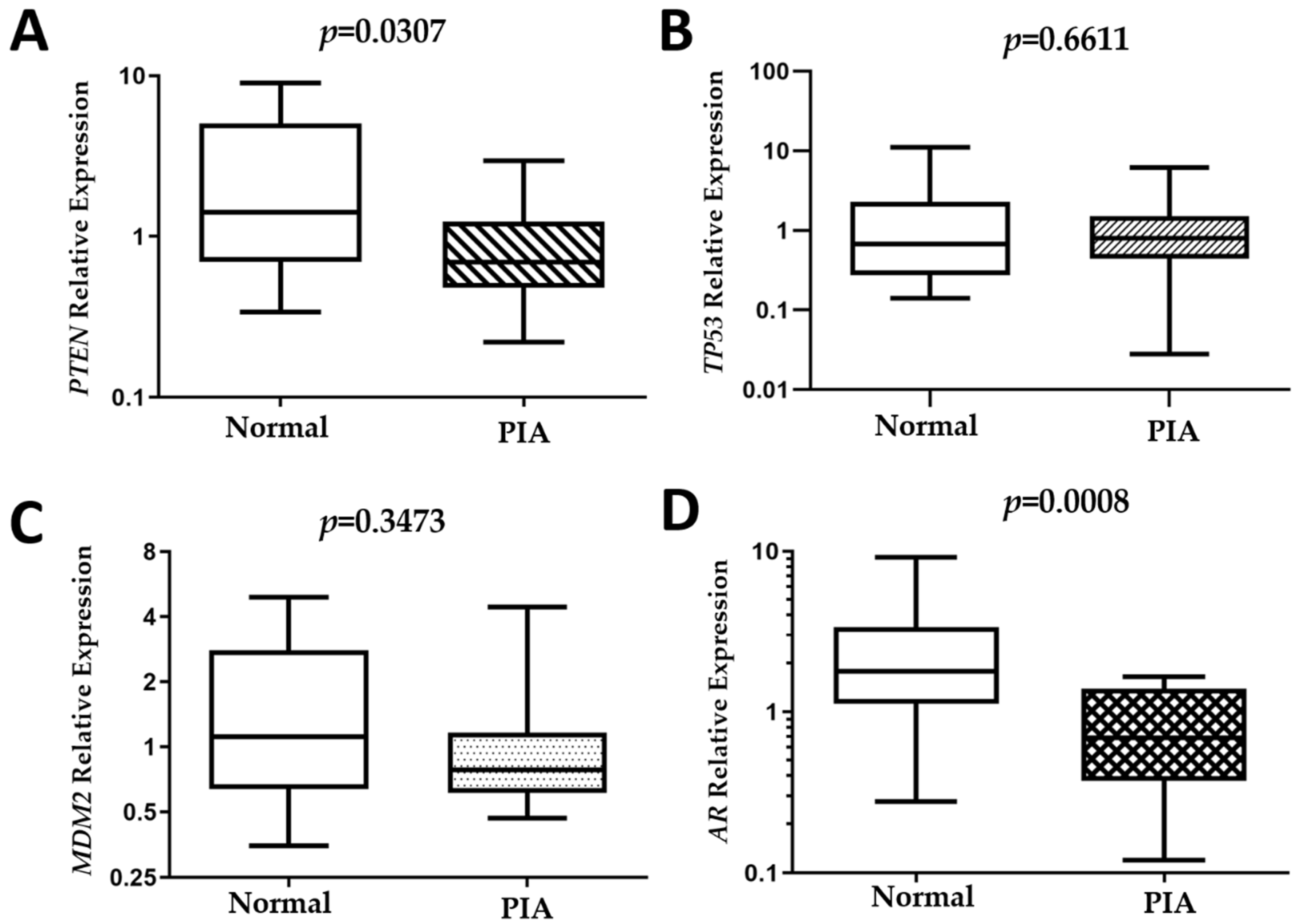
2.4. Gene Expression
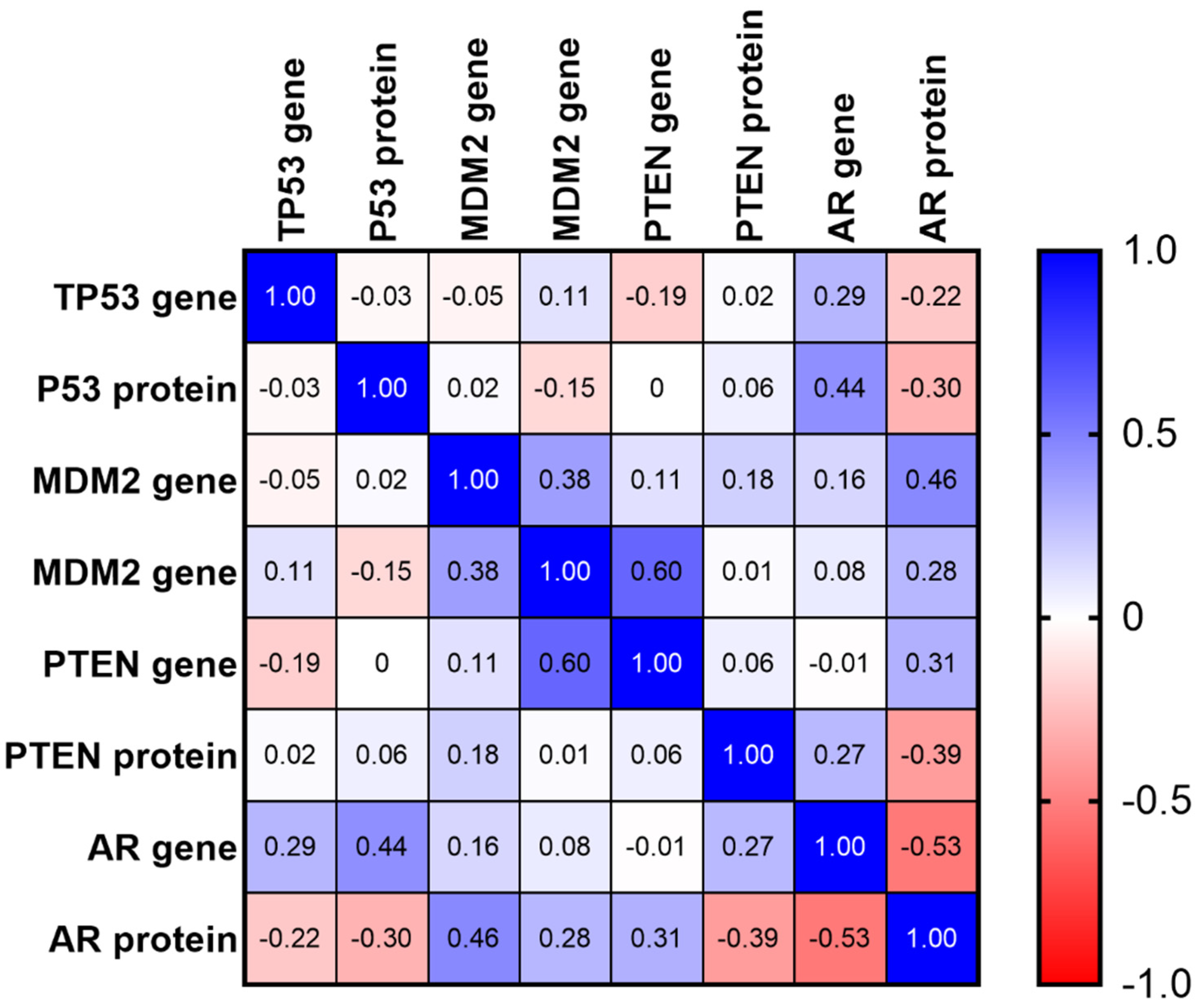
2.5. Matrix of Multiple Correlation
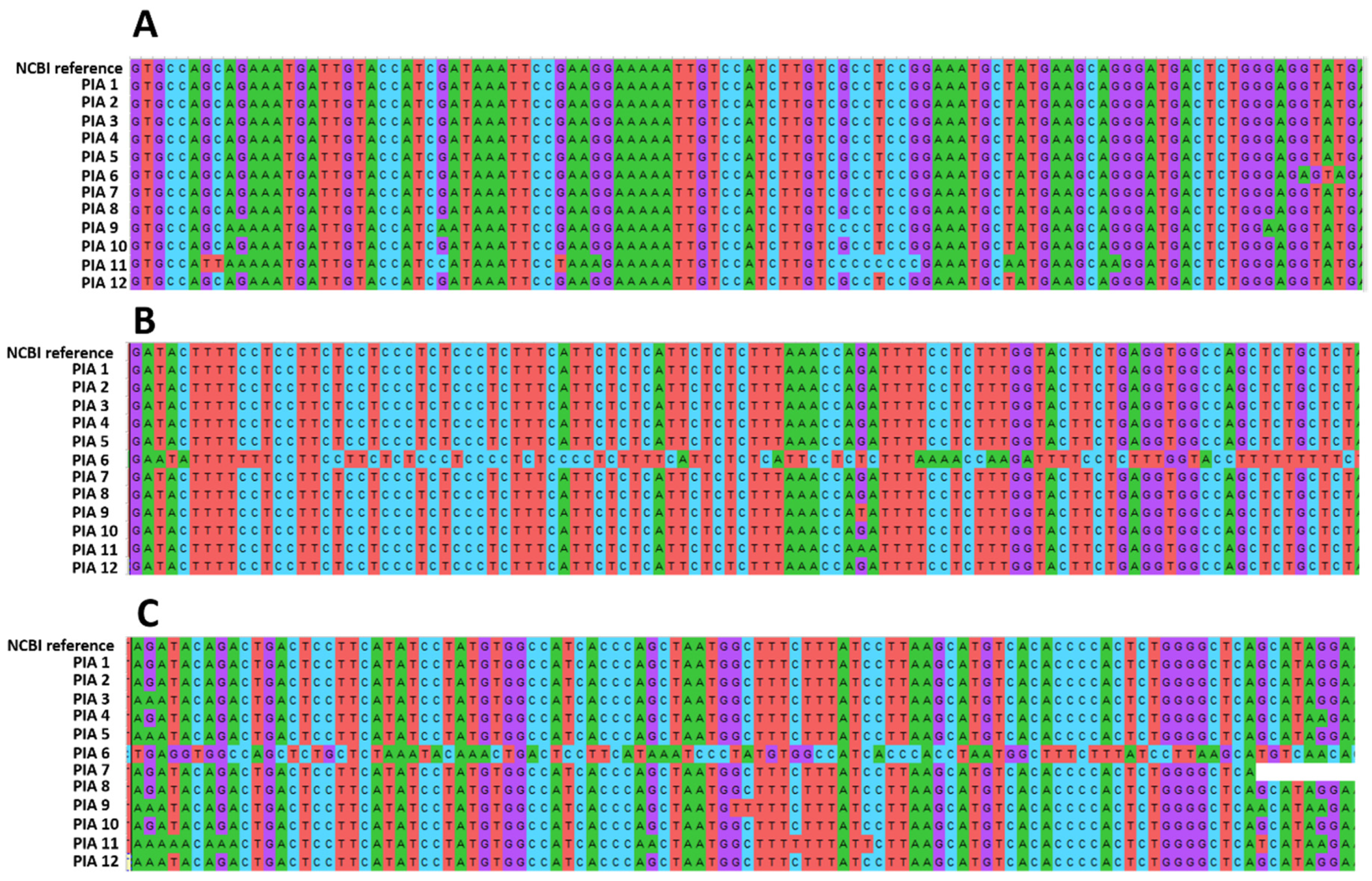
2.6. AR Sequencing
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Tissue Selection
4.3. PIA Morphological Features
4.4. Immunohistochemistry
4.5. Immunohistochemical Score
4.6. Laser-Capture Microdissection and qPCR
4.7. DNA Extraction and Sequencing
4.8. Data Analysis
4.9. Data Availability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palapattu, G.S.; Sutcliffe, S.; Bastian, P.J.; Platz, E.A.; De Marzo, A.M.; Isaacs, W.B.; Nelson, W.G. Prostate carcinogenesis and inflammation: Emerging insights. Carcinogenesis 2005, 26, 1170–1181. [Google Scholar] [CrossRef]
- de Bono, J.S.; Guo, C.; Gurel, B.; De Marzo, A.M.; Sfanos, K.S.; Mani, R.S.; Gil, J.; Drake, C.G.; Alimonti, A. Prostate carcinogenesis: Inflammatory storms. Nat. Rev. Cancer 2020, 20, 455–469. [Google Scholar] [CrossRef]
- Montironi, R.; Mazzucchelli, R.; Lopez-Beltran, A.; Cheng, L.; Scarpelli, M. Mechanisms of disease: High-grade prostatic intraepithelial neoplasia and other proposed preneoplastic lesions in the prostate. Nat. Clin. Pract. Urol. 2007, 4, 321–332. [Google Scholar] [CrossRef]
- De Marzo, A.M.; Marchi, V.L.; Epstein, J.I.; Nelson, W.G. Proliferative inflammatory atrophy of the prostate: Implications for prostatic carcinogenesis. Am. J. Pathol. 1999, 155, 1985–1992. [Google Scholar] [CrossRef]
- De Marzo, A.M.; Platz, E.A.; Epstein, J.I.; Ali, T.; Billis, A.; Chan, T.Y.; Cheng, L.; Datta, M.; Egevad, L.; Ertoy-Baydar, D.; et al. A working group classification of focal prostate atrophy lesions. Am. J. Surg Pathol. 2006, 30, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wei, L.; Wang, C.; Huang, Y.; Li, W.; Li, T.; Mo, C.; Qin, H.; Zhong, X.; Wang, Y.; et al. Chronic prostatitis alters the prostatic microenvironment and accelerates preneoplastic lesions in C57BL/6 mice. Biol. Res. 2019, 52, 30. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bergh, A.; Damber, J.E. Cyclooxygenase-2 expression correlates with local chronic inflammation and tumor neovascularization in human prostate cancer. Clin. Cancer Res. 2005, 11, 3250–3256. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.; Keener, R.; Rubenstein, M.; Stookey, S.; Bajpai, S.; Hicks, J.; Alme, A.K.; Drake, C.G.; Zheng, Q.; Trabzonlu, L.; et al. Consequences of interleukin 1β-triggered chronic inflammation in the mouse prostate gland: Altered architecture associated with prolonged CD4. Prostate 2019, 79, 732–745. [Google Scholar] [CrossRef]
- Sugar, L.M. Inflammation and prostate cancer. Can. J. Urol. 2006, 13 (Suppl. 1), 46–47. [Google Scholar]
- Karaivanov, M.; Todorova, K.; Kuzmanov, A.; Hayrabedyan, S. Quantitative immunohistochemical detection of the molecular expression patterns in proliferative inflammatory atrophy. J. Mol. Histol. 2007, 38, 1–11. [Google Scholar] [CrossRef]
- De Marzo, A.M.; Platz, E.A.; Sutcliffe, S.; Xu, J.; Grönberg, H.; Drake, C.G.; Nakai, Y.; Isaacs, W.B.; Nelson, W.G. Inflammation in prostate carcinogenesis. Nat. Rev. Cancer 2007, 7, 256–269. [Google Scholar] [CrossRef]
- Palmieri, C.; Story, M.; Lean, F.Z.X.; Akter, S.H.; Grieco, V.; De Marzo, A.M. Diagnostic Utility of Cytokeratin-5 for the Identification of Proliferative Inflammatory Atrophy in the Canine Prostate. J. Comp. Pathol. 2018, 158, 1–5. [Google Scholar] [CrossRef]
- Fonseca-Alves, C.E.; Rodrigues, M.M.; de Moura, V.M.; Rogatto, S.R.; Laufer-Amorim, R. Alterations of C-MYC, NKX3.1, and E-cadherin expression in canine prostate carcinogenesis. Microsc. Res. Tech. 2013, 76, 1250–1256. [Google Scholar] [CrossRef]
- Palmieri, C.; Lean, F.Z.; Akter, S.H.; Romussi, S.; Grieco, V. A retrospective analysis of 111 canine prostatic samples: Histopathological findings and classification. Res. Vet. Sci. 2014, 97, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Alves, C.E.; Kobayashi, P.E.; Rivera-Calderón, L.G.; Laufer-Amorim, R. Evidence of epithelial-mesenchymal transition in canine prostate cancer metastasis. Res. Vet. Sci. 2015, 100, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Laufer-Amorim, R.; Fonseca-Alves, C.E.; Villacis, R.A.R.; Linde, S.A.D.; Carvalho, M.; Larsen, S.J.; Marchi, F.A.; Rogatto, S.R. Comprehensive Genomic Profiling of Androgen-Receptor-Negative Canine Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 1555. [Google Scholar] [CrossRef]
- Fonseca-Alves, C.D.; Busso, A.F.; Silveira, S.M.; Rogatto, S.R.; Laufer-Amorim, R. Genomic gains in prostatic carcinoma and proliferative inflammatory atrophy in dogs. Cancer Res. 2012, 72, 5260. [Google Scholar] [CrossRef]
- Waters, D.J.; Bostwick, D.G. Prostatic intraepithelial neoplasia occurs spontaneously in the canine prostate. J. Urol. 1997, 157, 713–716. [Google Scholar] [CrossRef]
- Waters, D.J.; Bostwick, D.G. The canine prostate is a spontaneous model of intraepithelial neoplasia and prostate cancer progression. Anticancer Res. 1997, 17, 1467–1470. [Google Scholar] [PubMed]
- Aquilina, J.W.; McKinney, L.; Pacelli, A.; Richman, L.K.; Waters, D.J.; Thompson, I.; Burghardt, W.F.; Bostwick, D.G. High grade prostatic intraepithelial neoplasia in military working dogs with and without prostate cancer. Prostate 1998, 36, 189–193. [Google Scholar] [CrossRef]
- Madewell, B.R.; Gandour-Edwards, R.; DeVere White, R.W. Canine prostatic intraepithelial neoplasia: Is the comparative model relevant? Prostate 2004, 58, 314–317. [Google Scholar] [CrossRef]
- Wang, W.; Bergh, A.; Damber, J.E. Morphological transition of proliferative inflammatory atrophy to high-grade intraepithelial neoplasia and cancer in human prostate. Prostate 2009, 69, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Alves, C.E.; Kobayashi, P.E.; Palmieri, C.; Laufer-Amorim, R. Investigation of c-KIT and Ki67 expression in normal, preneoplastic and neoplastic canine prostate. BMC Vet. Res. 2017, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Woenckhaus, J.; Fenic, I. Proliferative inflammatory atrophy: A background lesion of prostate cancer? Andrologia 2008, 40, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Leav, I.; Schelling, K.H.; Adams, J.Y.; Merk, F.B.; Alroy, J. Role of canine basal cells in prostatic post natal development, induction of hyperplasia, sex hormone-stimulated growth; and the ductal origin of carcinoma. Prostate 2001, 47, 149–163. [Google Scholar] [CrossRef]
- Romanucci, M.; Frattone, L.; Ciccarelli, A.; Bongiovanni, L.; Malatesta, D.; Benazzi, C.; Brachelente, C.; Della Salda, L. Immunohistochemical expression of heat shock proteins, p63 and androgen receptor in benign prostatic hyperplasia and prostatic carcinoma in the dog. Vet. Comp. Oncol. 2016, 14, 337–349. [Google Scholar] [CrossRef]
- Akter, S.H.; Lean, F.Z.; Lu, J.; Grieco, V.; Palmieri, C. Different Growth Patterns of Canine Prostatic Carcinoma Suggests Different Models of Tumor-Initiating Cells. Vet. Pathol. 2015, 52, 1027–1033. [Google Scholar] [CrossRef]
- Leis-Filho, A.F.; Fonseca-Alves, C.E. Anatomy, histology, and physiology of the canine prostate gland. In Veterinary Anatomy and Physiology; IntechOpen: London, UK, 2018; pp. 47–67. [Google Scholar] [CrossRef]
- Fonseca-Alves, C.E.; Kobayashi, P.E.; Rivera Calderón, L.G.; Felisbino, S.L.; Rinaldi, J.C.; Drigo, S.A.; Rogatto, S.R.; Laufer-Amorim, R. Immunohistochemical panel to characterize canine prostate carcinomas according to aberrant p63 expression. PLoS ONE 2018, 13, e0199173. [Google Scholar] [CrossRef]
- Gupta, A.; Behl, T.; Heer, H.R.; Deshmukh, R.; Sharma, P.L. Mdm2-P53 Interaction Inhibitor with Cisplatin Enhances Apoptosis in Colon and Prostate Cancer Cells In-Vitro. Asian Pac. J. Cancer Prev. 2019, 20, 3341–3351. [Google Scholar] [CrossRef]
- McClurg, U.L.; Chit, N.C.T.H.; Azizyan, M.; Edwards, J.; Nabbi, A.; Riabowol, K.T.; Nakjang, S.; McCracken, S.R.; Robson, C.N. Molecular mechanism of the TP53-MDM2-AR-AKT signalling network regulation by USP12. Oncogene 2018, 37, 4679–4691. [Google Scholar] [CrossRef]
- Chopra, H.; Khan, Z.; Contreras, J.; Wang, H.; Sedrak, A.; Zhu, Y. Activation of p53 and destabilization of androgen receptor by combinatorial inhibition of MDM2 and MDMX in prostate cancer cells. Oncotarget 2018, 9, 6270–6281. [Google Scholar] [CrossRef] [PubMed]
- Bouali, S.; Chrétien, A.S.; Ramacci, C.; Rouyer, M.; Marchal, S.; Galenne, T.; Juin, P.; Becuwe, P.; Merlin, J.L. P53 and PTEN expression contribute to the inhibition of EGFR downstream signaling pathway by cetuximab. Cancer Gene Ther. 2009, 16, 498–507. [Google Scholar] [CrossRef]
- Yang, W.; Wang, K.; Ma, J.; Hui, K.; Lv, W.; Ma, Z.; Huan, M.; Luo, L.; Wang, X.; Li, L.; et al. Inhibition of Androgen Receptor Signaling Promotes Prostate Cancer Cell Migration via Upregulation of Annexin A1 Expression. Arch. Med. Res. 2020. [Google Scholar] [CrossRef]
- Nakano, M.; Taura, Y.; Inoue, M. Protein expression of Mdm2 and p53 in hyperplastic and neoplastic lesions of the canine circumanal gland. J. Comp. Pathol. 2005, 132, 27–32. [Google Scholar] [CrossRef]
- Mayo, L.D.; Donner, D.B. The PTEN, Mdm2, p53 tumor suppressor-oncoprotein network. Trends Biochem. Sci. 2002, 27, 462–467. [Google Scholar] [CrossRef]
- Pant, V.; Lozano, G. Dissecting the p53-Mdm2 feedback loop in vivo: Uncoupling the role in p53 stability and activity. Oncotarget 2014, 5, 1149–1156. [Google Scholar] [CrossRef][Green Version]
- Rivera-Calderón, L.G.; Fonseca-Alves, C.E.; Kobayashi, P.E.; Carvalho, M.; Drigo, S.A.; de Oliveira Vasconcelos, R.; Laufer-Amorim, R. Alterations in PTEN, MDM2, TP53 and AR protein and gene expression are associated with canine prostate carcinogenesis. Res. Vet. Sci. 2016, 106, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.; Krishna, N.S.; Grigor, K.M.; Bartlett, J.M. Androgen receptor gene amplification and protein expression in hormone refractory prostate cancer. Br. J. Cancer 2003, 89, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Dobi, A.; Mohamed, A.; Li, H.; Thangapazham, R.L.; Furusato, B.; Shaheduzzaman, S.; Tan, S.H.; Vaidyanathan, G.; Whitman, E.; et al. TMPRSS2-ERG fusion, a common genomic alteration in prostate cancer activates C-MYC and abrogates prostate epithelial differentiation. Oncogene 2008, 27, 5348–5353. [Google Scholar] [CrossRef]
- Attard, G.; Swennenhuis, J.F.; Olmos, D.; Reid, A.H.; Vickers, E.; A’Hern, R.; Levink, R.; Coumans, F.; Moreira, J.; Riisnaes, R.; et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 2009, 69, 2912–2918. [Google Scholar] [CrossRef] [PubMed]
- Burcham, P.C.; Raso, A.; Henry, P.J. Airborne acrolein induces keratin-8 (Ser-73) hyperphosphorylation and intermediate filament ubiquitination in bronchiolar lung cell monolayers. Toxicology 2014, 319, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Ittmann, M.; Huang, J.; Radaelli, E.; Martin, P.; Signoretti, S.; Sullivan, R.; Simons, B.W.; Ward, J.M.; Robinson, B.D.; Chu, G.C.; et al. Animal models of human prostate cancer: The consensus report of the New York meeting of the Mouse Models of Human Cancers Consortium Prostate Pathology Committee. Cancer Res. 2013, 73, 2718–2736. [Google Scholar] [CrossRef] [PubMed]
- Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Eble, J.N.; Sauter, G.; Epstein, J.I.; Sesterhenn, I.A. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. In Classification of Tumours; WHO, Ed.; WHO: Geneva, Switzerland, 2004; pp. 255–257. [Google Scholar]
- Hewitt, S.M.; Baskin, D.G.; Frevert, C.W.; Stahl, W.L.; Rosa-Molinar, E. Controls for immunohistochemistry: The Histochemical Society’s standards of practice for validation of immunohistochemical assays. J. Histochem. Cytochem. 2014, 62, 693–697. [Google Scholar] [CrossRef]
- Ito, S.; Ohga, T.; Saeki, H.; Nakamura, T.; Watanabe, M.; Tanaka, S.; Kakeji, Y.; Maehara, Y. p53 mutation profiling of multiple esophageal carcinoma using laser capture microdissection to demonstrate field carcinogenesis. Int. J. Cancer 2005, 113, 22–28. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Calderón, L.G.; Fonseca-Alves, C.E.; Kobayashi, P.E.; Carvalho, M.; Vasconcelos, R.O.; Laufer-Amorim, R. p-mTOR, p-4EBP-1 and eIF4E expression in canine prostatic carcinoma. Res. Vet. Sci. 2019, 122, 86–92. [Google Scholar] [CrossRef]
- Lai, C.L.; van den Ham, R.; Mol, J.; Teske, E. Immunostaining of the androgen receptor and sequence analysis of its DNA-binding domain in canine prostate cancer. Vet. J. 2009, 181, 256–260. [Google Scholar] [CrossRef]
- Kumar, U.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]

| Score | ||||||
|---|---|---|---|---|---|---|
| Group | 1 | 2 | 3 | 4 | p | |
| PTEN | Normal | 0% (0/20) | 0% (0/20) | 0% (0/20) | 100% (20/20) | p = 0.003 |
| PIA | 40% (8/20) | 25% (5/20) | 25% (5/20) | 10% (2/20) | ||
| P53 | Normal | 0% (0/20) | 0% (0/20) | 55% (11/20) | 45% (9/20) | p = 0.3568 |
| PIA | 0% (0/20) | 20% (4/20) | 40% (8/20) | 40% (8/20) | ||
| MDM2 | Normal | 0% (0/20) | 65% (13/20) | 30% (6/20) | 5% (1/20) | p = 0.5784 |
| PIA | 0% (0/20) | 55% (11/20) | 30% (6/20) | 15% (3/20) | ||
| AR | Normal | 0% (0/20) | 0% (0/20) | 0% (0/20) | 100% (20/20) | p = 0.01 |
| PIA | 0% (0/20) | 45% (9/20) | 55% (11/20) | 0% (0/20) | ||
| Gene Symbol | Location | Primer Sequences |
|---|---|---|
| AR | Chromosome 24 | F: 5′-CGCCCCTGACCTGGTTT-3′ |
| R: 5′-GGCTGTACATCCGGGACTTG-3′ | ||
| PTEN | Chromosome 26 | F: 5′-CGACGGGAAGACAAGTTCATG-3′ |
| R: 5′-TCACCGCACACAGGCAAT-3′ | ||
| MDM2 | Chromosome 10 | F: 5′-GGGCCCCTTCGTGAGAATTG-3′ |
| R: 5′-GGTGTGGCTTTTCTCAGGGATT-3′ | ||
| TP53 | Chromosome 5 | F: 5′-GAACGCTGCTCTGACAGTAGTGA-3′ |
| R: 5′-CCCGCAAATTTCCTTCCA-3′ | ||
| HPRT | Chromosome X | F: 5′-AGCTTGCTGGTGAAAAGGAC-3′ |
| R: 5′-TTATAGTCAAGGGCATATCC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Godoy Fernandes, G.; Pedrina, B.; de Faria Lainetti, P.; Kobayashi, P.E.; Govoni, V.M.; Palmieri, C.; de Moura, V.M.B.D.; Laufer-Amorim, R.; Fonseca-Alves, C.E. Morphological and Molecular Characterization of Proliferative Inflammatory Atrophy in Canine Prostatic Samples. Cancers 2021, 13, 1887. https://doi.org/10.3390/cancers13081887
de Godoy Fernandes G, Pedrina B, de Faria Lainetti P, Kobayashi PE, Govoni VM, Palmieri C, de Moura VMBD, Laufer-Amorim R, Fonseca-Alves CE. Morphological and Molecular Characterization of Proliferative Inflammatory Atrophy in Canine Prostatic Samples. Cancers. 2021; 13(8):1887. https://doi.org/10.3390/cancers13081887
Chicago/Turabian Stylede Godoy Fernandes, Giovana, Bruna Pedrina, Patrícia de Faria Lainetti, Priscila Emiko Kobayashi, Verônica Mollica Govoni, Chiara Palmieri, Veridiana Maria Brianezi Dignani de Moura, Renée Laufer-Amorim, and Carlos Eduardo Fonseca-Alves. 2021. "Morphological and Molecular Characterization of Proliferative Inflammatory Atrophy in Canine Prostatic Samples" Cancers 13, no. 8: 1887. https://doi.org/10.3390/cancers13081887
APA Stylede Godoy Fernandes, G., Pedrina, B., de Faria Lainetti, P., Kobayashi, P. E., Govoni, V. M., Palmieri, C., de Moura, V. M. B. D., Laufer-Amorim, R., & Fonseca-Alves, C. E. (2021). Morphological and Molecular Characterization of Proliferative Inflammatory Atrophy in Canine Prostatic Samples. Cancers, 13(8), 1887. https://doi.org/10.3390/cancers13081887










