The Role of Elective Neck Treatment in the Management of Sinonasal Carcinomas: A Systematic Review of the Literature and a Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
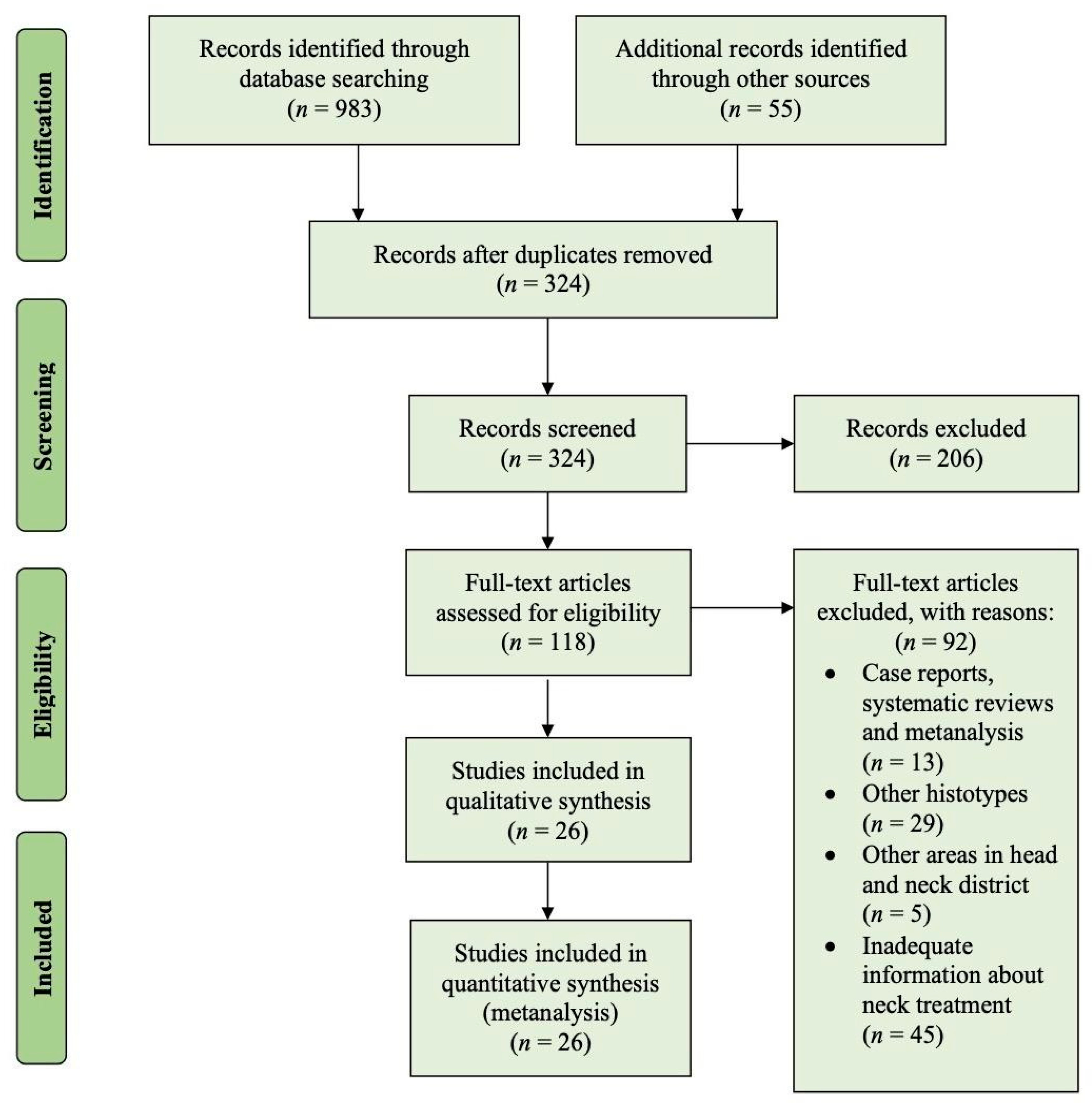
2. Materials and Methods
2.1. Searching Methods
2.2. Eligibility Criteria
2.3. Data Collection and Outcome Definitions
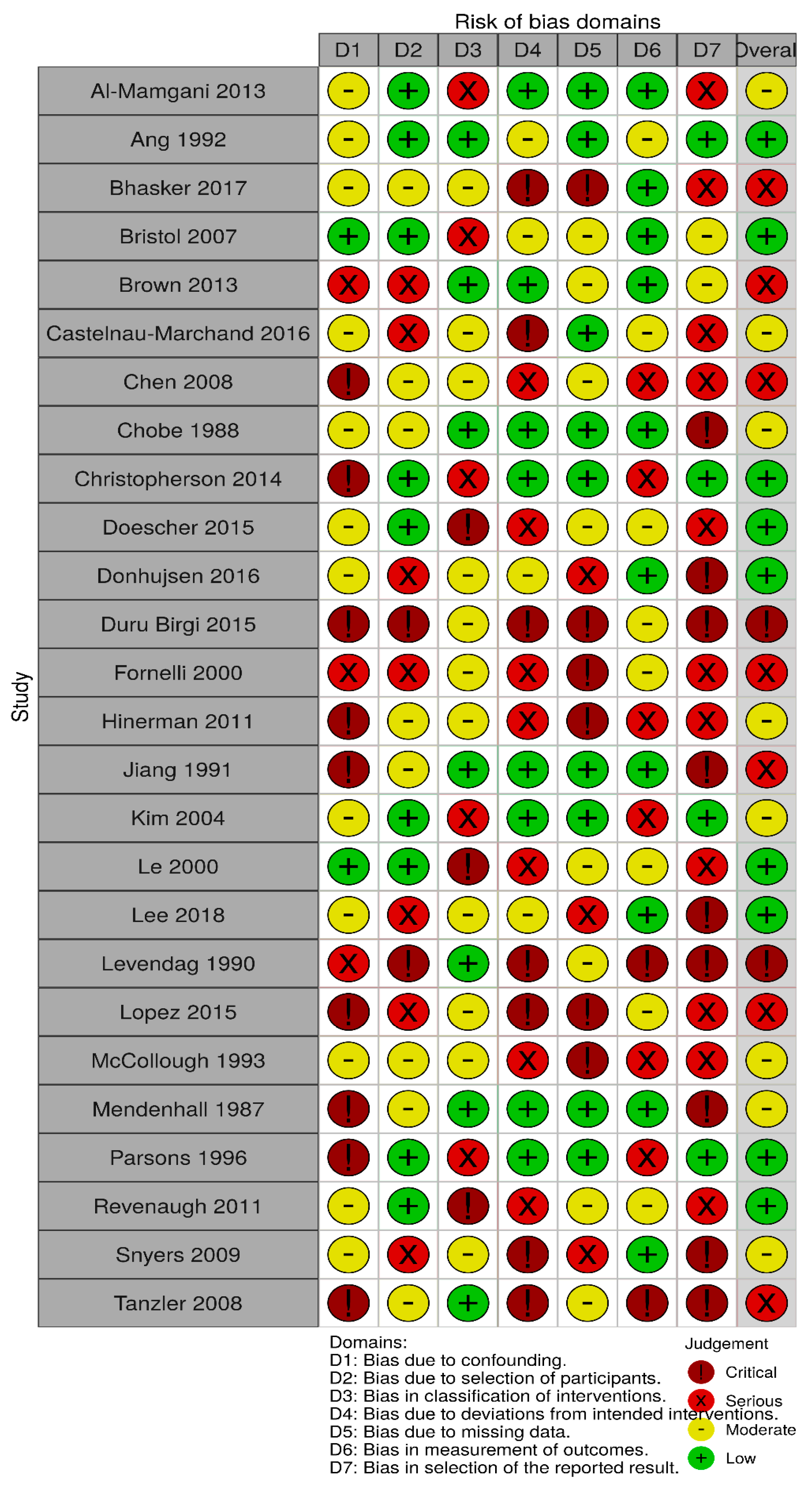
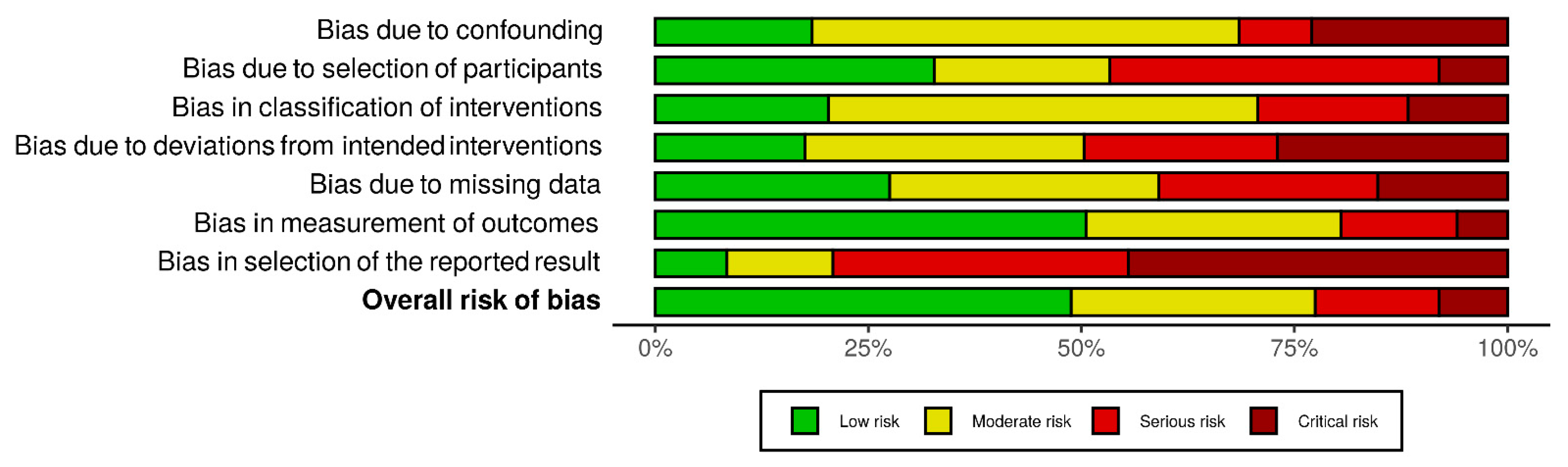
2.4. Quality Assessment
2.5. Statistical Methods
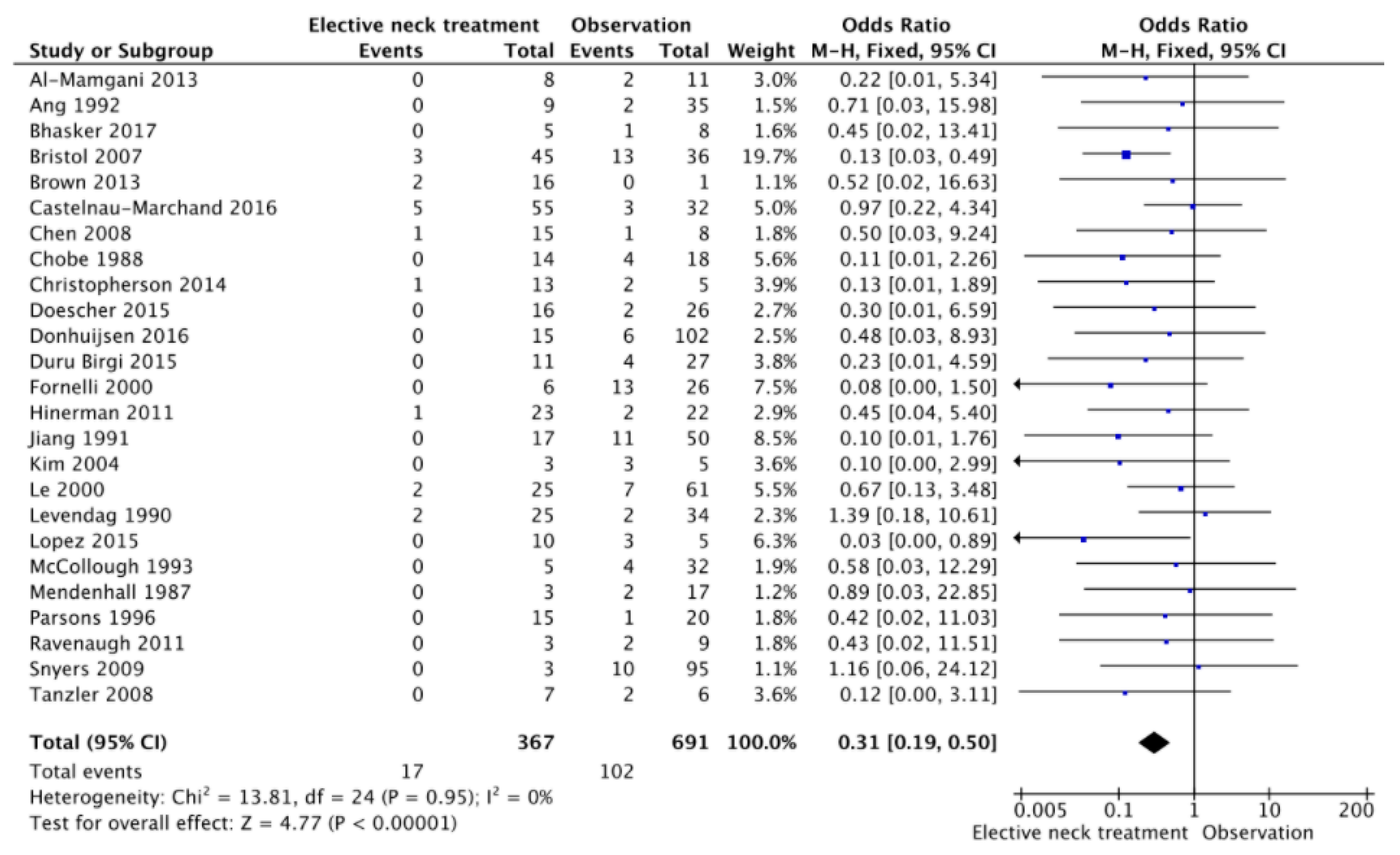
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Llorente, J.L.; López, J.F.M.; Suárez, C.; Hermsen, M.A. Sinonasal carcinoma: Clinical, pathological, genetic and therapeutic advances. Nat. Rev. Clin. Oncol. 2014, 11, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Paranasal Sinus and Nasal Cavity Cancer Treatment (Adult) (PDQ®). PDQ Cancer Information Summaries; National Cancer Institute: Bethesda, MD, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK82221/ (accessed on 19 August 2019).
- Robin, T.P.; Jones, B.L.; Ba, O.M.G.; Phan, A.; Abbott, D.; McDermott, J.D.; Goddard, J.A.; Raben, D.; Lanning, R.M.; Karam, S.D. A comprehensive comparative analysis of treatment modalities for sinonasal malignancies. Cancer 2017, 123, 3040–3049. [Google Scholar] [CrossRef]
- Ferrari, M.; Migliorati, S.; Tomasoni, M.; Crisafulli, V.; Nocivelli, G.; Paderno, A.; Rampinelli, V.; Taboni, S.; Schreiber, A.; Mattavelli, D.; et al. Sinonasal cancer encroaching the orbit: Ablation or preservation? Oral Oncol. 2021, 114, 105185. [Google Scholar] [CrossRef]
- Youlden, D.R.; Cramb, S.M.; Peters, S.; Porceddu, S.V.; Møller, H.; Fritschi, L.; Baade, P.D. International comparisons of the incidence and mortality of sinonasal cancer. Cancer Epidemiol. 2013, 37, 770–779. [Google Scholar] [CrossRef]
- Turner, J.H.; Reh, D.D. Incidence and survival in patients with sinonasal cancer: A historical analysis of population-based data. Head Neck 2012, 34, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Gangl, K.; Nemec, S.; Altorjai, G.; Pammer, J.; Grasl, M.C.; Erovic, B.M. Prognostic survival value of retropharyngeal lymph node involvement in sinonasal tumors: A retrospective, descriptive, and exploratory study. Head Neck 2017, 34, 877–1427. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.M.S.; Santaolalla, F.; Del Rey, A.S.; Martínez-Ibargüen, A.; González, A.; Iriarte, M.R. Preliminary study of the lymphatic drainage system of the nose and paranasal sinuses and its role in detection of sentinel metastatic nodes. Acta Oto-Laryngol. 2005, 125, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Peck, B.W.; Van Abel, K.M.; Moore, E.J.; Price, D.L. Rates and Locations of Regional Metastases in Sinonasal Malignancies: The Mayo Clinic Experience. J. Neurol. Surg. Part B Skull Base 2017, 79, 282–288. [Google Scholar] [CrossRef]
- Lund, V.J.; Chisholm, E.J.; Takes, R.P.; Suárez, C.; Mendenhall, W.M.; Rinaldo, A.; Llorente, J.L.; Terhaard, C.H.J.; Rodrigo, J.P.; Maughan, E.; et al. Evidence for treatment strategies in sinonasal adenocarcinoma. Head Neck 2011, 34, 1168–1178. [Google Scholar] [CrossRef]
- Abu-Ghanem, S.; Horowitz, G.; Abergel, A.; Yehuda, M.; Gutfeld, O.; Carmel, N.-N.; Fliss, D.M. Elective neck irradiation versus observation in squamous cell carcinoma of the maxillary sinus with N0 neck: A meta-analysis and review of the literature. Head Neck 2015, 37, 1823–1828. [Google Scholar] [CrossRef]
- Faisal, M.; Seemann, R.; Lill, C.; Hamzavi, S.; Wutzl, A.; Erovic, B.M.; Janik, S. Elective neck treatment in sinonasal undifferentiated carcinoma: Systematic review and meta-analysis. Head Neck 2020, 42, 1057–1066. [Google Scholar] [CrossRef]
- Moher, D.; Altman, D.G.; Liberati, A.; Tetzlaff, J. PRISMA Statement. Epidemiology 2011, 22, 128. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamgani, A.; Van Rooij, P.; Mehilal, R.; Tans, L.; Levendag, P.C. Combined-modality treatment improved outcome in sinonasal undifferentiated carcinoma: Single-institutional experience of 21 patients and review of the literature. Eur. Arch. Oto-RhinoLaryngol. 2013, 270, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.; Jiang, G.-L.; Frankenthaler, R.A.; Kaanders, J.H.; Garden, A.S.; Delclos, L.; Peters, L.J. Carcinomas of the nasal cavity. Radiother. Oncol. 1992, 24, 163–168. [Google Scholar] [CrossRef]
- Bhasker, S.; Mallick, S.; Benson, R.; Bhanuprasad, V.; Sharma, A.; Thakar, A. A multimodality approach to sinonasal undifferentiated carcinoma: A single institute experience. J. Laryngol. Otol. 2016, 131, 19–25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bristol, I.J.; Ahamad, A.; Garden, A.S.; Morrison, W.H.; Hanna, E.Y.; Papadimitrakopoulou, V.A.; Rosenthal, D.I.; Ang, K.K. Postoperative Radiotherapy for Maxillary Sinus Cancer: Long-Term Outcomes and Toxicities of Treatment. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; Bekiroglu, F.; Shaw, R.J.; Woolgar, J.A.; Triantafyllou, A.; Rogers, S.N. First report of elective selective neck dissection in the management of squamous cell carcinoma of the maxillary sinus. Br. J. Oral Maxillofac. Surg. 2013, 51, 103–107. [Google Scholar] [CrossRef]
- Castelnau-Marchand, P.; Levy, A.; Moya-Plana, A.; Mirghani, H.; Nguyen, F.; Del Campo, E.R.; Janot, F.; Kolb, F.; Ferrand, F.-R.; Temam, S.; et al. Sinonasal squamous cell carcinoma without clinical lymph node involvement Sinunasales Plattenepithelkarzinom ohne klinische Lymphknotenbeteiligung. Strahlenther. Onkol. 2016, 192, 537–544. [Google Scholar] [CrossRef]
- Chen, A.M.; Daly, M.E.; El-Sayed, I.; Garcia, J.; Lee, N.Y.; Bucci, M.K.; Kaplan, M.J. Patterns of Failure After Combined-Modality Approaches Incorporating Radiotherapy for Sinonasal Undifferentiated Carcinoma of the Head and Neck. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 338–343. [Google Scholar] [CrossRef]
- Chobe, R.; McNeese, M.; Weber, R.; Fletcher, G.H. Radiation Therapy for Carcinoma of the Nasal Vestibule. Otolaryngol. Neck Surg. 1988, 98, 67–71. [Google Scholar] [CrossRef]
- Christopherson, K.; Werning, J.W.; Malyapa, R.S.; Morris, C.G.; Mendenhall, W.M. Radiotherapy for sinonasal undifferentiated carcinoma. Am. J. Otolaryngol. 2014, 35, 141–146. [Google Scholar] [CrossRef]
- Doescher, J.; Piontek, G.; Wirth, M.; Bettstetter, M.; Schlegel, J.; Haller, B.; Brockhoff, G.; Reiter, R.; Pickhard, A. Epstein-Barr virus infection is strictly associated with the metastatic spread of sinonasal squamous-cell carcinomas. Oral Oncol. 2015, 51, 929–934. [Google Scholar] [CrossRef]
- Donhuijsen, K.; Kollecker, I.; Petersen, P.; Gaßler, N.; Schulze, J.; Schroeder, H.-G. Metastatic behaviour of sinonasal adenocarcinomas of the intestinal type (ITAC). Eur. Arch. Oto-Rhino-Laryngol. 2015, 273, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Duru Birgi, S.D.; Teo, M.; Dyker, K.E.; Sen, M.; Prestwich, R.J.D. Definitive and adjuvant radiotherapy for sinonasal squamous cell carcinomas: A single institutional experience. Radiat. Oncol. 2015, 10, 190. [Google Scholar] [CrossRef]
- Fornelli, R.A.; Fedok, F.G.; Wilson, E.P.; Rodman, S.M. Squamous Cell Carcinoma of the Anterior Nasal Cavity: A Dual Institution Review. Otolaryngol. Neck Surg. 2000, 123, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Hinerman, R.W.; Indelicato, D.J.; Morris, C.G.; Kirwan, J.M.; Werning, J.W.; Vaysberg, M.; Mendenhall, W.M. Radiotherapy with or without surgery for maxillary sinus squamous cell carcinoma: Should the clinical N0 neck be treated? Am. J. Clin. Oncol. 2011, 34, 483–487. [Google Scholar] [CrossRef]
- Jiang, G.; Ang, K.; Peters, L.; Wendt, C.; Oswald, M.; Goepfert, H. Maxillary sinus carcinomas: Natural history and results of postoperative radiotherapy. Radiother. Oncol. 1991, 21, 193–200. [Google Scholar] [CrossRef]
- Kim, B.S.; Vongtama, R.; Juillard, G. Sinonasal undifferentiated carcinoma: Case series and literature review. Am. J. Otolaryngol. 2004, 25, 162–166. [Google Scholar] [CrossRef]
- Le, Q.-T.; Fu, K.K.; Kaplan, M.J.; Terris, D.J.; E Fee, W.; Goffinet, D.R. Lymph node metastasis in maxillary sinus carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2000, 46, 541–549. [Google Scholar] [CrossRef]
- Lee, W.H.; Choi, S.H.; Kim, S.-H.; Choi, E.C.; Lee, C.G.; Keum, K.C. Elective neck treatment in clinically node-negative paranasal sinus carcinomas: Impact on treatment outcome. Radiat. Oncol. J. 2018, 36, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Levendag, P.; Pomp, J. Radiation therapy of squamous cell carcinoma of the nasal vestibule. Int. J. Radiat. Oncol. 1990, 19, 1363–1367. [Google Scholar] [CrossRef]
- Lopez, F.; Suarez, V.; Vivanco, B.; Suarez, C.; Llorente, J. Current management of sinonasal undifferentiated carcinoma. Rhinol. J. 2015, 53, 212–220. [Google Scholar] [CrossRef]
- Mccollough, W.; Mendenhall, N.P.; Parsons, J.T.; Mendenhall, W.M.; Stringer, S.P.; Cassisi, N.J.; Million, R.R. Radiotherapy alone for squamous cell carcinoma of the nasal vestibule: Management of the primary site and regional lymphatics. Int. J. Radiat. Oncol. 1993, 26, 73–79. [Google Scholar] [CrossRef]
- Mendenhall, N.P.; Parsons, J.T.; Cassisi, N.J.; Million, R.R. Carcinoma of the nasal vestibule treated with radiation therapy. Laryngoscope 1987, 97, 626–632. [Google Scholar] [CrossRef]
- Parsons, J.T.; Mendenhall, W.M.; Stringer, S.P.; Cassisi, N.J.; Million, R.R. Management of minor salivary gland carcinomas. Int. J. Radiat. Oncol. Biol. Phys. 1996, 35, 443–454. [Google Scholar] [CrossRef]
- Revenaugh, P.C.; Seth, R.; Pavlovich, J.B.; Knott, P.D.; Batra, P.S. Minimally invasive endoscopic resection of sinonasal undifferentiated carcinoma. Am. J. Otolaryngol. 2011, 32, 464–469. [Google Scholar] [CrossRef]
- Snyers, A.; Janssens, G.O.R.J.; Twickler, M.B.; Hermus, A.R.; Takes, R.P.; Kappelle, A.C.; Merkx, M.A.W.; Dirix, P.; Kaanders, J.H.A.M. Malignant Tumors of the Nasal Cavity and Paranasal Sinuses: Long-Term Outcome and Morbidity with Emphasis on Hypothalamic-Pituitary Deficiency. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1343–1351. [Google Scholar] [CrossRef]
- Tanzler, E.D.; Morris, C.G.; Orlando, C.A.; Werning, J.W.; Mendenhall, W.M. Management of sinonasal undifferentiated carcinoma. Head Neck 2008, 30, 595–599. [Google Scholar] [CrossRef]
- Dooley, L.; Shah, J. Management of the neck in maxillary sinus carcinomas. Curr. Opin. Otolaryngol. Head Neck Surg. 2015, 23, 107–114. [Google Scholar] [CrossRef]
- Ranasinghe, V.J.; Stubbs, V.C.; Reny, D.C.; Fathy, R.; Brant, J.A.; Newman, J.G. Predictors of nodal metastasis in sinonasal squamous cell carcinoma: A national cancer database analysis. World J. Otorhinolaryngol. Head Neck Surg. 2020, 6, 137–141. [Google Scholar] [CrossRef]
- Mirghani, H.; Hartl, D.; Mortuaire, G.; Armas, G.L.; Auperin, A.; Chevalier, D.; Lefebvre, J.L. Nodal recurrence of sinonasal cancer: Does the risk of cervical relapse justify a prophylactic neck treatment? Oral Oncol. 2013, 49, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Atallah, S.; Moya-Plana, A.; Malard, O.; Poissonnet, G.; Fakhry, N.; Bettoni, J.; Gallet, P.; Ransy, P.; Vergez, S.; De Gabory, L.; et al. Should a neck dissection be performed on patients with cN0 adenoid cystic carcinoma? A REFCOR propensity score matching study. Eur. J. Cancer 2020, 130, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Sethi, R.K.; Feng, A.L.; Fontanarosa, J.B.; Deschler, D.G. The role of elective neck dissection in patients with adenoid cystic carcinoma of the head and neck. Laryngoscope 2019, 129, 2094–2104. [Google Scholar] [CrossRef] [PubMed]
- Kashiwazaki, R.; Turner, M.T.; Geltzeiler, M.; Fernandez-Miranda, J.C.; Gardner, P.A.; Snyderman, C.H.; Wang, E.W. The endoscopic endonasal approach for sinonasal and nasopharyngeal adenoid cystic carcinoma. Laryngoscope 2020, 130, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Gamez, M.E.; Lal, D.; Halyard, M.Y.; Wong, W.W.; Vargas, C.; Ma, D.; Ko, S.J.; Foote, R.L.; Patel, S.H. Outcomes and patterns of failure for sinonasal undifferentiated carcinoma (SNUC): The Mayo Clinic Experience. Head Neck 2017, 39, 1819–1824. [Google Scholar] [CrossRef] [PubMed]
- Morand, G.B.; Anderegg, N.; Vital, D.; Ikenberg, K.; Huber, G.F.; Soyka, M.B.; Egger, M.; Holzmann, D. Outcome by treatment modality in sinonasal undifferentiated carcinoma (SNUC): A case-series, systematic review and meta-analysis. Oral Oncol. 2017, 75, 28–34. [Google Scholar] [CrossRef]
- Chweya, C.M.; Low, C.M.; Van Gompel, J.J.; Van Abel, K.M.; Stokken, J.K.; Choby, G. Population-based analysis on the effect of nodal and distant metastases in sinonasal adenocarcinoma. Head Neck 2021, 43, 128–136. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®), Head and Neck Cancers, v 1.2021, © 2021. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx#head-and-neck (accessed on 12 April 2021).
- Weiss, M.H.; Harrison, L.B.; Isaacs, R.S. Use of Decision Analysis in Planning a Management Strategy for the Stage NO Neck. Arch. Otolaryngol. Head Neck Surg. 1994, 120, 699–702. [Google Scholar] [CrossRef]
- Michel, J.; Fakhry, N.; Mancini, J.; Braustein, D.; Moreddu, E.; Giovanni, A.; Dessi, P. Sinonasal squamous cell carcinomas: Clinical outcomes and predictive factors. Int. J. Oral Maxillofac. Surg. 2014, 43, 1–6. [Google Scholar] [CrossRef]
- Sanghvi, S.; Khan, M.N.; Patel, N.R.; Bs, S.Y.; Baredes, S.; Eloy, J.A. Epidemiology of sinonasal squamous cell carcinoma: A comprehensive analysis of 4994 patients. Laryngoscope 2014, 124, 76–83. [Google Scholar] [CrossRef]
- Bruno, C.; Fiori, G.; Locatello, L.G.; Cannavicci, A.; Gallo, O.; Maggiore, G. The role of Narrow Band Imaging (NBI) in the diagnosis of sinonasal diseases. Rhinology 2021, 59, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, H.; Hao, D.; Ge, Y.; Wan, G.; Zhang, J.; Liu, S.; Zhang, Y.; Xu, D. An MRI -Based Radiomic Nomogram for Discrimination between Malignant and Benign Sinonasal Tumors. J. Magn. Reson. Imaging 2021, 53, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.K.; Clair, J.M.-S.; El-Sayed, I. AHNS Series: Do you know your guidelines? Principles for treatment of cancer of the paranasal sinuses: A review of the National Comprehensive Cancer Network guidelines. Head Neck 2018, 40, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Takes, R.P.; Ferlito, A.; Silver, C.E.; Rinaldo, A.; Medina, J.E.; Robbins, K.T.; Rodrigo, J.P.; Hamoir, M.; Suárez, C.; Zbären, P.; et al. The controversy in the management of the N0 neck for squamous cell carcinoma of the maxillary sinus. Eur. Arch. Oto-Rhino-Laryngol. 2013, 271, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Cantu, G.; Bimbi, G.; Miceli, R.; Mariani, L.; Colombo, S.; Riccio, S.; Squadrelli, M.; Battisti, A.; Pompilio, M.; Rossi, M. Lymph Node Metastases in Malignant Tumors of the Paranasal Sinuses. Arch. Otolaryngol. Head Neck Surg. 2008, 134, 170–177. [Google Scholar] [CrossRef] [PubMed]

| No. of Patients (Sex) | Age (Years: Mean; Range) | Years | Final Histopathology | Subsite | T Stage | N Stage | N Evaluation | Treatment | Follow-Up Time (Months: Mean, Range) | OS | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al-Mamgani et al. (2013) [16] | 21 (11 M—10 F) | 52; 26–78 | 1996–2010 | 21 SNUC | 5 maxillary, 16 ethmoidal | 6 T3, 6 T4a, 9 T4b | 19 N0, 2 N+ | US, CT or MRI | 7 (C)RT, 14 Surgery +(C)RT | 54, 26–78 | 5-year OS 74% |
| Ang et al. (1992) [17] | 45 (29 M—16 F) | 58; 19–73 | 1969–1985 | 30 SCC, 9 AC, 5 ACC, 1 SNUC | 45 nasal cavity | NR | 44 N0, 1 N+ | physical examination, plain X-rays, polytomograms (after 1975 CT) | 18 (C)RT, 27 Surgery + (C)RT | 132, 36–204 | 5-year OS 75% |
| Bhasker et al. (2017) [18] | 16 (13 M—3 F) | 47.5; 8–65 | 2004–2012 | 16 SNUC | 7 nasal cavity, 5 maxillary, 3 ethmoidal, 1 sphenoidal | 1 T3, 15 T4 | 13 N0, 3 N+ | CT and/or MRI | 1 Surgery, 10 (C)RT, 3 Surgery + (C)RT | 10, 1–43 | 2-year OS 47% |
| Bristol et al. (2007) [19] | 146 (86 M—60 F) | 59; 26–90 | 1969–2002 | 89 SCC, 6 AC, 33 ACC, 11 SNUC, 7 Others | 146 maxillary | 22 T1-T2, 47 T3, 77 T4 | 126 N0, 20 N+ | physical examination, plain X-rays, (after 1975 CT) | 146 Surgery + (C)RT | 46, 4–357 | 5-year OS 55% |
| Brown et al. (2013) [20] | 18 (10 M—8 F) | 62; 39–89 | 1992–2008 | 18 SCC | 18 maxillary | 2 T2, 2 T3, 14 T4a | 17 N0, 1 N1 | NR | 3 Surgery, 15 Surgery + (C)RT | 102, 33–206 | 5-year OS 37% |
| Castelnau-Marchand et al. (2016) [21] | 104 (62 M—42 F) | 64; 17–92 | 1998–2012 | 104 SCC | 20 nasal cavity, 70 maxillary, 14 ethmoidal | 8 T1, 9 T2, 18 T3, 69 T4 | 87 N0, 4 N1, 3 N2a, 6 N2b, 4 N2c | CT and/or MRI | 14 Surgery, 30 (C)RT, 60 Surgery + (C)RT | 54, NR | 5-year OS 50% |
| Chen et al. (2008) [22] | 21 (14 M—7 F) | 47; 33–71 | 1990–2004 | 21 SNUC | 11 nasal cavity, 5 maxillary, 5 ethmoidal | 4 T3, 9 T4a, 8 T4b | 19 N0, 2 N+ | CT and MRI | 4 (C)RT, 17 Surgery + (C)RT | 58, 12–70 | 5-year OS 43% |
| Chobe et al. (1988) [23] | 32 (NR) | NR; 43–77 | 1963–1984 | 32 SCC | 32 nasal cavity | NR | 32 N0 | physical examination | 32 (C)RT | NR | NR |
| Christopherson et al. (2014) [24] | 23 (14 M—9 F) | 56.5; 23–83 | 1992–2010 | 23 SNUC | NR | NR | 18 N0, 5 N+ | NR | 8 (C)RT, 15 Surgery + (C)RT | 36, 12–240 | 5-year OS 32% |
| Doescher et al. (2015) [25] | 44 (33 M—11 F) | 61; 37–84 | 1994–2013 | 44 SCC | NR | 22 T1, 11 T2, 6 T3, 5 T4 | 42 N0, 2 N+ | NR | 30 Surgery, 1 (C)RT, 13 Surgery + (C)RT | 84, NR | 5-year OS 69% |
| Donhujsen et al. (2016) [26] | 117 (NR) | NR | 2000–2013 | 117 AC | NR | 12 T1, 60 T2, 20 T3, 25 T4 | 117 N0 | NR | 36 Surgery, 4 (C)RT, 77 Surgery + (C)RT | 60, NR | 5-year OS 26% |
| Duru Birgi et al. (2015) [27] | 43 (25 M—18 F) | 67; 41–86 | 2007–2012 | 43 SCC | 22 nasal cavity, 20 maxillary, 1 ethmoidal | 6 T1, 6 T2, 2 T3, 23 T4a, 6 T4b | 38 N0, 4 N1, 1 N2c | CT and/or MRI | 18 (C)RT, 25 Surgery + (C)RT | NR | 2-year OS 71% |
| Fornelli et al. (2000) [28] | 32 (21 M—11 F) | 65; NR | 1976–1993 | 32 SCC | 32 nasal cavity | NR | 32 N0 | NR | 15 Surgery, 9 (C)RT, 8 Surgery + (C)RT | 42, 9–156 | 5-year OS 50% |
| Hinerman et al. (2011) [29] | 54 (34 M—20 F) | 62; 36–79 | 1969–2006 | 54 SCC | 54 maxillary | 2 T2, 13 T3, 22 T4a, 17 T4b | 45 N0, 5 N1, 1 N2a, 2 N2b, 1 N2c | NR | 32 (C)RT, 22 Surgery + (C)RT | 18, NR | 5-year OS 41% |
| Jiang et al. (1991) [30] | 73 (41 M—32 F) | 53; 27–76 | 1969–1985 | 36 SCC, 6 AC, 20 ACC, 9 SNUC, 2 Others | 73 maxillary | 3 T1, 16 T2, 32 T3, 22 T4 | 67 N0, 4 N1, 2 N2 | physical examination, plain X-rays, (after 1975 CT, occasionally MRI) | 73 Surgery + (C)RT | 83, 9–182 | 5-year OS 48% |
| Kim et al. (2004) [31] | 8 (6 M—2 F) | 48; 27–68 | 1995–2002 | 8 SNUC | NR | NR | 8 N0 | NR | 1 Surgery, 3 (C)RT, 4 Surgery + (C)RT | NR | 2-year OS 75% |
| Le et al. (2000) [32] | 97 (67 M—30 F) | 58; 20–85 | 1959–1996 | 58 SCC, 4 AC, 19 ACC, 16 SNUC | 97 maxillary | 8 T2, 36 T3, 53 T4 | 86 N0, 6 N1, 3 N2b, 2 N2c | physical examination (after 1977 CT, occasionally MRI) | 36 (C)RT, 61 Surgery + (C)RT | 78, 18–276 | 5-year OS 31% |
| Lee et al. (2018) [33] | 124 (85 M—39 F) | 57.5; 33–82 | 2000–2015 | 82 SCC, 5 AC, 23 ACC, 14 Others | 109 maxillary, 13 ethmoidal, 2 sphenoidal | 13 T2, 58 T3, 53 T4 | 124 N0 | CT or MRI | 26 (C)RT, 98 Surgery + (C)RT | 54, 2–288 | 5-year OS 67% |
| Levendang et al. (1990) [34] | 63 (57 M—6 F) | 64; 33–84 | 1978–1988 | 63 SCC | 63 nasal cavity | 36 T1, 24 T2 | 59 N0, 2 N1, 2 N2a | NR | 63 (C)RT | 46, NR | 5-year OS 65% |
| Lopez et al. (2015) [35] | 17 (9 M—8 F) | 53; 28–73 | 2001–2013 | 17 SNUC | 17 ethmoidal | 1 T3, 4 T4a, 12 T4b | 15 N0, 2 N+ | CT or MRI | 3 (C)RT, 14 Surgery + (C)RT | 48, 6–96 | 5-year OS 58% |
| McCollough et al. (1993) [36] | 39 (23 M—16 F) | 65; 40.84 | 1968–1988 | 39 SCC | 39 nasal cavity | 13 T1, 8 T2, 18 T4 | 37 N0, 1 N1, 1 N2b | NR | 39 (C)RT | 24, NR | 5-year OS 75% |
| Mendenhall et al. (1987) [37] | 22 (NR) | NR | 1964–1984 | 22 SCC | 22 nasal cavity | 7 T1, 2 T2, 2 T3, 11 T4 | 20 N0, 2 N1 | NR | 22 (C)RT | 90, NR | 5-year OS 75% |
| Parsons et al. (1996) [38] | 35 (NR) | NR | 1964–1992 | 20 ACC, 15 Others | 14 nasal cavity, 12 maxillary, 7 ethmoidal, 2 sphenoidal | 4 T1-T2, 10 T3, 21 T4 | 35 N0 | NR | 18 (C)RT, 17 Surgery + (C)RT | NR | 5-year OS 43% |
| Revenaugh et al. (2011) [39] | 13 (7 M—6 F) | 49; 16–78 | 2002–2009 | 13 SNUC | 3 maxillary, 4 ethmoidal, 4 sphenoidal, 2 frontal | 1 T1, 3 T4a, 9 T4b | 12 N0, 1 N2c | NR | 5 (C)RT, 8 Surgery + (C)RT | 23, 3–62 | 2-year OS 80% |
| Snyers et al. (2009) [40] | 98 (NR) | NR | 1986–2006 | 55 SCC, 43 AC | 32 nasal cavity, 22 maxillary, 22 ethmoidal | 1 T1, 9 T2, 22 T3, 21 T4a, 23 T4b | 73 N0, 1 N1, 2 N2b | physical examination (in some cases CT, MRI or US + FNAC) | NR | 69, 3–253 | 5-year OS 35% |
| Tanzler et al. (2008) [41] | 15 (10 M—5 F) | 57; 23–82 | 1992–2005 | 15 SNUC | NR | 8 T4a, 7 T4b | 13 N0, 1 N1, 1 N2c | CT and/or MRI | 1 Surgery, 5 (C)RT, 9 Surgery + (C)RT | 30, 11–151 | 3-year OS 67% |
| Total | 1320 (657 M—359 F—304 NR) | 801 SCC, 190 AC, 120 ACC, 171 SNUC, 38 Others | 339 nasal cavity, 639 maxillary, 102 ethmoidal, 9 sphenoidal, 2 frontal | 305 T1-T2, 280 T3, 570 T4 (110 T4a, 91 T4b, 369 not specified) | 1198 N0, 31 N1, 32 N2 (6 N2a, 14 N2b, 10 N2c, 2 not specified) | 101 Surgery, 393 (C)RT, 726 Surgery + (C)RT |
| No. Patients | Regional Recurrence | ENT Group | Observation Group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Recurrences | Levels | Salvage Neck Strategy | Survival | Recurrences | Levels | Salvage Neck Strategy | Survival | |||
| Al-Mamgani et al. [16] | 19 | 2 | 0/8 | - | - | - | 2/11 (2 IRR) | NR | ND and PORT | no DOD |
| Ang et al. [17] | 44 | 2 | 0/9 | - | - | - | 2/35 (2 IRR) | Subdigastrics | ND and PORT | no DOD |
| Bhasker et al. [18] | 13 | 1 | 0/5 | - | - | - | 1/8 (1 IRR) | NR | BSC | 1 DIRR |
| Bristol et al. [19] | 81 | 16 | 3/45 (0 IRR) | II ipsilateral | ND and PORT | no DOD | 13/36 (8 IRR) | 7 II ipsilateral, 4 Ib ipsilateral, 1 II bilateral, 1 II contralateral | NR | NR |
| Brown et al. [20] | 17 | 2 | 2/16 (1 IRR) | ipsilateral | ND | 1 DLRD, 1 DIRR | 0/1 | - | - | - |
| Castelnau-Marchand et al. [21] | 87 | 8 | 5/55 (0 IRR) | Ib-II-III + intraparotid | NR | 3/32 (2 IRR) | Ib-II-III + intraparotid | NR | NR | |
| Chen et al. [22] | 19 | 2 | 1/15 (0 IRR) | NR | NR | 1 DLRD | 1/4 (0 IRR) | NR | NR | 1 DLRD |
| Chobe et al. [23] | 32 | 4 | 0/14 | - | - | - | 4/18 (2 IRR) | NR | 2 ND and PORT | 2 DLRD |
| Christopherson et al. [24] | 18 | 3 | 4/13 (NR) | NR | NR | NR | 2/5 (NR) | NR | NR | NR |
| Doescher et al. [25] | 42 | 2 | 0/16 | - | - | - | 2/26 (NR) | NR | NR | NR |
| Donhujsen et al. [26] | 117 | 6 | 0/15 | - | - | - | 6/102 (3 IRR) | NR | NR | NR |
| Duru Birgi et al. [27] | 38 | 4 | 0/11 | - | - | - | 4/27 (3 IRR) | 1 II, 2 I, 1 facial | 1 ND and PORT | 3 DIRR |
| Fornelli et al. [28] | 32 | 13 | 0/6 | - | - | - | 13/26 (NR) | most frequently level I | 2 ND and PORT | 11 DOD |
| Hinerman et al. [29] | 45 | 3 | 1/23 (1 IRR) | II contralateral | NR | NR | 2/22 (2 IRR) | 1 I, 1 II | NR | NR |
| Jiang et al. [30] | 67 | 11 | 0/17 | - | - | - | 11/50 (9 IRR) | 3 Ib ipsilateral, 1 Ib bilateral, 7 subdigastric ipsilateral | 2 BSC, 4 RT, 5 ND and PORT | 7 DOD (5 of IRR group) |
| Kim et al. [31] | 8 | 3 | 0/3 | - | - | - | 3/5 (3 IRR) | NR | NR | NR |
| Le et al. [32] | 86 | 9 | 2/25 (NR) | I-II | NR | NR | 7/61 (NR) | I-II | NR | NR |
| Lee et al. [33] | 124 | 21 | 7/40 (NR) | 5 in treated neck, 2 contralateral | NR | NR | 14/84 (NR) | 7 I ipsilateral, 6 II ipsilateral, 2 III ipsilateral, 1 IV ipsilateral, 1 I contralateral, 1 II contralateral, 1 I bilateral, 1 II III IV bilateral, 1 IV bilateral, 1 nr ipsilateral | NR | NR |
| Levendang et al. [34] | 59 | 4 | 2/25 (NR) | NR | NR | NR | 2/34 (NR) | NR | NR | NR |
| Lopez et al. [35] | 15 | 3 | 0/10 | - | - | - | 3/5 (NR) | NR | 1 ND and PORT | 1 DIRR, 1 DLRD |
| McCollough et al. [36] | 37 | 4 | 0/5 | - | - | - | 4/32 (NR) | 1 jugulodigastric ipsilateral, 2 submaxillary, 1 facial | ND +/- PORT | no DOD |
| Mendenhall et al. [37] | 20 | 2 | 0/3 | - | - | - | 2/17 (NR) | Ib | ND | no DOD |
| Parsons et al. [38] | 35 | 1 | 0/15 | - | - | - | 1/20 (NR) | NR | NR | NR |
| Revenaugh et al. [39] | 12 | 2 | 0/3 | - | - | - | 1/9 (NR) | II ipsilateral | ND | no DOD |
| Snyers et al. [40] | 98 | 10 | 0/3 | - | - | - | 10/95 (NR) | NR | NR | NR |
| Tanzler et al. [41] | 13 | 2 | 0/7 | - | - | - | 2/6 (NR) | NR | NR | 1 DIRR, 1 DLRD |
| Total | 1178 | 140 | 24/407 | 115/771 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galloni, C.; Locatello, L.G.; Bruno, C.; Cannavicci, A.; Maggiore, G.; Gallo, O. The Role of Elective Neck Treatment in the Management of Sinonasal Carcinomas: A Systematic Review of the Literature and a Meta-Analysis. Cancers 2021, 13, 1842. https://doi.org/10.3390/cancers13081842
Galloni C, Locatello LG, Bruno C, Cannavicci A, Maggiore G, Gallo O. The Role of Elective Neck Treatment in the Management of Sinonasal Carcinomas: A Systematic Review of the Literature and a Meta-Analysis. Cancers. 2021; 13(8):1842. https://doi.org/10.3390/cancers13081842
Chicago/Turabian StyleGalloni, Costanza, Luca Giovanni Locatello, Chiara Bruno, Angelo Cannavicci, Giandomenico Maggiore, and Oreste Gallo. 2021. "The Role of Elective Neck Treatment in the Management of Sinonasal Carcinomas: A Systematic Review of the Literature and a Meta-Analysis" Cancers 13, no. 8: 1842. https://doi.org/10.3390/cancers13081842
APA StyleGalloni, C., Locatello, L. G., Bruno, C., Cannavicci, A., Maggiore, G., & Gallo, O. (2021). The Role of Elective Neck Treatment in the Management of Sinonasal Carcinomas: A Systematic Review of the Literature and a Meta-Analysis. Cancers, 13(8), 1842. https://doi.org/10.3390/cancers13081842






