Recent Development of Gold Nanoparticles as Contrast Agents for Cancer Diagnosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Gold Nanoparticles in Cancer Diagnosis
2.1. Magnetic Resonance Imaging (MRI)
2.2. CT and Nuclear Imaging
2.3. Fluorescence Imaging
2.4. Photoacoustic Imaging
2.5. X-ray Fluorescence Imaging
2.6. Other Imaging Modelity
3. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Xie, J.; Gong, L.; Zhu, S.; Yong, Y.; Gu, Z.; Zhao, Y. Emerging strategies of nanomaterial-mediated tumor radiosensitization. Adv. Mater. 2019, 31, e1802244. [Google Scholar] [CrossRef]
- Song, G.; Cheng, L.; Chao, Y.; Yang, K.; Liu, Z. Emerging nanotechnology and advanced materials for cancer radiation therapy. Adv. Mater. 2017, 29, 1700996. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Du, B.; Huang, Y.; Zheng, J. Ultrasmall noble metal nanoparticles: Breakthroughs and biomedical implications. Nano Today 2018, 21, 106–125. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wang, X.; Walker, E.; Wang, J.; Springer, S.; Lou, J.; Ramamurthy, G.; Burda, C.; Basilion, J.P. Nanoparticles yield increased drug uptake and therapeutic efficacy upon sequential near-infrared irradiation. ACS Nano 2020, 14, 15193–15203. [Google Scholar] [CrossRef]
- Goswami, N.; Luo, Z.; Yuan, X.; Leong, D.T.; Xie, J. Engineering gold-based radiosensitizers for cancer radiotherapy. Mater. Horiz. 2017, 4, 817–831. [Google Scholar] [CrossRef]
- Gong, F.; Yang, N.; Wang, X.; Zhao, Q.; Chen, Q.; Liu, Z.; Cheng, L. Tumor microenvironment-responsive intelligent nanoplatforms for cancer theranostics. Nano Today 2020, 32, 100851. [Google Scholar] [CrossRef]
- Vankayala, R.; Hwang, K.C. Near-infrared-light-activatable nanomaterial-mediated phototheranostic nanomedicines: An emerging paradigm for cancer treatment. Adv. Mater. 2018, 30, e1706320. [Google Scholar] [CrossRef]
- Ehlerding, E.B.; Chen, F.; Cai, W. Biodegradable and renal clearable inorganic nanoparticles. Adv. Sci. 2016, 3, 3. [Google Scholar] [CrossRef]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Peng, X.; Wang, Y.Q.; Wang, Y.X.; Shin, D.M.; El-Sayed, M.A.; Nie, S. A reexamination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands. ACS Nano 2010, 4, 5887–5896. [Google Scholar] [CrossRef] [PubMed]
- Spaas, C.; Dok, R.; Deschaume, O.; De Roo, B.; Vervaele, M.; Seo, J.W.; Bartic, C.; Hoet, P.; Van den Heuvel, F.; Nuyts, S. Dependence of gold nanoparticle radiosensitization on functionalizing layer thickness. Radiat. Res. 2016, 185, 384–392. [Google Scholar] [CrossRef]
- Mangadlao, J.D.; Wang, X.; McCleese, C.; Escamilla, M.; Ramamurthy, G.; Wang, Z.; Govande, M.; Basilion, J.P.; Burda, C. Prostate-specific membrane antigen targeted gold nanoparticles for theranostics of prostate cancer. ACS Nano 2018, 12, 3714–3725. [Google Scholar] [CrossRef]
- Mosquera, J.; Garcia, I.; Liz-Marzan, L.M. Cellular uptake of nanoparticles versus small molecules: A matter of size. Acc. Chem. Res. 2018, 51, 2305–2313. [Google Scholar] [CrossRef]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef]
- Daniel, M.C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Bastus, N.G.; Comenge, J.; Puntes, V. Kinetically controlled seeded growth synthesis of citrate-stabilized gold nanoparticles of up to 200 nm: Size focusing versus ostwald ripening. Langmuir 2011, 27, 11098–11105. [Google Scholar] [CrossRef]
- Cheng, Y.; Samia, A.C.; Li, J.; Kenney, M.E.; Resnick, A.; Burda, C. Delivery and efficacy of a cancer drug as a function of the bond to the gold nanoparticle surface. Langmuir 2010, 26, 2248–2255. [Google Scholar] [CrossRef]
- Hostetler, M.J.; Wingate, J.E.; Zhong, C.J.; Harris, J.E.; Vachet, R.W.; Clark, M.R.; Londono, J.D.; Green, S.J.; Stokes, J.J.; Wignall, G.D.; et al. Alkanethiolate gold cluster molecules with core diameters from 1.5 to 5.2 nm: Core and monolayer properties as a function of core size. Langmuir 1998, 14, 17–30. [Google Scholar] [CrossRef]
- Tan, Y.N.; Lee, J.Y.; Wang, D.I.C. Uncovering the design rules for peptide synthesis of metal nanoparticles. J. Am. Chem. Soc. 2010, 132, 5677–5686. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef]
- Luo, D.; Hasan, M.S.; Shahid, S.; Khlebtsov, B.; Cattell, M.J.; Sukhorukov, G.B. Gold nanorod mediated chlorhexidine microparticle formation and near-infrared light induced release. Langmuir 2017, 33, 7982–7993. [Google Scholar] [CrossRef]
- von Maltzahn, G.; Park, J.H.; Agrawal, A.; Bandaru, N.K.; Das, S.K.; Sailor, M.J.; Bhatia, S.N. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009, 69, 3892–3900. [Google Scholar] [CrossRef]
- Bhattarai, S.R.; Derry, P.J.; Aziz, K.; Singh, P.K.; Khoo, A.M.; Chadha, A.S.; Liopo, A.; Zubarev, E.R.; Krishnan, S. Gold nanotriangles: Scale up and X-ray radiosensitization effects in mice. Nanoscale 2017, 9, 5085–5093. [Google Scholar] [CrossRef] [PubMed]
- Borzenkov, M.; Chirico, G.; D’Alfonso, L.; Sironi, L.; Collini, M.; Cabrini, E.; Dacarro, G.; Milanese, C.; Pallavicini, P.; Taglietti, A.; et al. Thermal and chemical stability of thiol bonding on gold nanostars. Langmuir 2015, 31, 8081–8091. [Google Scholar] [CrossRef]
- Tabish, T.A.; Dey, P.; Mosca, S.; Salimi, M.; Palombo, F.; Matousek, P.; Stone, N. Smart gold nanostructures for light mediated cancer theranostics: Combining optical diagnostics with photothermal therapy. Adv. Sci. 2020, 7, 1903441. [Google Scholar] [CrossRef]
- Schwartz-Duval, A.S.; Konopka, C.J.; Moitra, P.; Daza, E.A.; Srivastava, I.; Johnson, E.V.; Kampert, T.L.; Fayn, S.; Haran, A.; Dobrucki, L.W.; et al. Intratumoral generation of photothermal gold nanoparticles through a vectorized biomineralization of ionic gold. Nat. Commun. 2020, 11, 4530. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, T.; Qin, X.; Qiao, Q.; Shang, L.; Song, Q.; Yang, C.; Zhang, Z. Intracellularly generated immunological gold nanoparticles for combinatorial photothermal therapy and immunotherapy against tumor. Nano Lett. 2019, 19, 6635–6646. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Guo, Y.; Li, X.; Li, X.; Wang, S.; Wang, L.; Lv, G.; Zhang, X.; Wang, H.; Gong, X.; et al. Size-tuning ionization to optimize gold nanoparticles for simultaneous enhanced CT imaging and radiotherapy. ACS Nano 2016, 10, 2536–2548. [Google Scholar] [CrossRef]
- Perrault, S.D.; Walkey, C.; Jennings, T.; Fischer, H.C.; Chan, W.C. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009, 9, 1909–1915. [Google Scholar] [CrossRef]
- Oh, E.; Delehanty, J.B.; Sapsford, K.E.; Susumu, K.; Goswami, R.; Blanco-Canosa, J.B.; Dawson, P.E.; Granek, J.; Shoff, M.; Zhang, Q.; et al. Cellular uptake and fate of PEGylated gold nanoparticles is dependent on both cell-penetration peptides and particle size. ACS Nano 2011, 5, 6434–6448. [Google Scholar] [CrossRef]
- Zhang, X.D.; Wu, D.; Shen, X.; Chen, J.; Sun, Y.M.; Liu, P.X.; Liang, X.J. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials 2012, 33, 6408–6419. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Li, X.; Wang, B.; Zhou, S.; Chen, W.; Chen, H.; Liang, S.; Zheng, L.; Yu, H.; Chu, R.; Wang, M.; et al. Surface chemistry governs the sub-organ transfer, clearance and toxicity of functional gold nanoparticles in the liver and kidney. J. Nanobiotechnol. 2020, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Doane, T.L.; Cheng, Y.; Lu, F.; Srinivasan, S.; Zhu, J.-J.; Burda, C. Control of surface ligand density on PEGylated gold nanoparticles for optimized cancer cell uptake. Part. Part. Syst. Charact. 2015, 32, 197–204. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Zhu, M.; Lin, G.; Liu, J.; Zhou, Z.; Tian, X.; Pan, Y. PEGylated Au@Pt nanodendrites as novel theranostic agents for computed tomography imaging and photothermal/radiation synergistic therapy. ACS Appl. Mater. Interfaces 2017, 9, 279–285. [Google Scholar] [CrossRef]
- Shiraishi, K.; Yokoyama, M. Toxicity and immunogenicity concerns related to PEGylated-micelle carrier systems: A review. Sci. Technol. Adv. Mater. 2019, 20, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Kong, F.; Gao, X.; Jiang, L.; Li, X.; Gao, W.; Xu, K.; Tang, B. Avoiding thiol compound interference: A nanoplatform based on high-fidelity Au-Se bonds for biological applications. Angew. Chem. Int. Ed. 2018, 57, 5306–5309. [Google Scholar] [CrossRef]
- Wu, J.; Han, H.; Jin, Q.; Li, Z.; Li, H.; Ji, J. Design and proof of programmed 5-aminolevulinic acid prodrug nanocarriers for targeted photodynamic cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 14596–14605. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, Y.; Yang, J.; Liu, Y.; Hu, F.; Zhu, K.; Jiang, X. Gold nanoclustersfor targeting methicillin-resistant staphylococcus aureus in vivo. Angew. Chem. Int. Ed. 2018, 57, 3958–3962. [Google Scholar] [CrossRef]
- Lei, Y.; Tang, L.; Xie, Y.; Xianyu, Y.; Zhang, L.; Wang, P.; Hamada, Y.; Jiang, K.; Zheng, W.; Jiang, X. Gold nanoclusters-assisted delivery of NGF siRNA for effective treatment of pancreatic cancer. Nat. Commun. 2017, 8, 15130. [Google Scholar] [CrossRef]
- Luo, M.; Xuan, M.; Huo, S.; Fan, J.; Chakraborty, G.; Wang, Y.; Zhao, H.; Herrmann, A.; Zheng, L. Four-dimensional deoxyribonucleic acid-gold nanoparticle assemblies. Angew. Chem. Int. Ed. 2020, 59, 1–7. [Google Scholar] [CrossRef]
- Sykes, E.A.; Chen, J.; Zheng, G.; Chan, W.C.W. Investigating the impact of nanoparticle size on active and passive tumor targeting efficiency. ACS Nano 2014, 8, 5696–5706. [Google Scholar] [CrossRef]
- Yue, T.; Zhang, X. Cooperative effect inreceptor-mediated endocytosis of multiple nanoparticles. ACS Nano 2012, 6, 3196–3205. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, H.; Huang, J.; Qian, W.; Martinson, D.E.; Ji, B.; Li, Y.; Wang, Y.A.; Yang, L.; Mao, H. Probing and enhancing ligand-mediated active targeting of tumors using sub-5 nm ultrafine iron oxide nanoparticles. Theranostics 2020, 10, 2479–2494. [Google Scholar] [CrossRef]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; O’Connor, M.J.; Dilmanian, F.A.; Slatkin, D.N.; Adams, D.J.; Smilowitz, H.M. Micro-CT enables microlocalisation and quantification of her2-targeted gold nanoparticles within tumour regions. Br. J. Radiol. 2011, 84, 526–533. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, N.; Shao, Z.; Qiu, H.; Yao, H.; Ji, J.; Wang, J.; Lu, W.; Chen, R.C.; Zhang, L.; et al. Folate-targeted nanoparticle delivery of androgen receptor shrna enhances the sensitivity of hormone-independent prostate cancer to radiotherapy. Nanomedicine 2017, 13, 1309–1321. [Google Scholar] [CrossRef]
- Meyers, J.D.; Cheng, Y.; Broome, A.M.; Agnes, R.S.; Schluchter, M.D.; Margevicius, S.; Wang, X.; Kenney, M.E.; Burda, C.; Basilion, J.P. Peptide-targeted gold nanoparticles for photodynamic therapy of brain cancer. Part. Part. Syst. Charact. 2015, 32, 448–457. [Google Scholar] [CrossRef]
- Liang, G.; Jin, X.; Zhang, S.; Xing, D. RGD peptide-modified fluorescent gold nanoclusters as highly efficient tumor-targeted radiotherapy sensitizers. Biomaterials 2017, 144, 95–104. [Google Scholar] [CrossRef]
- Wang, X.; Huang, S.S.; Heston, W.D.; Guo, H.; Wang, B.C.; Basilion, J.P. Development of targeted near-infrared imaging agents for prostate cancer. Mol. Cancer Ther. 2014, 13, 2595–2606. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Huo, S.; Zhang, X.; Liu, J.; Tan, A.; Li, S.; Jin, S.; Xue, X.; Zhao, Y.Y.; Ji, T.; et al. Neuropilin-1-targeted gold nanoparticles enhance therapeutic efficacy of platinum(iv) drug for prostate cancer treatment. ACS Nano 2014, 8, 4205–4220. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Liang, J.; Chen, H.; Geng, D.; Jiao, L.; Yang, J.Y.; Qian, H.; Zhang, C.; Ding, Y. Performance of doxorubicin-conjugated gold nanoparticles: Regulation of drug location. ACS Appl. Mater. Interfaces 2017, 9, 8569–8580. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, C.X.; Chen, L.G.; Yan, X.P. Dual-stimuli responsive and reversibly activatable theranostic nanoprobe for precision tumor-targeting and fluorescence-guided photothermal therapy. Nat. Commun. 2017, 8, 14998. [Google Scholar] [CrossRef]
- Cheng, Y.; Meyers, J.D.; Agnes, R.S.; Doane, T.L.; Kenney, M.E.; Broome, A.M.; Burda, C.; Basilion, J.P. Addressing brain tumors with targeted gold nanoparticles: A new gold standard for hydrophobic drug delivery? Small 2011, 7, 2301–2306. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.D.; Jin, S.B.; Ma, X.W.; Xue, X.D.; Yang, K.N.; Kumar, A.; Wang, P.C.; Zhang, J.; Hu, Z.; Liang, X.J. Ultrasmall gold nanoparticles as carriers for nucleus-based size-dependent nuclear entry. ACS Nano 2014, 8, 5852–5862. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Thompson, L.B.; Boulos, S.P.; Sisco, P.N.; Murphy, C.J. Gold nanorods: Their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv. Drug Deliv. Rev. 2012, 64, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Mackey, M.A.; Huang, X.; Kang, B.; El-Sayed, M.A. Beating cancer in multiple ways using nanogold. Chem. Soc. Rev. 2011, 40, 3391–3404. [Google Scholar] [CrossRef]
- Chen, J.; Ning, C.; Zhou, Z.; Yu, P.; Zhu, Y.; Tan, G.; Mao, C. Nanomaterials as photothermal therapeutic agents. Prog. Mater. Sci. 2019, 99, 1–26. [Google Scholar] [CrossRef]
- Fan, M.; Han, Y.; Gao, S.; Yan, H.; Cao, L.; Li, Z.; Liang, X.J.; Zhang, J. Ultrasmall gold nanoparticles in cancer diagnosis and therapy. Theranostics 2020, 10, 4944–4957. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.C.; Jo, E.; Kwon, Y.W.; Lee, B.; Ryu, J.H.; Lee, E.J.; Kim, K.; Lee, J. Superparamagnetic gold nanoparticles synthesized on protein particle scaffolds for cancer theragnosis. Adv. Mater. 2017, 29, 1701146. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Cho, M.K.; Lee, E.J.; Ahn, K.Y.; Lee, K.E.; Jung, J.H.; Cho, Y.; Han, S.S.; Kim, Y.K.; Lee, J. A highly sensitive and selective diagnostic assay based on virus nanoparticles. Nat. Nanotechnol. 2009, 4, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Johnson, A.; Wang, X.; Li, H.; Erokwu, B.O.; Springer, S.; Lou, J.; Ramamurthy, G.; Flask, C.A.; Burda, C.; et al. Targeted radiosensitizers for MR-guided radiation therapy of prostate cancer. Nano Lett. 2020, 20, 7159–7167. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wang, X.; Zeng, S.; Ramamurthy, G.; Burda, C.; Basilion, J.P. Prostate-specific membrane antigen targeted gold nanoparticles for prostate cancer radiotherapy: Does size matter for targeted particles? Chem. Sci. 2019, 10, 8119–8128. [Google Scholar] [CrossRef]
- Zhou, C.; Hao, G.; Thomas, P.; Liu, J.; Yu, M.; Sun, S.; Oz, O.K.; Sun, X.; Zheng, J. Near-infrared emitting radioactive gold nanoparticles with molecular pharmacokinetics. Angew. Chem. Int. Ed. 2012, 51, 10118–10122. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sultan, D.; Detering, L.; Luehmann, H.; Liu, Y. Facile synthesis, pharmacokinetic and systemic clearance evaluation, and positron emission tomography cancer imaging of 64Cu-Au alloy nanoclusters. Nanoscale 2014, 6, 13501–13509. [Google Scholar] [CrossRef]
- Zhao, Y.; Detering, L.; Sultan, D.; Cooper, M.L.; You, M.; Cho, S.; Meier, S.L.; Luehmann, H.; Sun, G.; Rettig, M.; et al. Gold nanoclusters doped with 64Cu for CXCR4 positron emission tomography imaging of breast cancer and metastasis. ACS Nano 2016, 10, 5959–5970. [Google Scholar] [CrossRef]
- Yang, J.; Wang, T.; Zhao, L.; Rajasekhar, V.K.; Joshi, S.; Andreou, C.; Pal, S.; Hsu, H.T.; Zhang, H.; Cohen, I.J.; et al. Gold/alpha-lactalbumin nanoprobes for the imaging and treatment of breast cancer. Nat. Biomed. Eng. 2020, 4, 686–703. [Google Scholar] [CrossRef]
- Han, S.; Bouchard, R.; Sokolov, K.V. Molecular photoacoustic imaging with ultra-small gold nanoparticles. Biomed. Opt. Express 2019, 10, 3472–3483. [Google Scholar] [CrossRef]
- Manohar, N.; Reynoso, F.J.; Diagaradjane, P.; Krishnan, S.; Cho, S.H. Quantitative imaging of gold nanoparticle distribution in a tumor-bearing mouse using benchtop X-ray fluorescence computed tomography. Sci. Rep. 2016, 6, 22079. [Google Scholar] [CrossRef]
- Zhou, Z.; Lu, Z.R. Molecular imaging of the tumor microenvironment. Adv. Drug Deliv. Rev. 2017, 113, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wu, X.; Roelle, S.; Chen, C.; Schiemann, W.P.; Lu, Z.R. Targeted gadofullerene for sensitive magnetic resonance imaging and risk-stratification of breast cancer. Nat. Commun. 2017, 8, 692. [Google Scholar] [CrossRef]
- Zhou, Z.; Deng, H.; Yang, W.; Wang, Z.; Lin, L.; Munasinghe, J.; Jacobson, O.; Liu, Y.; Tang, L.; Ni, Q.; et al. Early stratification of radiotherapy response by activatable inflammation magnetic resonance imaging. Nat. Commun. 2020, 11, 3032. [Google Scholar] [CrossRef]
- Na, H.B.; Song, I.C.; Hyeon, T. Inorganic nanoparticles for MRI contrast agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Wang, Z.; Qiao, R.; Tang, N.; Lu, Z.; Wang, H.; Zhang, Z.; Xue, X.; Huang, Z.; Zhang, S.; Zhang, G. Active targeting theranostic iron oxide nanoparticles for MRI and magnetic resonance-guided focused ultrasound ablation of lung cancer. Biomaterials 2017, 127, 25–35. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Hofmann, H.; Rothen-Rutishauser, B.; Petri-Fink, A. Assessing the in vitro and in vivo toxicity of superparamagnetic iron oxide nanoparticles. Chem. Rev. 2012, 112, 2323–2338. [Google Scholar] [CrossRef]
- Qin, W.; Lohrman, J.; Ren, S. Magnetic and optoelectronic properties of gold nanocluster-thiophene assembly. Angew. Chem. Int. Ed. 2014, 53, 7316–7319. [Google Scholar] [CrossRef] [PubMed]
- Hembury, M.; Chiappini, C.; Bertazzo, S.; Kalber, T.L.; Drisko, G.L.; Ogunlade, O.; Walker-Samuel, S.; Krishna, K.S.; Jumeaux, C.; Beard, P.; et al. Gold-silica quantum rattles for multimodal imaging and therapy. Proc. Natl. Acad. Sci. USA 2015, 112, 1959–1964. [Google Scholar] [CrossRef]
- Kwon, K.C.; Ryu, J.H.; Lee, J.H.; Lee, E.J.; Kwon, I.C.; Kim, K.; Lee, J. Proteinticle/gold core/shell nanoparticles for targeted cancer therapy without nanotoxicity. Adv. Mater. 2014, 26, 6436–6441. [Google Scholar] [CrossRef] [PubMed]
- Aldeek, F.; Safi, M.; Zhan, N.; Palui, G.; Mattoussi, H. Understanding the self-assembly of proteins onto gold nanoparticles and quantum dots driven by metal-histidine coordination. ACS Nano 2013, 7, 10197–10210. [Google Scholar] [CrossRef] [PubMed]
- Alric, C.; Taleb, J.; Duc, G.L.; Mandon, C.; Billotey, C.; Meur-Herland, A.L.; Brochard, T.; Vocanson, F.; Janier, M.; Perriat, P.; et al. Gadolinium chelate coated gold nanoparticles as contrast agents for both x-ray computed tomography and magnetic resonance imaging. J. Am. Chem. Soc. 2008, 130, 5908–5915. [Google Scholar] [CrossRef]
- Liang, G.; Ye, D.; Zhang, X.; Dong, F.; Chen, H.; Zhang, S.; Li, J.; Shen, X.; Kong, J. One-pot synthesis of Gd3+-functionalized gold nanoclusters for dual model (fluorescence/magnetic resonance) imaging. J. Mater. Chem. B 2013, 1, 3545. [Google Scholar] [CrossRef]
- Rotz, M.W.; Holbrook, R.J.; MacRenaris, K.W.; Meade, T.J. A markedly improved synthetic approach for the preparation of multifunctional Au-DNA nanoparticle conjugates modified with optical and mr imaging probes. Bioconjugate Chem. 2018, 29, 3544–3549. [Google Scholar] [CrossRef]
- Rammohan, N.; Holbrook, R.J.; Rotz, M.W.; MacRenaris, K.W.; Preslar, A.T.; Carney, C.E.; Reichova, V.; Meade, T.J. Gd(III)-gold nanoconjugates provide remarkable cell labeling for high field magnetic resonance imaging. Bioconjugate Chem. 2017, 28, 153–160. [Google Scholar] [CrossRef]
- Song, Y.; Xu, X.; MacRenaris, K.W.; Zhang, X.Q.; Mirkin, C.A.; Meade, T.J. Multimodal gadolinium-enriched DNA-gold nanoparticle conjugates for cellular imaging. Angew. Chem. Int. Ed. 2009, 48, 9143–9147. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, R.J.; Rammohan, N.; Rotz, M.W.; MacRenaris, K.W.; Preslar, A.T.; Meade, T.J. Gd(III)-dithiolane gold nanoparticles for t1-weighted magnetic resonance imaging of the pancreas. Nano Lett. 2016, 16, 3202–3209. [Google Scholar] [CrossRef]
- Rotz, M.W.; Culver, K.S.B.; Parigi, G.; MacRenaris, K.W.; Luchinat, C.; Odom, T.W.; Meade, T.J. High relaxivity Gd(III)-DNA gold nanostars: Investigation of shape effects on proton relaxation. ACS Nano 2015, 9, 3385–3396. [Google Scholar] [CrossRef]
- Tsvirkun, D.; Ben-Nun, Y.; Merquiol, E.; Zlotver, I.; Meir, K.; Weiss-Sadan, T.; Matok, I.; Popovtzer, R.; Blum, G. Ct imaging of enzymatic activity in cancer using covalent probes reveal a size-dependent pattern. J. Am. Chem. Soc. 2018, 140, 12010–12020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wen, S.; Zhao, L.; Li, D.; Liu, C.; Jiang, W.; Gao, X.; Gu, W.; Ma, N.; Zhao, J.; et al. Ultrastable polyethyleneimine-stabilized gold nanoparticles modified with polyethylene glycol for blood pool, lymph node and tumor CT imaging. Nanoscale 2016, 8, 5567–5577. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Dilmanian, F.A.; Slatkin, D.N.; Smilowitz, H.M. Radiotherapy enhancement with gold nanoparticles. J. Pharm. Pharmacol. 2008, 60, 977–985. [Google Scholar] [CrossRef]
- Dong, Y.C.; Hajfathalian, M.; Maidment, P.S.N.; Hsu, J.C.; Naha, P.C.; Si-Mohamed, S.; Breuilly, M.; Kim, J.; Chhour, P.; Douek, P.; et al. Effect of gold nanoparticle size on their properties as contrast agents for computed tomography. Sci. Rep. 2019, 9, 14912. [Google Scholar] [CrossRef]
- Huang, P.; Bao, L.; Zhang, C.; Lin, J.; Luo, T.; Yang, D.; He, M.; Li, Z.; Gao, G.; Gao, B.; et al. Folic acid-conjugated silica-modified gold nanorods for x-ray/CT imaging-guided dual-mode radiation and photo-thermal therapy. Biomaterials 2011, 32, 9796–9809. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, L.; Peng, C.; Shen, M.; Shi, X.; Zhang, G. Folic acid-modified dendrimer-entrapped gold nanoparticles as nanoprobes for targeted CT imaging of human lung adencarcinoma. Biomaterials 2013, 34, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Long, M.; Qin, Y.; Sun, X.; Zheng, J. Luminescent gold nanoparticles with efficient renal clearance. Angew. Chem. Int. Ed. 2011, 50, 3168–3172. [Google Scholar] [CrossRef]
- Zhang, X.D.; Luo, Z.; Chen, J.; Shen, X.; Song, S.; Sun, Y.; Fan, S.; Fan, F.; Leong, D.T.; Xie, J. Ultrasmall Au10-12(SG)10-12 nanomolecules for high tumor specificity and cancer radiotherapy. Adv. Mater. 2014, 26, 4565–4568. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wang, X.; Zeng, S.; Ramamurthy, G.; Burda, C.; Basilion, J.P. Targeted gold nanocluster-enhanced radiotherapy of prostate cancer. Small 2019, 15, 1900968. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.J.; Ferdani, R. Copper-64 radiopharmaceuticals for pet imaging of cancer: Advances in preclinical and clinical research. Cancer Biother. Radiopharm. 2009, 24, 379–393. [Google Scholar] [CrossRef]
- Zhu, M.; Aikens, C.M.; Hollander, F.J.; Schatz, G.C.; Jin, R. Correlating the crystal structure of a thiol-protected Au25 cluster and optical properties. J. Am. Chem. Soc. 2008, 130, 5883–5885. [Google Scholar] [CrossRef]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically precise colloidal metal nanoclusters and nanoparticles: Fundamentals and opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef]
- Shang, L.; Dong, S.; Nienhaus, G.U. Ultra-small fluorescent metal nanoclusters: Synthesis and biological applications. Nano Today 2011, 6, 401–418. [Google Scholar] [CrossRef]
- Liu, J.; Yu, M.; Zhou, C.; Yang, S.; Ning, X.; Zheng, J. Passive tumor targeting of renal-clearable luminescent gold nanoparticles: Long tumor retention and fast normal tissue clearance. J. Am. Chem. Soc. 2013, 135, 4978–4981. [Google Scholar] [CrossRef] [PubMed]
- Pyo, K.; Ly, N.H.; Yoon, S.Y.; Shen, Y.; Choi, S.Y.; Lee, S.Y.; Joo, S.W.; Lee, D. Highly luminescent folate-functionalized Au22 nanoclusters for bioimaging. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Montana, D.M.; Wei, H.; Cordero, J.M.; Schneider, M.; Le Guevel, X.; Chen, O.; Bruns, O.T.; Bawendi, M.G. Shortwave infrared in vivo imaging with gold nanoclusters. Nano Lett. 2017, 17, 6330–6334. [Google Scholar] [CrossRef]
- Ho-Wu, R.; Yau, S.H.; Goodson, I.T. Efficient singlet oxygen generation in metal nanoclusters for two-photon photodynamic therapy applications. J. Phys. Chem. B 2017, 121, 10073–10080. [Google Scholar] [CrossRef]
- Miyata, S.; Miyaji, H.; Kawasaki, H.; Yamamoto, M.; Nishida, E.; Takita, H.; Akasaka, T.; Ushijima, N.; Iwanaga, T.; Sugaya, T. Antimicrobial photodynamic activity and cytocompatibility of Au25(Capt)18 clusters photoexcited by blue led light irradiation. Int. J. Nanomed. 2017, 12, 2703–2716. [Google Scholar] [CrossRef]
- Ye, J.; Fu, G.; Yan, X.; Liu, J.; Wang, X.; Cheng, L.; Zhang, F.; Sun, P.Z.; Liu, G. Noninvasive magnetic resonance/photoacoustic imaging for photothermal therapy response monitoring. Nanoscale 2018, 10, 5864–5868. [Google Scholar] [CrossRef]
- Cheng, X.; Sun, R.; Yin, L.; Chai, Z.; Shi, H.; Gao, M. Light-triggered assembly of gold nanoparticles for photothermal therapy and photoacoustic imaging of tumors in vivo. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Li, W.; Chen, X. Gold nanoparticles for photoacoustic imaging. Nanomedicine 2015, 10, 299–320. [Google Scholar] [CrossRef]
- Song, J.; Kim, J.; Hwang, S.; Jeon, M.; Jeong, S.; Kim, C.; Kim, S. “Smart” gold nanoparticles for photoacoustic imaging: An imaging contrast agent responsive to the cancer microenvironment and signal amplification via pH-induced aggregation. Chem. Commun. 2016, 52, 8287–8290. [Google Scholar] [CrossRef]
- Viator, J.A.; Gupta, S.; Goldschmidt, B.S.; Bhattacharyya, K.; Kannan, R.; Shukla, R.; Dale, P.S.; Boote, E.; Katti, K. Gold nanoparticle mediated detection of prostate cancer cells using photoacoustic flowmetry with optical reflectance. J. Biomed. Nanotechnol. 2010, 6, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.M. Estimation of strontium in animal bone using X-ray fluorescence analysis. Nature 1959, 183, 887–888. [Google Scholar] [CrossRef] [PubMed]
- Larsson, J.C.; Vogt, C.; Vagberg, W.; Toprak, M.S.; Dzieran, J.; Arsenian-Henriksson, M.; Hertz, H.M. High-spatial-resolution X-ray fluorescence tomography with spectrally matched nanoparticles. Phys. Med. Biol. 2018, 63, 164001. [Google Scholar] [CrossRef]
- Bazalova, M.; Kuang, Y.; Pratx, G.; Xing, L. Investigation of X-ray fluorescence computed tomography (XFCT) and k-edge imaging. IEEE Trans. Med. Imaging 2012, 31, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, L.; Chen, J.; Chen, Z.; Zhang, W.; Lu, H. Quantitative imaging of Gd nanoparticles in mice using benchtop cone-beam X-ray fluorescence computed tomography system. Int. J. Mol. Sci. 2019, 20, 2315. [Google Scholar] [CrossRef]
- Nicolson, F.; Kircher, M.F.; Stone, N.; Matousek, P. Spatially offset raman spectroscopy for biomedical applications. Chem. Soc. Rev. 2021, 50, 556–568. [Google Scholar] [CrossRef]
- Yu, M.; Zheng, J. Clearance pathways and tumor targeting of imaging nanoparticles. ACS Nano 2015, 9, 6655–6674. [Google Scholar] [CrossRef]
- Loynachan, C.N.; Soleimany, A.P.; Dudani, J.S.; Lin, Y.; Najer, A.; Bekdemir, A.; Chen, Q.; Bhatia, S.N.; Stevens, M.M. Renal clearable catalytic gold nanoclusters for in vivo disease monitoring. Nat. Nanotechnol. 2019, 14, 883–890. [Google Scholar] [CrossRef]
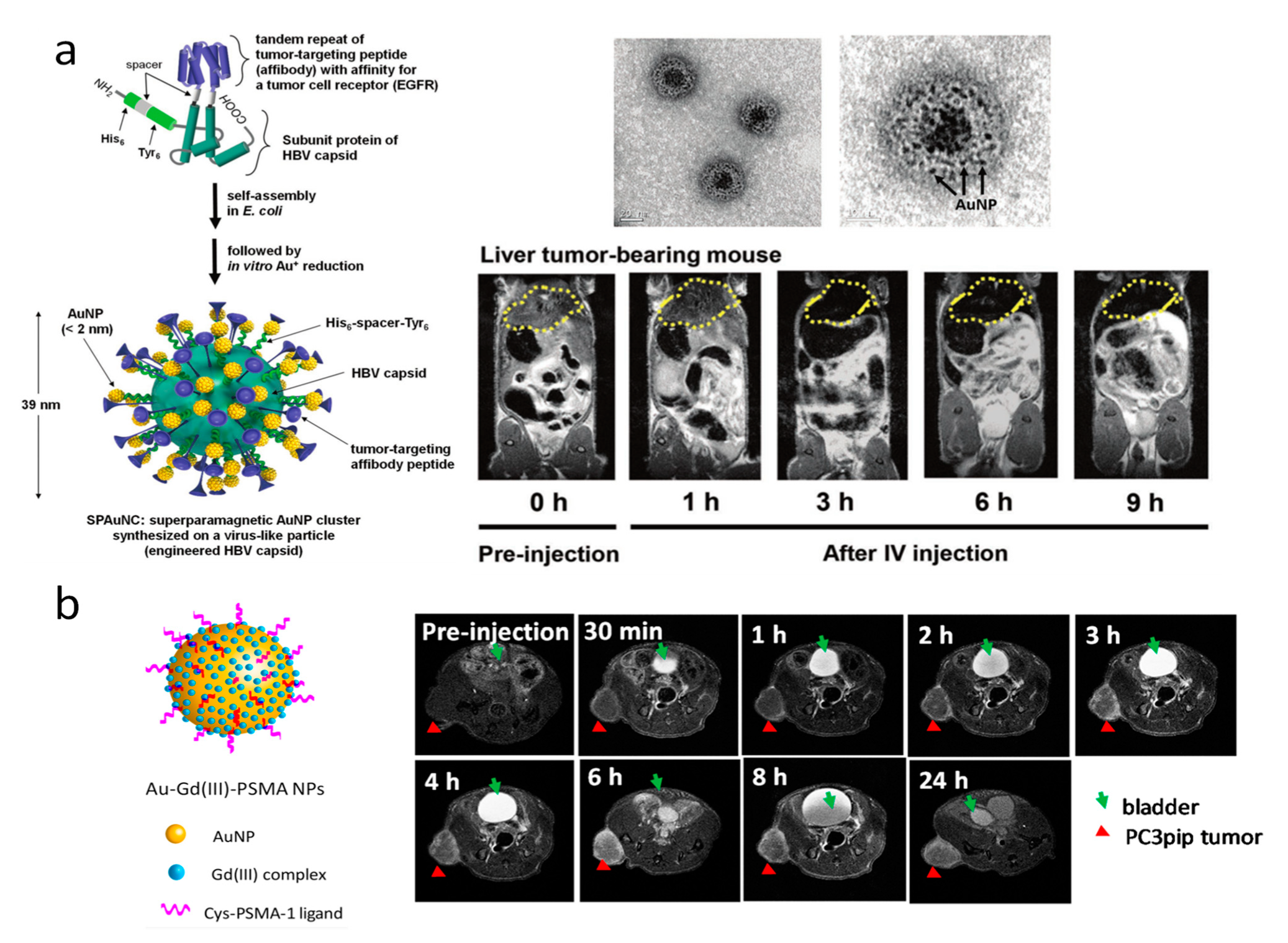
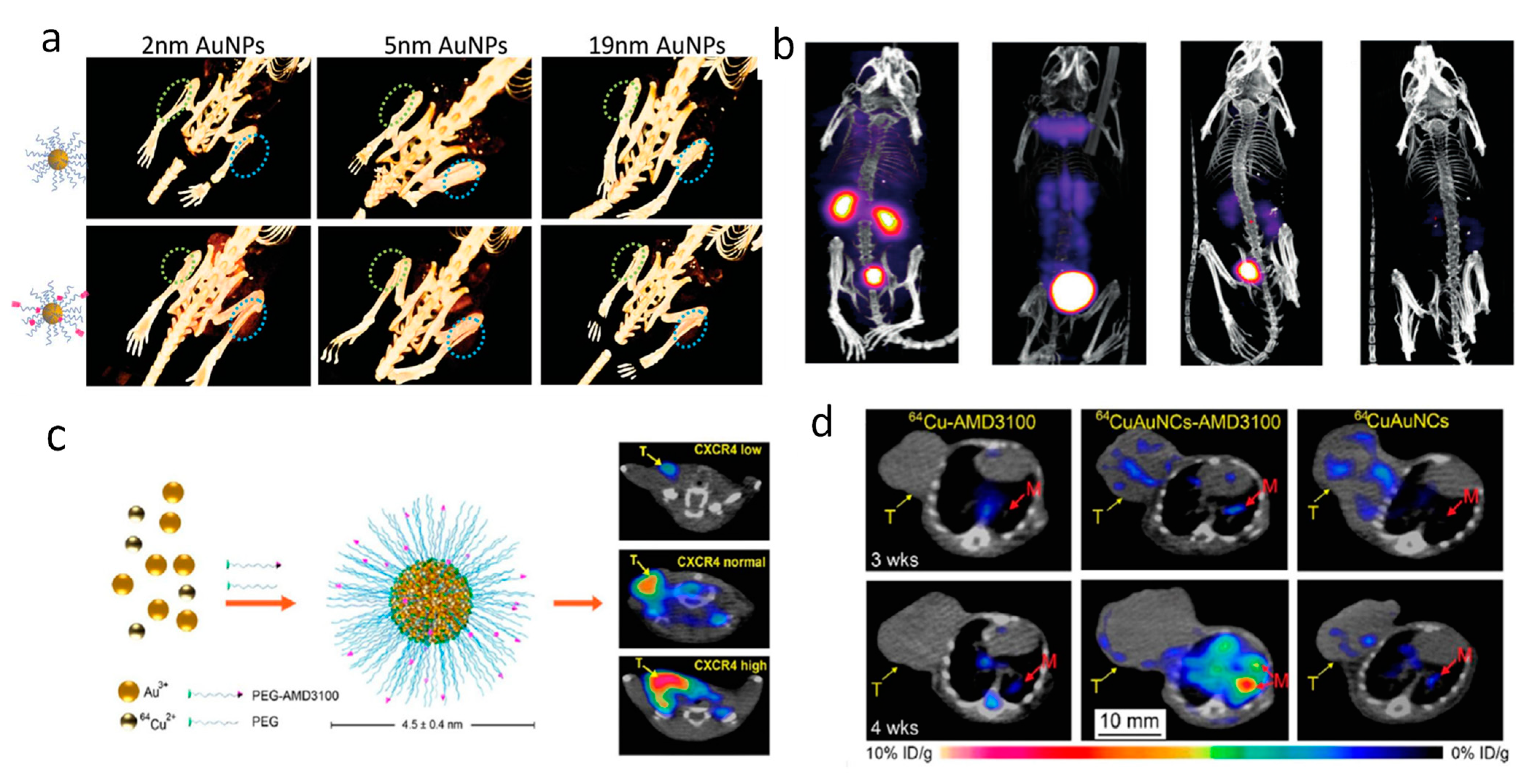

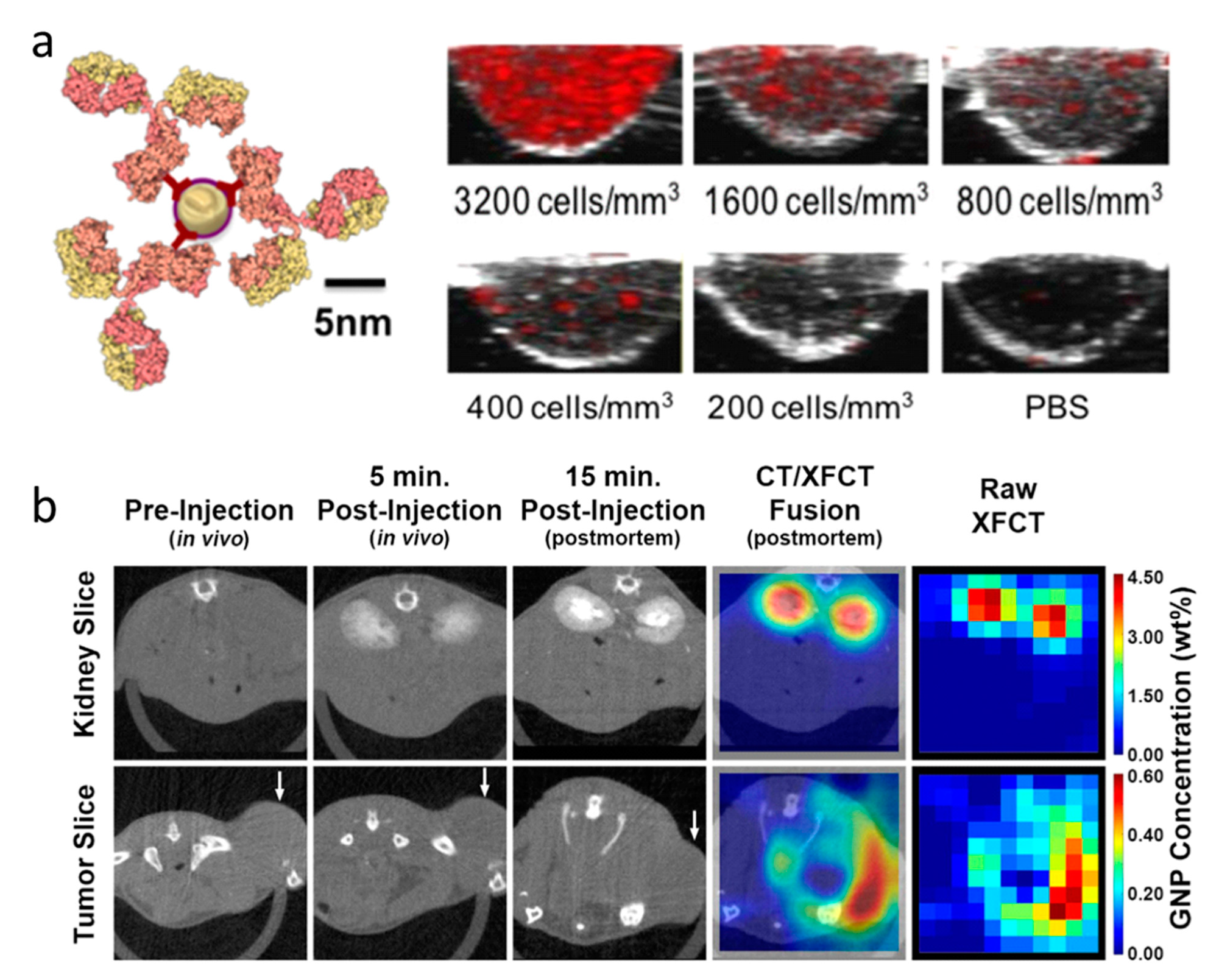
| Particle Type | Size | Surface | Imaging Modality | Tumor Models | References |
|---|---|---|---|---|---|
| Superparamagnetic AuNCs (SPAuNCs) | 3 nm | EGFR | MRI (T2) | liver tumor (MDA-MB-468) | [62,63] |
| AuNPs | 5 nm | PSMA | MRI (T1) | prostate tumor (PC3) | [64] |
| AuNPs | 2, 5, 19 nm | PSMA | CT | prostate tumor (PC3) | [65] |
| [198Au]AuNCs | 3 nm | GSH | SPECT | - | [66] |
| 64CuAuNCs | 4.5 nm | AMD3100 | PET | breast tumor (4T1) | [66,67,68] |
| AuNCs | 2–6 nm | alpha-lactalbumin | fluorescence | breast tumor (MDA-MB-231) | [69] |
| AuNPs | 5 nm | EGFR | PAI | A431 cells | [70] |
| AuNPs | 1.9 nm | - | XRF | prostate tumor (PC3) | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, D.; Wang, X.; Burda, C.; Basilion, J.P. Recent Development of Gold Nanoparticles as Contrast Agents for Cancer Diagnosis. Cancers 2021, 13, 1825. https://doi.org/10.3390/cancers13081825
Luo D, Wang X, Burda C, Basilion JP. Recent Development of Gold Nanoparticles as Contrast Agents for Cancer Diagnosis. Cancers. 2021; 13(8):1825. https://doi.org/10.3390/cancers13081825
Chicago/Turabian StyleLuo, Dong, Xinning Wang, Clemens Burda, and James P. Basilion. 2021. "Recent Development of Gold Nanoparticles as Contrast Agents for Cancer Diagnosis" Cancers 13, no. 8: 1825. https://doi.org/10.3390/cancers13081825
APA StyleLuo, D., Wang, X., Burda, C., & Basilion, J. P. (2021). Recent Development of Gold Nanoparticles as Contrast Agents for Cancer Diagnosis. Cancers, 13(8), 1825. https://doi.org/10.3390/cancers13081825







