Validation of the T Descriptor (TNM-8) in T3N0 Non-Small-Cell Lung Cancer Patients; a Bicentric Cohort Analysis with Arguments for Redefinition
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Preoperative Staging, Surgery, and Adjuvant Therapy
2.3. Follow-Up and Statistical Analysis
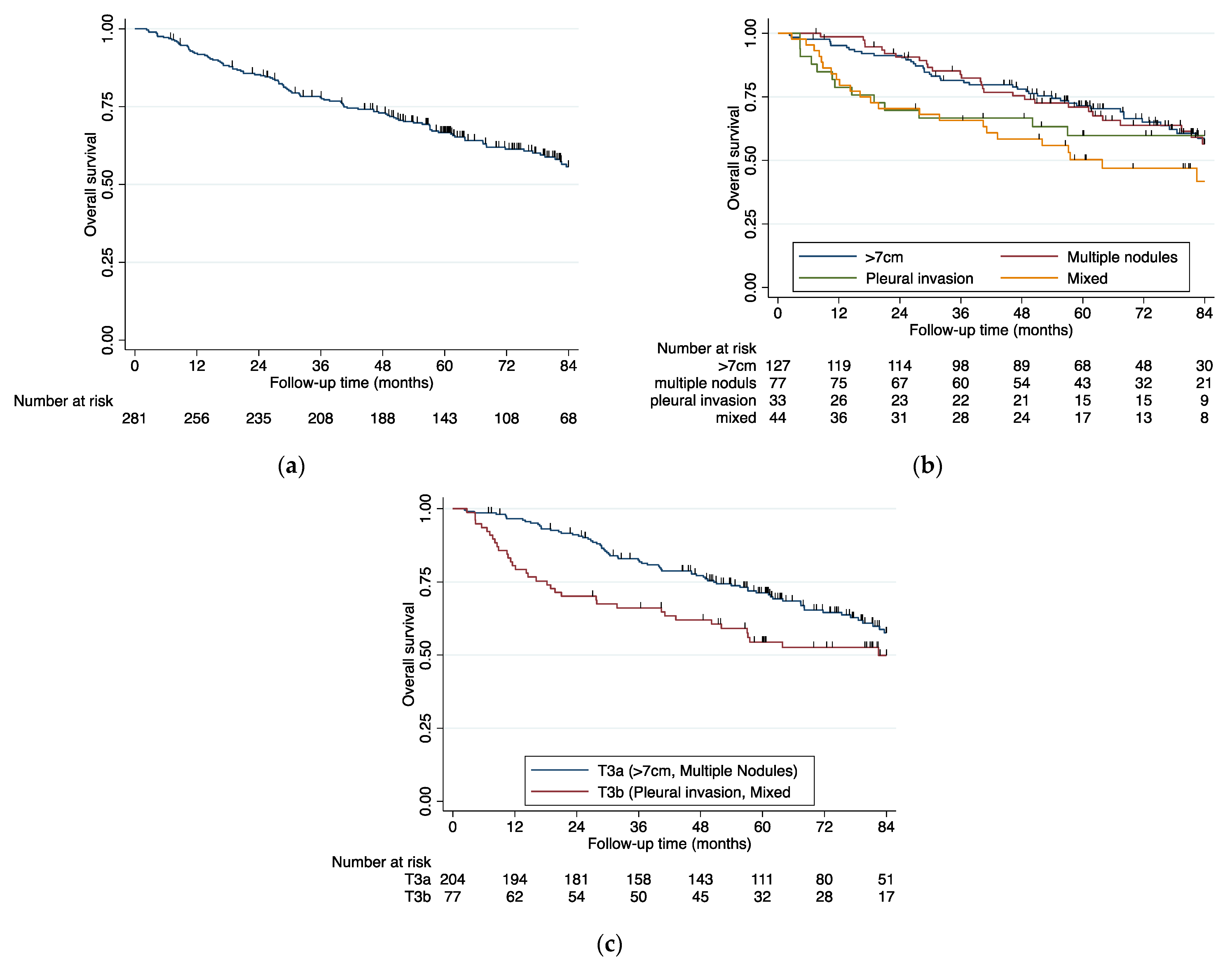
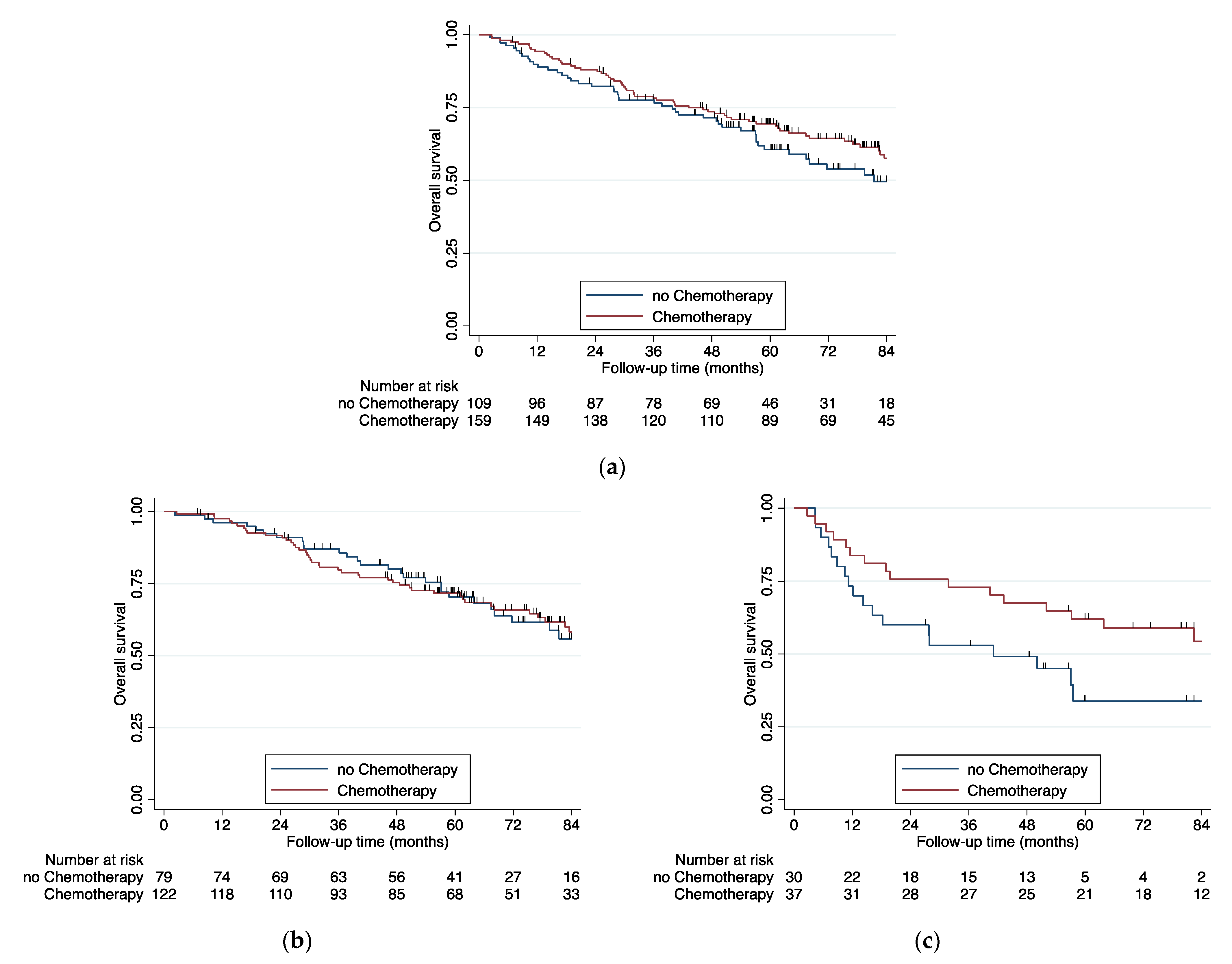
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Rami-Porta, R.; Bolejack, V.; Crowley, J.; Ball, D.; Kim, J.; Lyons, G.; Rice, T.; Suzuki, K.; Thomas, C.F.; Travis, W.D.; et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2015, 10, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- Mountain, C.F. Revisions in the International System for Staging Lung Cancer. Chest 1997, 111, 1710–1717. [Google Scholar] [CrossRef] [PubMed]
- Rami-Porta, R.; Ball, D.; Crowley, J.; Giroux, D.J.; Jett, J.; Travis, W.D.; Tsuboi, M.; Vallières, E.; Goldstraw, P. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the T Descriptors in the Forthcoming (Seventh) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2007, 2, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Kris, M.G.; Gaspar, L.E.; Chaft, J.E.; Kennedy, E.B.; Azzoli, C.G.; Ellis, P.M.; Lin, S.H.; Pass, H.I.; Seth, R.; Shepherd, F.A.; et al. Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I to IIIA Completely Resected Non–Small-Cell Lung Cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 2960–2974. [Google Scholar] [CrossRef]
- Bradbury, P.; Sivajohanathan, D.; Chan, A.; Kulkarni, S.; Ung, Y.; Ellis, P.M. Postoperative Adjuvant Systemic Therapy in Completely Resected Non–Small-Cell Lung Cancer: A Systematic Review. Clin. Lung Cancer 2017, 18, 259–273.e8. [Google Scholar] [CrossRef]
- Douillard, J.-Y.; Tribodet, H.; Aubert, D.; Shepherd, F.A.; Rosell, R.; Ding, K.; Veillard, A.-S.; Seymour, L.; Le Chevalier, T.; Spiro, S.; et al. Adjuvant Cisplatin and Vinorelbine for Completely Resected Non-small Cell Lung Cancer: Subgroup Analysis of the Lung Adjuvant Cisplatin Evaluation. J. Thorac. Oncol. 2010, 5, 220–228. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J.; Wu, Y.-L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Baum, P.; Diers, J.; Haag, J.; Klotz, L.; Eichhorn, F.; Eichhorn, M.; Wiegering, A.; Winter, H. Nationwide effect of high procedure volume in lung cancer surgery on in-house mortality in Germany. Lung Cancer 2020, 149, 78–83. [Google Scholar] [CrossRef]
- Lardinois, D.; De Leyn, P.; Van Schil, P.; Porta, R.R.; A Waller, D.; Passlick, B.; Zielinski, M.; Junker, K.; Rendina, E.A.; Ris, H.-B. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur. J. Cardio-Thoracic Surg. 2006, 30, 787–792. [Google Scholar] [CrossRef]
- Schemper, M.; Smith, T.L. A note on quantifying follow-up in studies of failure time. Control. Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef]
- Betensky, R.A. Measures of follow-up in time-to-event studies: Why provide them and what should they be? Clin. Trials 2015, 12, 403–408. [Google Scholar] [CrossRef]
- Chen, K.; Chen, H.; Yang, F.; Sui, X.; Li, X.; Wang, J. Validation of the Eighth Edition of the TNM Staging System for Lung Cancer in 2043 Surgically Treated Patients With Non–small-cell Lung Cancer. Clin. Lung Cancer 2017, 18, e457–e466. [Google Scholar] [CrossRef]
- Hwang, J.K.; Page, B.J.; Flynn, D.; Passmore, L.; McCaul, E.; Brady, J.; Yang, I.A.; Marshall, H.; Windsor, M.; Bowman, R.V.; et al. Validation of the Eighth Edition TNM Lung Cancer Staging System. J. Thorac. Oncol. 2020, 15, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Chansky, K.; Detterbeck, F.C.; Nicholson, A.G.; Rusch, V.W.; Vallières, E.; Groome, P.; Kennedy, C.; Krasnik, M.; Peake, M.; Shemanski, L.; et al. The IASLC Lung Cancer Staging Project: External Validation of the Revision of the TNM Stage Groupings in the Eighth Edition of the TNM Classification of Lung Cancer. J. Thorac. Oncol. 2017, 12, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Jiang, W.; Chen, H.; Yang, F.; Wang, J.; Wang, Q. Validation of the Stage Groupings in the Eighth Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2017, 12, 1679–1686. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, S.; Mao, Y.; Mu, J.; Xue, Q.; Feng, X.; He, J. Surgical Outcomes of Synchronous Multiple Primary Non-Small Cell Lung Cancers. Sci. Rep. 2016, 6, 23252. [Google Scholar] [CrossRef] [PubMed]
- Blaauwgeers, H.; Damhuis, R.; Lissenberg-Witte, B.I.; De Langen, A.J.; Senan, S.; Thunnissen, E. A Population-Based Study of Outcomes in Surgically Resected T3N0 Non–Small Cell Lung Cancer in The Netherlands, Defined Using TNM-7 and TNM-8; Justification of Changes and an Argument to Incorporate Histology in the Staging Algorithm. J. Thorac. Oncol. 2019, 14, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef]
- Zhong, C.; Liu, H.; Jiang, L.; Zhang, W.; Yao, F. Chemotherapy Plus Best Supportive Care versus Best Supportive Care in Patients with Non-Small Cell Lung Cancer: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2013, 8, e58466. [Google Scholar] [CrossRef]
- NSCLC Meta-analyses Collaborative Group. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: Two meta-analyses of individual patient data. Lancet 2010, 375, 1267–1277. [Google Scholar] [CrossRef]
- Cortés, Á.A.; Urquizu, L.C.; Cubero, J.H. Adjuvant chemotherapy in non-small cell lung cancer: State-of-the-art. Transl. Lung Cancer Res. 2015, 4, 191–197. [Google Scholar]
- Cuffe, S.; Bourredjem, A.; Graziano, S.; Pignon, J.-P.; Domerg, C.; Ezzalfani, M.; Seymour, L.; Strevel, E.; Burkes, R.; Capelletti, M.; et al. A Pooled Exploratory Analysis of the Effect of Tumor Size and KRAS Mutations on Survival Benefit From Adjuvant Platinum-Based Chemotherapy in Node-Negative Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2012, 7, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, R.; Dunant, A.; Pignon, J.-P.; Bergman, B.; Chabowski, M.; Grunenwald, D.; Kozlowski, M.; Le Péchoux, C.; Pirker, R.; Pinel, M.-I.S.; et al. Long-Term Results of the International Adjuvant Lung Cancer Trial Evaluating Adjuvant Cisplatin-Based Chemotherapy in Resected Lung Cancer. J. Clin. Oncol. 2010, 28, 35–42. [Google Scholar] [CrossRef]
- Eberle, A.; Jansen, L.; Castro, F.; Krilaviciute, A.; Luttmann, S.; Emrich, K.; Holleczek, B.; Nennecke, A.; Katalinic, A.; Brenner, H. Lung cancer survival in Germany: A population-based analysis of 132,612 lung cancer patients. Lung Cancer 2015, 90, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Giroux, D.J.; Van Schil, P.; Asamura, H.; Rami-Porta, R.; Chansky, K.; Crowley, J.J.; Rusch, V.W.; Kernstine, K.; Araujo, L.H.; Beckett, P.; et al. The IASLC Lung Cancer Staging Project: A Renewed Call to Participation. J. Thorac. Oncol. 2018, 13, 801–809. [Google Scholar] [CrossRef] [PubMed]
| T-Descriptor | TNM-6 | TNM-7 | TNM-8 |
|---|---|---|---|
| Diameter 5–7 cm | T2 | T2b | T3 |
| <2 cm distance from the carina | T3 | T3 | T2 |
| Complete atelectasis | T3 | T3 | T2 |
| Separate tumor nodules in same lobe | T4 | T3 | T3 |
| Parietal pleural/chest wall invasion | T3 | T3 | T3 |
| Diameter >7 cm | T2 | T3 | T4 |
| Diaphragm invasion | T3 | T3 | T4 |
| >7 cm | Multiple Nodules | Parietal Pleural Invasion | Mixed | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | p Value | |
| All | 127 | 45.2 | 77 | 27.4 | 33 | 11.7 | 44 | 15.7 | 281 | 100.0 | |
| Gender | 0.231 | ||||||||||
| Male | 78 | 61.4% | 41 | 53.2% | 20 | 60.6% | 31 | 70.5% | 170 | 60.5% | |
| Female | 49 | 38.6% | 36 | 46.8% | 13 | 39.4% | 13 | 29.5% | 111 | 39.5% | |
| ECOG | 0.060 | ||||||||||
| 0 | 106 | 83.5% | 58 | 75.3% | 28 | 84.8% | 39 | 88.6% | 231 | 82.2% | |
| ≥1 | 21 | 16.5% | 19 | 24.7% | 5 | 15.2% | 5 | 11.4% | 50 | 17.8% | |
| Age | 0.602 | ||||||||||
| <60 | 14 | 11.0% | 6 | 7.8% | 4 | 12.1% | 8 | 18.2% | 32 | 11.4% | |
| 60–69 | 24 | 18.9% | 19 | 24.7% | 9 | 27.3% | 9 | 20.5% | 61 | 21.7% | |
| ≥70 | 89 | 70.1% | 52 | 67.5% | 20 | 60.6% | 27 | 61.4% | 188 | 66.9% | |
| Surgery | <0.001 | ||||||||||
| Lobectomy | 106 | 83.5% | 62 | 80.5% | 32 | 97.0% | 36 | 81.8% | 236 | 84.0% | |
| Sleeve Resection | 5 | 3.9% | 1 | 1.3% | 0 | 0.0% | 0 | 0.0% | 6 | 2.1% | |
| Sublobar Resection | 0 | 0.0% | 12 | 15.6% | 1 | 3.0% | 0 | 0.0% | 13 | 4.6% | |
| Bilobectomy | 8 | 6.3% | 0 | 0.0% | 0 | 0.0% | 3 | 6.8% | 11 | 3.9% | |
| Pneumonectomy | 8 | 6.3% | 2 | 2.6% | 0 | 0.0% | 5 | 11.4% | 15 | 5.3% | |
| Resection side * | 0.380 | ||||||||||
| Right | 74 | 58.3% | 48 | 62.3% | 22 | 66.7% | 25 | 58.1% | 169 | 60.4% | |
| Left | 53 | 41.7% | 29 | 37.7% | 11 | 33.3% | 18 | 41.9% | 111 | 39.6% | |
| Residue | 0.001 | ||||||||||
| 0 | 123 | 96.9% | 77 | 100.0% | 29 | 87.9% | 38 | 86.4% | 267 | 95.0% | |
| 1 | 4 | 3.1% | 0 | 0.0% | 4 | 12.1% | 6 | 13.6% | 14 | 5.0% | |
| Histology | 0.239 | ||||||||||
| Adenocarcinoma | 55 | 43.3% | 42 | 54.5% | 17 | 51.5% | 18 | 40.9% | 132 | 47.0% | |
| Squamous cell carcinoma | 50 | 39.4% | 31 | 40.3% | 11 | 33.3% | 20 | 45.5% | 112 | 39.9% | |
| Other | 22 | 17.3% | 4 | 5.2% | 5 | 15.2% | 6 | 13.6% | 37 | 13.2% | |
| Adjuvant therapy | 0.001 | ||||||||||
| No therapy | 39 | 30.7% | 40 | 51.9% | 13 | 39.4% | 17 | 38.6% | 109 | 38.8% | |
| Chemotherapy | 86 | 67.7% | 36 | 46.8% | 15 | 45.5% | 22 | 50.0% | 159 | 56.6% | |
| Radio–Chemotherapy | 1 | 0.8% | 1 | 1.3% | 4 | 12.1% | 4 | 9.1% | 10 | 3.6% | |
| Radiotherapy | 1 | 0.8% | 0 | 0.0% | 1 | 3.0% | 1 | 2.3% | 3 | 1.1% | |
| IASLC Database | Netherland Database | Heidelberg and Berlin Database | |
|---|---|---|---|
| All pT3N0 (Stadium IIB) | 56% | 47.9% | 66.7% |
| >7 cm | 41% | 47.2% | 71.5% |
| multiple nodules | 49% | 62.8% | 71.0% |
| parietal pleural invasion | 49% | 45.3% | 59.8% |
| mixed | 49% | 28.7% | 50.3% |
| HR | St. Err. | p-Value | (95% Conf. | Interval) | |
|---|---|---|---|---|---|
| Age | 1.05 | 0.02 | 0.021 | 1.01 | 1.09 |
| Gender (RC: male) | 0.61 | 0.22 | 0.185 | 0.29 | 0.28 |
| ECOG >0 (RC: ECOG = 0) | 0.87 | 0.51 | 0.752 | 0.27 | 2.80 |
| Squamous-cell-C (RC: Adeno-C) | 1.76 | 0.67 | 0.144 | 0.83 | 3.73 |
| Other C (RC: Adeno-C) | 2.13 | 1.06 | 0.124 | 0.81 | 5.58 |
| CTX (RC: no CTX) | 0.51 | 0.19 | 0.068 | 0.24 | 1.05 |
| HR | St. Err. | p-Value | (95% Conf. | Interval) | |
|---|---|---|---|---|---|
| T3b (RC: T3a) | 2.34 | 0.48 | <0.01 | 1.57 | 3.49 |
| Age | 1.06 | 0.01 | <0.01 | 1.03 | 1.08 |
| Gender (RC: male) | 0.76 | 0.15 | 0.179 | 0.51 | 1.13 |
| ECOG >0 (RC: ECOG = 0) | 1.45 | 0.40 | 0.171 | 0.85 | 2.48 |
| Squamous-cel-C (RC: Adeno-C) | 1.17 | 0.25 | 0.463 | 0.78 | 1.79 |
| Other CA (RC: Adeno-CA) | 1.56 | 0.47 | 0.137 | 0.87 | 2.82 |
| CTX (RC: no CTX) | 0.92 | 0.20 | 0.708 | 0.60 | 1.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baum, P.; Taber, S.; Erdmann, S.; Muley, T.; Kriegsmann, M.; Christopoulos, P.; Thomas, M.; Winter, H.; Pfannschmidt, J.; Eichhorn, M.E. Validation of the T Descriptor (TNM-8) in T3N0 Non-Small-Cell Lung Cancer Patients; a Bicentric Cohort Analysis with Arguments for Redefinition. Cancers 2021, 13, 1812. https://doi.org/10.3390/cancers13081812
Baum P, Taber S, Erdmann S, Muley T, Kriegsmann M, Christopoulos P, Thomas M, Winter H, Pfannschmidt J, Eichhorn ME. Validation of the T Descriptor (TNM-8) in T3N0 Non-Small-Cell Lung Cancer Patients; a Bicentric Cohort Analysis with Arguments for Redefinition. Cancers. 2021; 13(8):1812. https://doi.org/10.3390/cancers13081812
Chicago/Turabian StyleBaum, Philip, Samantha Taber, Stella Erdmann, Thomas Muley, Mark Kriegsmann, Petros Christopoulos, Michael Thomas, Hauke Winter, Joachim Pfannschmidt, and Martin E. Eichhorn. 2021. "Validation of the T Descriptor (TNM-8) in T3N0 Non-Small-Cell Lung Cancer Patients; a Bicentric Cohort Analysis with Arguments for Redefinition" Cancers 13, no. 8: 1812. https://doi.org/10.3390/cancers13081812
APA StyleBaum, P., Taber, S., Erdmann, S., Muley, T., Kriegsmann, M., Christopoulos, P., Thomas, M., Winter, H., Pfannschmidt, J., & Eichhorn, M. E. (2021). Validation of the T Descriptor (TNM-8) in T3N0 Non-Small-Cell Lung Cancer Patients; a Bicentric Cohort Analysis with Arguments for Redefinition. Cancers, 13(8), 1812. https://doi.org/10.3390/cancers13081812








