Association between Skeletal Muscle Loss and the Response to Neoadjuvant Chemotherapy for Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Population
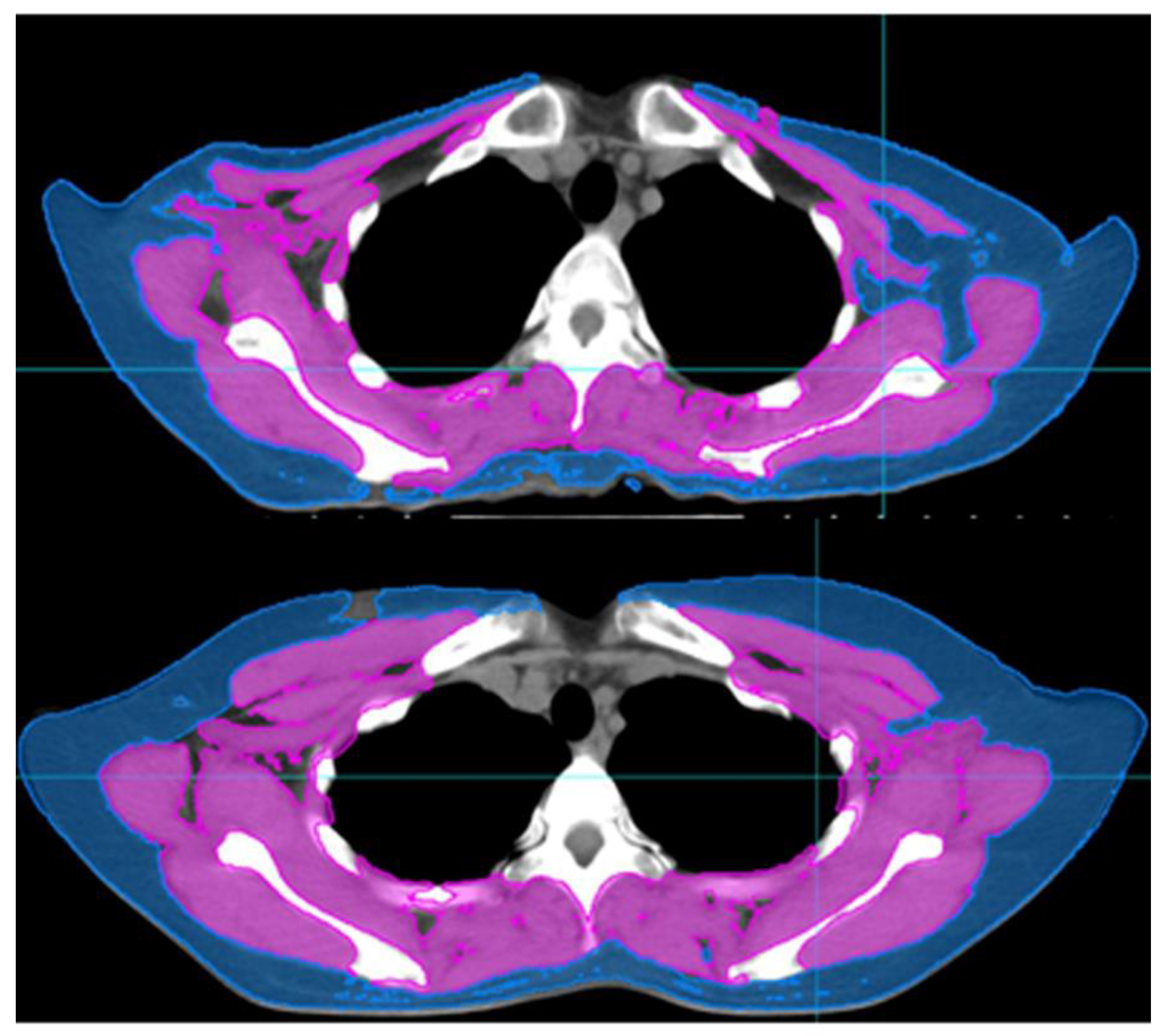
2.2. Measurement of Body Composition and the Definition of Skeletal Muscle Loss
2.3. Statistical Analysis
3. Results
3.1. Patient and Tumor Characteristics
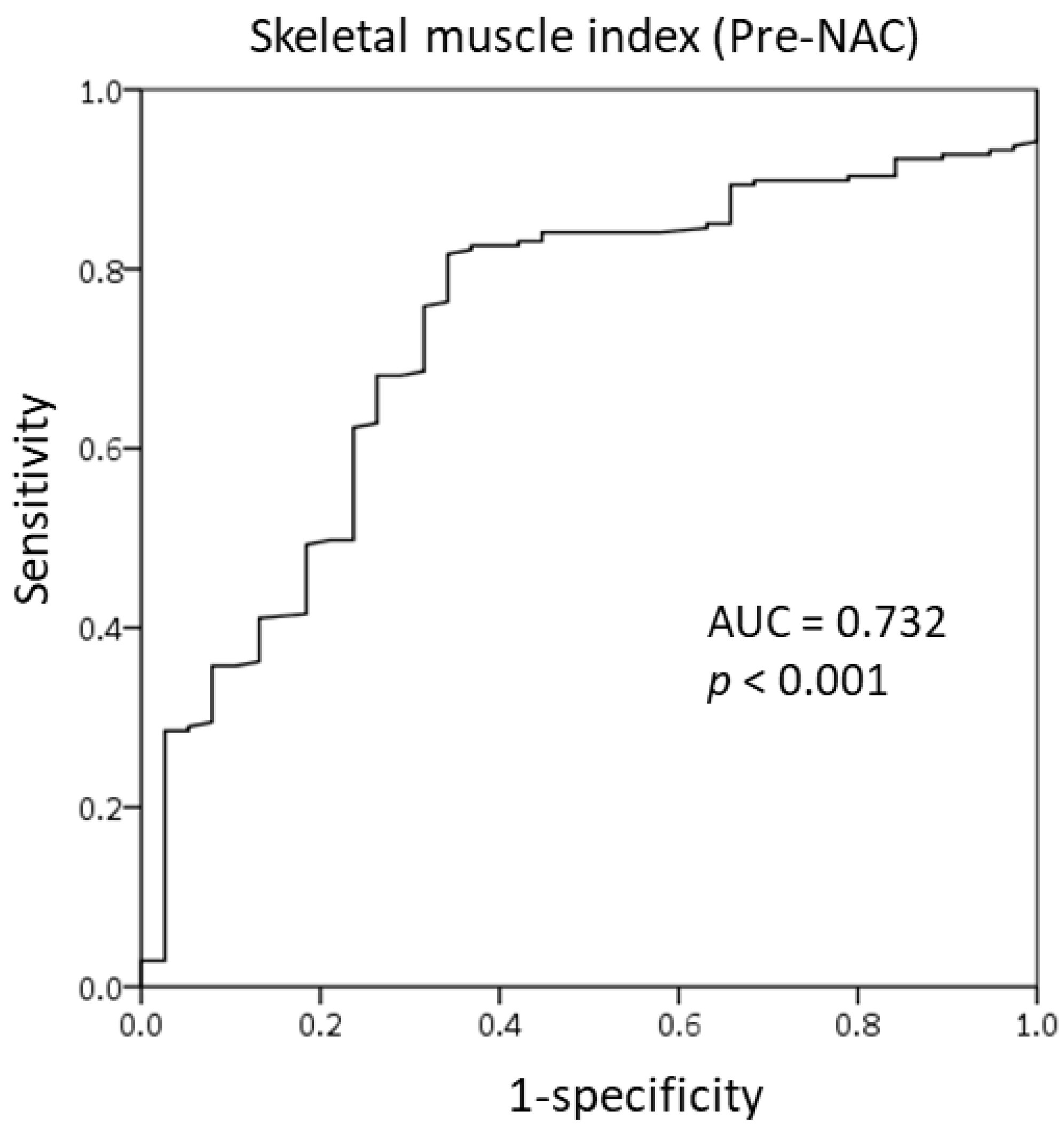
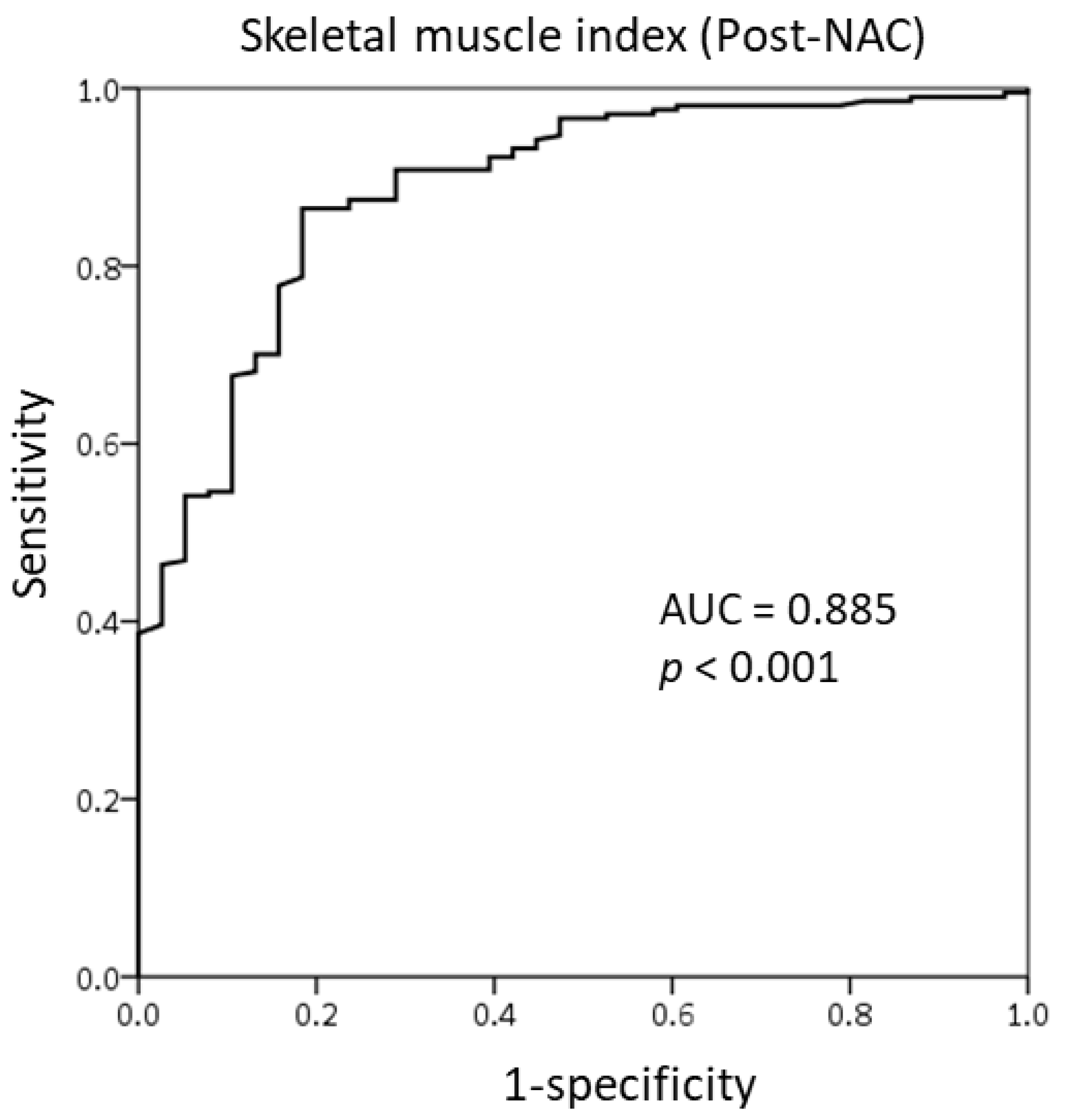
3.2. Cut-Off Value of the SMI for Skeletal Muscle Loss Group
3.3. Predictive Value of Subcutaneous Adipose Tissue Index
3.4. Comparison of Patient and Tumor Characteristics Between the Responder and Non-Responder Groups
3.5. Univariate and Multivariate Analyses of the Response to Neoadjuvant Chemotherapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwartz, G.F.; Birchansky, C.A.; Komarnicky, L.T.; Mansfield, C.M.; Cantor, R.I.; Biermann, W.A.; Fellin, F.M.; McFarlane, J. Induction chemotherapy followed by breast conservation for locally advanced carcinoma of the breast. Cancer 1994, 73, 362–369. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Ames, F.C.; Buzdar, A.U.; Kau, S.W.; McNeese, M.D.; Paulus, D.; Hug, V.; Holmes, F.A.; Romsdahl, M.M.; Fraschini, G.; et al. Management of stage iii primary breast cancer with primary chemotherapy, surgery, and radiation therapy. Cancer 1988, 62, 2507–2516. [Google Scholar] [CrossRef]
- Fisher, B.; Brown, A.; Mamounas, E.; Wieand, S.; Robidoux, A.; Margolese, R.G.; Cruz, A.B., Jr.; Fisher, E.R.; Wickerham, D.L.; Wolmark, N.; et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: Findings from national surgical adjuvant breast and bowel project b-18. J. Clin. Oncol. 1997, 15, 2483–2493. [Google Scholar] [CrossRef]
- Semiglazov, V.; Eiermann, W.; Zambetti, M.; Manikhas, A.; Bozhok, A.; Lluch, A.; Tjulandin, S.; Sabadell, M.D.; Caballero, A.; Valagussa, P.; et al. Surgery following neoadjuvant therapy in patients with her2-positive locally advanced or inflammatory breast cancer participating in the neoadjuvant herceptin (noah) study. Eur. J. Surg. Oncol. 2011, 37, 856–863. [Google Scholar] [CrossRef]
- Antoun, S.; Baracos, V.E.; Birdsell, L.; Escudier, B.; Sawyer, M.B. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann. Oncol. 2010, 21, 1594–1598. [Google Scholar] [CrossRef]
- Prado, C.M.M.; Baracos, V.E.; McCargar, L.J.; Reiman, T.; Mourtzakis, M.; Tonkin, K.; Mackey, J.R.; Koski, S.; Pituskin, E.; Sawyer, M.B. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin. Cancer Res. 2009, 15, 2920–2926. [Google Scholar] [CrossRef]
- Kazemi-Bajestani, S.M.; Mazurak, V.C.; Baracos, V. Computed tomography-defined muscle and fat wasting are associated with cancer clinical outcomes. Semin. Cell Dev. Biol. 2016, 54, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Van der Kroft, G.; van Dijk, D.P.J.; Rensen, S.S.; van Tiel, F.H.; de Greef, B.; West, M.; Ostridge, K.; Dejong, C.H.C.; Neumann, U.P.; Damink, S.W.M.O. Low thoracic muscle radiation attenuation is associated with postoperative pneumonia following partial hepatectomy for colorectal metastasis. HPB Oxf. 2020, 22, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, S.; Popuri, K.; Feliciano, E.M.C.; Caan, B.J.; Baracos, V.E.; Beg, M.F. Muscle segmentation in axial computed tomography (ct) images at the lumbar (l3) and thoracic (t4) levels for body composition analysis. Comput. Med. Imaging Graph. 2019, 75, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised recist guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.H.; Park, J.S.; Shin, H.J.; Cha, J.H.; Chae, E.Y.; Choi, W.J. Prediction of pathological complete response of breast cancer patients undergoing neoadjuvant chemotherapy: Usefulness of breast mri computer-aided detection. Br. J. Radiol. 2014, 87, 20140142. [Google Scholar] [CrossRef]
- Choi, J.H.; Lim, H.I.; Lee, S.K.; Kim, W.W.; Kim, S.M.; Cho, E.; Ko, E.Y.; Han, B.K.; Park, Y.H.; Ahn, J.S.; et al. The role of pet ct to evaluate the response to neoadjuvant chemotherapy in advanced breast cancer: Comparison with ultrasonography and magnetic resonance imaging. J. Surg. Oncol. 2010, 102, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, R.; Mao, S.; Zhang, Y.; Dai, Y.; Guo, Q.; Song, X.; Zhang, Q.; Li, L.; Chen, Q. Metabolic biomarker signature for predicting the effect of neoadjuvant chemotherapy of breast cancer. Ann. Transl. Med. 2019, 7, 670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Huang, M.; Zhou, H.; Chen, K.; Jin, J.; Wu, Y.; Ying, L.; Ding, X.; Su, D.; Zou, D. A nomogram to predict the pathologic complete response of neoadjuvant chemotherapy in triple-negative breast cancer based on simple laboratory indicators. Ann. Surg. Oncol. 2019, 26, 3912–3919. [Google Scholar] [CrossRef] [PubMed]
- Desmedt, C.; Haibe-Kains, B.; Wirapati, P.; Buyse, M.; Larsimont, D.; Bontempi, G.; Delorenzi, M.; Piccart, M.; Sotiriou, C. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin. Cancer Res. 2008, 14, 5158–5165. [Google Scholar] [CrossRef] [PubMed]
- Goorts, B.; van Nijnatten, T.J.A.; de Munck, L.; Moossdorff, M.; Heuts, E.M.; de Boer, M.; Lobbes, M.B.I.; Smidt, M.L. Clinical tumor stage is the most important predictor of pathological complete response rate after neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res. Treat. 2017, 163, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Bonadonna, G.; Veronesi, U.; Brambilla, C.; Ferrari, L.; Luini, A.; Greco, M.; Bartoli, C.; de Yoldi, G.C.; Zucali, R.; Rilke, F.; et al. Primary chemotherapy to avoid mastectomy in tumors with diameters of three centimeters or more. J. Natl. Cancer Inst. 1990, 82, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Gajdos, C.; Tartter, P.I.; Estabrook, A.; Gistrak, M.A.; Jaffer, S.; Bleiweiss, I.J. Relationship of clinical and pathologic response to neoadjuvant chemotherapy and outcome of locally advanced breast cancer. J. Surg. Oncol. 2002, 80, 4–11. [Google Scholar] [CrossRef]
- Loi, S.; Milne, R.L.; Friedlander, M.L.; McCredie, M.R.; Giles, G.G.; Hopper, J.L.; Phillips, K.A. Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 1686–1691. [Google Scholar] [CrossRef]
- Kroenke, C.H.; Chen, W.Y.; Rosner, B.; Holmes, M.D. Weight, weight gain, and survival after breast cancer diagnosis. J. Clin. Oncol. 2005, 23, 1370–1378. [Google Scholar] [CrossRef]
- Del Fabbro, E.; Parsons, H.; Warneke, C.L.; Pulivarthi, K.; Litton, J.K.; Dev, R.; Palla, S.L.; Brewster, A.; Bruera, E. The relationship between body composition and response to neoadjuvant chemotherapy in women with operable breast cancer. Oncologist 2012, 17, 1240–1245. [Google Scholar] [CrossRef]
- Litton, J.K.; Gonzalez-Angulo, A.M.; Warneke, C.L.; Buzdar, A.U.; Kau, S.W.; Bondy, M.; Mahabir, S.; Hortobagyi, G.N.; Brewster, A.M. Relationship between obesity and pathologic response to neoadjuvant chemotherapy among women with operable breast cancer. J. Clin. Oncol. 2008, 26, 4072–4077. [Google Scholar] [CrossRef]
- Wulan, S.N.; Westerterp, K.R.; Plasqui, G. Ethnic differences in body composition and the associated metabolic profile: A comparative study between asians and caucasians. Maturitas 2010, 65, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Malietzis, G.; Lee, G.H.; Bernardo, D.; Blakemore, A.I.; Knight, S.C.; Moorghen, M.; Al-Hassi, H.O.; Jenkins, J.T. The prognostic significance and relationship with body composition of ccr7-positive cells in colorectal cancer. J. Surg. Oncol. 2015, 112, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, E.M.C.; Kroenke, C.H.; Meyerhardt, J.A.; Prado, C.M.; Bradshaw, P.T.; Kwan, M.L.; Xiao, J.; Alexeeff, S.; Corley, D.; Weltzien, E.; et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: Results from the c scans study. JAMA Oncol. 2017, 3, e172319. [Google Scholar] [CrossRef]
- Hubbard, J.M.; Cohen, H.J.; Muss, H.B. Incorporating biomarkers into cancer and aging research. J. Clin. Oncol. 2014, 32, 2611–2616. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G.; Park, W.; Kim, H.; Choi, D.H.; Park, H.C.; Kim, S.H.; Cho, Y.B.; Yun, S.H.; Kim, H.C.; Lee, W.Y.; et al. Baseline neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in rectal cancer patients following neoadjuvant chemoradiotherapy. Tumori J. 2019, 105, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Takeuchi, M.; Saitoh, M.; Takeda, S. Neutrophil-to-lymphocyte ratio after four weeks of nivolumab administration as a predictive marker in patients with pretreated non-small-cell lung cancer. Thorac. Cancer 2018, 9, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Oflazoglu, U.; Alacacioglu, A.; Varol, U.; Kucukzeybek, Y.; Salman, T.; Onal, H.T.; Yilmaz, H.E.; Yildiz, Y.; Taskaynatan, H.; Saray, S.; et al. The role of inflammation in adjuvant chemotherapy-induced sarcopenia (izmir oncology group (izog) study). Support. Care Cancer 2020, 28, 3965–3977. [Google Scholar] [CrossRef]
- Chemama, S.; Bayar, M.A.; Lanoy, E.; Ammari, S.; Stoclin, A.; Goere, D.; Elias, D.; Raynard, B.; Antoun, S. Sarcopenia is associated with chemotherapy toxicity in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal cancer. Ann. Surg. Oncol. 2016, 23, 3891–3898. [Google Scholar] [CrossRef]
- Tan, B.; Brammer, K.; Randhawa, N.; Welch, N.; Parsons, S.; James, E.; Catton, J. Sarcopenia is associated with toxicity in patients undergoing neo-adjuvant chemotherapy for oesophago-gastric cancer. Brit. J. Surg. 2014, 101, 40. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Kim, J.W.; Keum, K.C.; Lee, C.G.; Jeung, H.C.; Lee, I.J. Prognostic significance of sarcopenia with inflammation in patients with head and neck cancer who underwent definitive chemoradiotherapy. Front. Oncol. 2018, 8, 457. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Number | % |
|---|---|---|
| Age | ||
| Median (IQR) | 48 (42–54) | |
| Hypertension | ||
| No | 215 | 87.40% |
| Yes | 31 | 12.60% |
| Diabetes mellitus | ||
| No | 229 | 93.10% |
| Yes | 17 | 6.90% |
| Exercise | ||
| No | 175 | 71.10% |
| Yes | 71 | 28.90% |
| Weight change | ||
| No change | 211 | 85.80% |
| Decrease | 14 | 5.70% |
| Increase | 21 | 8.50% |
| Smoking status | ||
| Non-smoker | 241 | 98.00% |
| Current smoker | 4 | 1.60% |
| Ex-smoker | 1 | 0.40% |
| Drinking status | ||
| Non-drinker | 219 | 88.90% |
| Current drinker | 21 | 8.60% |
| Ex-drinker | 6 | 2.50% |
| Skeletal muscle mass_pre-NAC | ||
| Skeletal muscle loss | 30 | 12.20% |
| Non-skeletal muscle loss | 216 | 87.80% |
| Skeletal muscle mass_post-NAC | ||
| Skeletal muscle loss | 33 | 13.50% |
| Non-skeletal muscle loss | 213 | 86.50% |
| Change in skeletal muscle mass | ||
| Skeletal muscle loss → Skeletal muscle loss | 18 | 7.30% |
| Skeletal muscle loss → Non-skeletal muscle loss | 12 | 4.90% |
| Non-skeletal muscle loss → Skeletal muscle loss | 15 | 6.10% |
| Non-skeletal muscle loss → Non-skeletal muscle loss | 201 | 81.70% |
| Body mass index | ||
| Underweight (<18.5) | 8 | 3.30% |
| Normal (18.5–23) | 128 | 52.00% |
| Overweight (23–25) | 43 | 17.50% |
| Obese (≥25) | 67 | 27.20% |
| Hemoglobin | ||
| Median (IQR) | 13.2 (12.5–13.9) | |
| Platelets | ||
| Median (IQR) | 268 (229–312) | |
| Albumin | ||
| Median (IQR) | 4.5 (4.3–4.6) | |
| Protein | ||
| Median (IQR) | 7.4 (7.1–7.7) | |
| Characteristics | Number | % |
|---|---|---|
| Pathology | ||
| IDC | 232 | 94.30% |
| ILC | 5 | 2.05% |
| Mucinous | 5 | 2.05% |
| Others | 4 | 1.60% |
| Clinical T stage | ||
| T1 | 46 | 18.70% |
| T2 | 139 | 56.50% |
| T3 | 34 | 13.80% |
| T4 | 27 | 11.00% |
| Clinical N stage | ||
| N0 | 58 | 23.60% |
| N1 | 98 | 39.80% |
| N2 | 49 | 19.90% |
| N3 | 41 | 16.70% |
| Stage | ||
| I | 9 | 3.70% |
| II | 123 | 50.00% |
| III | 114 | 46.30% |
| Operation | ||
| Breast-conserving surgery | 129 | 52.40% |
| Modified radical mastectomy | 117 | 47.60% |
| RT modality | ||
| 3D CRT | 57 | 23.20% |
| IMRT | 189 | 76.80% |
| Dose scheme | ||
| 180 cGy per fraction | 226 | 91.90% |
| 200 cGy per fraction | 8 | 3.20% |
| 267 cGy per fraction | 12 | 4.90% |
| ER | ||
| Negative | 99 | 40.20% |
| Positive | 147 | 59.80% |
| PR | ||
| Negative | 171 | 69.50% |
| Positive | 75 | 30.50% |
| HER2 | ||
| Negative | 170 | 69.10% |
| Positive | 76 | 30.90% |
| Ki-67 | ||
| <15 | 112 | 45.50% |
| ≥15 | 78 | 31.70% |
| Unknown | 56 | 22.80% |
| Chemotherapy regimen | ||
| Adriamycin based | 5 | 2.00% |
| Adriamycin and taxol based | 161 | 65.40% |
| Taxol based | 8 | 3.30% |
| Trastuzumab based | 72 | 29.30% |
| Selective estrogen receptor modulator | ||
| No | 143 | 58.10% |
| Yes | 103 | 41.90% |
| Aromatase inhibitor | ||
| No | 220 | 89.40% |
| Yes | 26 | 10.60% |
| Responder Group (n = 208) | Non-Responder Group (n = 38) | ||||
|---|---|---|---|---|---|
| Characteristics | Number | % | Number | % | p-Value |
| Age | |||||
| Median (IQR) | 48 | 47 | |||
| Hypertension | 0.911 | ||||
| No | 182 | 87.50% | 33 | 86.80% | |
| Yes | 26 | 12.50% | 5 | 13.20% | |
| Diabetes mellitus | 0.663 | ||||
| No | 193 | 92.80% | 36 | 94.70% | |
| Yes | 15 | 7.20% | 2 | 5.30% | |
| Exercise | 0.706 | ||||
| No | 147 | 70.70% | 28 | 73.70% | |
| Yes | 61 | 29.30% | 10 | 26.30% | |
| Weight change | 0.811 | ||||
| No change | 179 | 86.00% | 32 | 84.20% | |
| Decrease | 11 | 5.30% | 3 | 7.90% | |
| Increase | 18 | 8.70% | 3 | 7.90% | |
| Smoking status | 0.796 | ||||
| Non-smoker | 204 | 98.10% | 37 | 97.40% | |
| Current smoker | 3 | 1.50% | 1 | 2.60% | |
| Ex-smoker | 1 | 0.50% | 0 | 0.00% | |
| Drinking status | 0.896 | ||||
| Non-drinker | 186 | 89.30% | 33 | 86.80% | |
| Current drinker | 17 | 8.30% | 4 | 10.50% | |
| Ex-drinker | 5 | 2.40% | 1 | 2.70% | |
| Skeletal muscle mass_pre-NAC | 0.019 | ||||
| Skeletal muscle loss | 21 | 10.10% | 9 | 23.70% | |
| Non- Skeletal muscle loss | 187 | 89.90% | 29 | 76.30% | |
| Skeletal muscle mass post-NAC | <0.001 | ||||
| Skeletal muscle loss | 12 | 5.80% | 21 | 55.30% | |
| Non- Skeletal muscle loss | 196 | 94.20% | 17 | 44.70% | |
| Change in skeletal muscle mass | <0.001 | ||||
| Skeletal muscle loss → Skeletal muscle loss | 9 | 4.30% | 9 | 23.70% | |
| Skeletal muscle loss → Non-skeletal muscle loss | 12 | 5.80% | 0 | 0.00% | |
| Non-skeletal muscle loss → Skeletal muscle loss | 3 | 1.40% | 12 | 31.60% | |
| Non-skeletal muscle loss → Non-skeletal muscle loss | 184 | 88.50% | 17 | 44.70% | |
| Body mass index | 0.903 | ||||
| Underweight | 7 | 3.40% | 1 | 2.60% | |
| Normal | 107 | 51.40% | 21 | 55.30% | |
| Overweight | 36 | 17.30% | 7 | 18.40% | |
| Obese | 58 | 27.90% | 9 | 23.70% | |
| Hemoglobin | 0.922 | ||||
| Median (IQR) | 13.2 | 13.4 | |||
| Platelets | 0.051 | ||||
| Median (IQR) | 264.5 | 292.5 | |||
| Albumin | 0.125 | ||||
| Median (IQR) | 4.5 | 4.6 | |||
| Protein | 0.937 | ||||
| Median (IQR) | 7.4 | 7.45 | |||
| Responder Group (n = 208) | Non-Responder Group (n = 38) | ||||
|---|---|---|---|---|---|
| Characteristics | Number | % | Number | % | p-Value |
| Pathology | 0.001 | ||||
| IDC | 201 | 96.60% | 31 | 81.60% | |
| ILC | 2 | 1.00% | 3 | 7.90% | |
| Mucinous | 2 | 1.00% | 3 | 7.90% | |
| Others | 3 | 1.40% | 1 | 2.60% | |
| T stage | 0.116 | ||||
| T1 | 42 | 20.20% | 4 | 10.50% | |
| T2 | 120 | 57.70% | 19 | 50.00% | |
| T3 | 26 | 12.50% | 8 | 21.10% | |
| T4 | 20 | 9.60% | 7 | 18.40% | |
| N stage | 0.076 | ||||
| N0 | 52 | 25.00% | 6 | 15.80% | |
| N1 | 87 | 41.80% | 11 | 28.90% | |
| N2 | 37 | 17.80% | 12 | 31.60% | |
| N3 | 32 | 15.40% | 9 | 23.70% | |
| Stage | 0.003 | ||||
| I | 9 | 4.30% | 0 | 0.00% | |
| II | 112 | 53.90% | 11 | 28.90% | |
| III | 87 | 41.80% | 27 | 71.10% | |
| Operation | <0.001 | ||||
| Breast-conserving surgery | 120 | 57.70% | 9 | 23.70% | |
| Modified radical mastectomy | 88 | 42.30% | 29 | 76.30% | |
| RT modality | 0.241 | ||||
| 3D CRT | 51 | 24.50% | 6 | 15.80% | |
| IMRT | 157 | 75.50% | 32 | 84.20% | |
| Dose scheme | 0.354 | ||||
| 180 cGy per fraction | 189 | 90.90% | 37 | 97.40% | |
| 200 cGy per fraction | 8 | 3.80% | 0 | 0.00% | |
| 267 cGy per fraction | 11 | 5.30% | 1 | 2.60% | |
| ER | 0.236 | ||||
| Negative | 87 | 41.80% | 12 | 31.60% | |
| Positive | 121 | 58.20% | 26 | 68.40% | |
| PR | 0.874 | ||||
| Negative | 145 | 69.70% | 26 | 68.40% | |
| Positive | 63 | 30.30% | 12 | 31.60% | |
| HER2 | 0.296 | ||||
| Negative | 141 | 67.80% | 29 | 76.30% | |
| Positive | 67 | 32.20% | 9 | 23.70% | |
| Ki-67 | 0.001 | ||||
| <15 | 92 | 44.20% | 20 | 52.60% | |
| ≥15 | 60 | 28.80% | 18 | 47.40% | |
| Unknown | 56 | 27.00% | 0 | 0.00% | |
| Chemotherapy regimen | <0.001 | ||||
| Adriamycin based | 1 | 0.50% | 4 | 10.50% | |
| Adriamycin and taxol based | 138 | 66.30% | 23 | 60.50% | |
| Taxol based | 5 | 2.40% | 3 | 7.90% | |
| Trastuzumab based | 64 | 30.80% | 8 | 21.10% | |
| Selective estrogen receptor modulator | 0.162 | ||||
| No | 117 | 56.25% | 26 | 68.40% | |
| Yes | 91 | 43.75% | 12 | 31.60% | |
| Aromatase inhibitor | 0.560 | ||||
| No | 185 | 88.90% | 35 | 92.10% | |
| Yes | 23 | 11.10% | 3 | 7.90% | |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Age (<48 vs. ≥48 years) | 1.322 | 0.66–2.64 | 0.430 | |||
| Smoking status | 0.877 | |||||
| Non-smoker vs. current smoker | 0.550 | 0.06–5.43 | 0.608 | |||
| Non-smoker vs. ex-smoker | NA | NA | NA | |||
| Drinking status | 0.896 | |||||
| Non-drinker vs. current drinker | 0.762 | 0.24–2.41 | 0.644 | |||
| Non-drinker vs. ex-drinker | 0.897 | 0.10–7.92 | 0.922 | |||
| Pre-NAC skeletal muscle mass (Skeletal muscle loss vs. non-skeletal muscle loss) | 2.749 | 1.15–6.58 | 0.023 | 0.193 | 0.04–0.94 | 0.042 |
| Post-NAC skeletal muscle mass (Skeletal muscle loss vs. non-skeletal muscle loss) | 20.074 | 8.45–47.69 | <0.001 | 64.566 | 15.13–275.58 | <0.001 |
| Change in skeletal muscle mass † | <0.001 | |||||
| Group 1 vs. 2 | N/A | N/A | 0.999 | |||
| Group 1 vs. 3 | 0.250 | 0.52–1.20 | 0.083 | |||
| Group 1 vs. 4 | 10.824 | 3.79–30.90 | <0.001 | |||
| Body mass index | 0.904 | |||||
| Underweight vs. Obese | 1.086 | 0.12–9.90 | 0.942 | |||
| Normal vs. Obese | 0.739 | 0.32–1.72 | 0.483 | |||
| Overweight vs. Obese | 0.798 | 0.27–2.33 | 0.680 | |||
| ER (negative vs. positive) | 0.723 | 0.35–1.49 | 0.381 | |||
| PR (negative vs. positive) | 1.028 | 0.51–2.07 | 0.938 | |||
| HER2 (negative vs. positive) | 1.465 | 0.66–3.27 | 0.352 | |||
| Ki-67 (<15 vs. ≥15) | 0.725 | 0.35–1.48 | 0.377 | |||
| Pathology | 0.009 | 0.056 | ||||
| IDC vs. ILC | 0.103 | 0.02–0.64 | 0.015 | 1.209 | 0.09–16.90 | 0.888 |
| IDC vs. mucinous | 0.103 | 0.02–0.64 | 0.015 | 0.067 | 0.01–0.47 | 0.006 |
| IDC vs. others | 0.463 | 0.05–4.59 | 0.510 | 0.640 | 0.03–12.71 | 0.770 |
| T stage | 0.131 | |||||
| T1 vs. T2 | 0.602 | 0.19–1.87 | 0.380 | |||
| T1 vs. T3 | 0.310 | 0.09–1.13 | 0.076 | |||
| T1 vs. T4 | 0.272 | 0.07–1.04 | 0.057 | |||
| N stage | 0.086 | |||||
| N0 vs. N1 | 0.913 | 0.32–2.61 | 0.865 | |||
| N0 vs. N2 | 0.356 | 0.12–1.03 | 0.058 | |||
| N0 vs. N3 | 0.410 | 0.13–1.26 | 0.120 | |||
| Stage (stage I/II vs. stage III) | 0.293 | 0.14–0.62 | 0.001 | 0.276 | 0.11–0.71 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.M.; Cho, Y.; Kim, J.W.; Ahn, S.G.; Kim, J.H.; Jeung, H.C.; Jeong, J.; Lee, I.J. Association between Skeletal Muscle Loss and the Response to Neoadjuvant Chemotherapy for Breast Cancer. Cancers 2021, 13, 1806. https://doi.org/10.3390/cancers13081806
Lee BM, Cho Y, Kim JW, Ahn SG, Kim JH, Jeung HC, Jeong J, Lee IJ. Association between Skeletal Muscle Loss and the Response to Neoadjuvant Chemotherapy for Breast Cancer. Cancers. 2021; 13(8):1806. https://doi.org/10.3390/cancers13081806
Chicago/Turabian StyleLee, Byung Min, Yeona Cho, Jun Won Kim, Sung Gwe Ahn, Jee Hung Kim, Hei Cheul Jeung, Joon Jeong, and Ik Jae Lee. 2021. "Association between Skeletal Muscle Loss and the Response to Neoadjuvant Chemotherapy for Breast Cancer" Cancers 13, no. 8: 1806. https://doi.org/10.3390/cancers13081806
APA StyleLee, B. M., Cho, Y., Kim, J. W., Ahn, S. G., Kim, J. H., Jeung, H. C., Jeong, J., & Lee, I. J. (2021). Association between Skeletal Muscle Loss and the Response to Neoadjuvant Chemotherapy for Breast Cancer. Cancers, 13(8), 1806. https://doi.org/10.3390/cancers13081806






