Could Primary Chemoradiotherapy in T2 Glottic Cancers Yield Results Comparable to Primary Radiotherapy in T1? Considerations from 531 German Early Stage Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
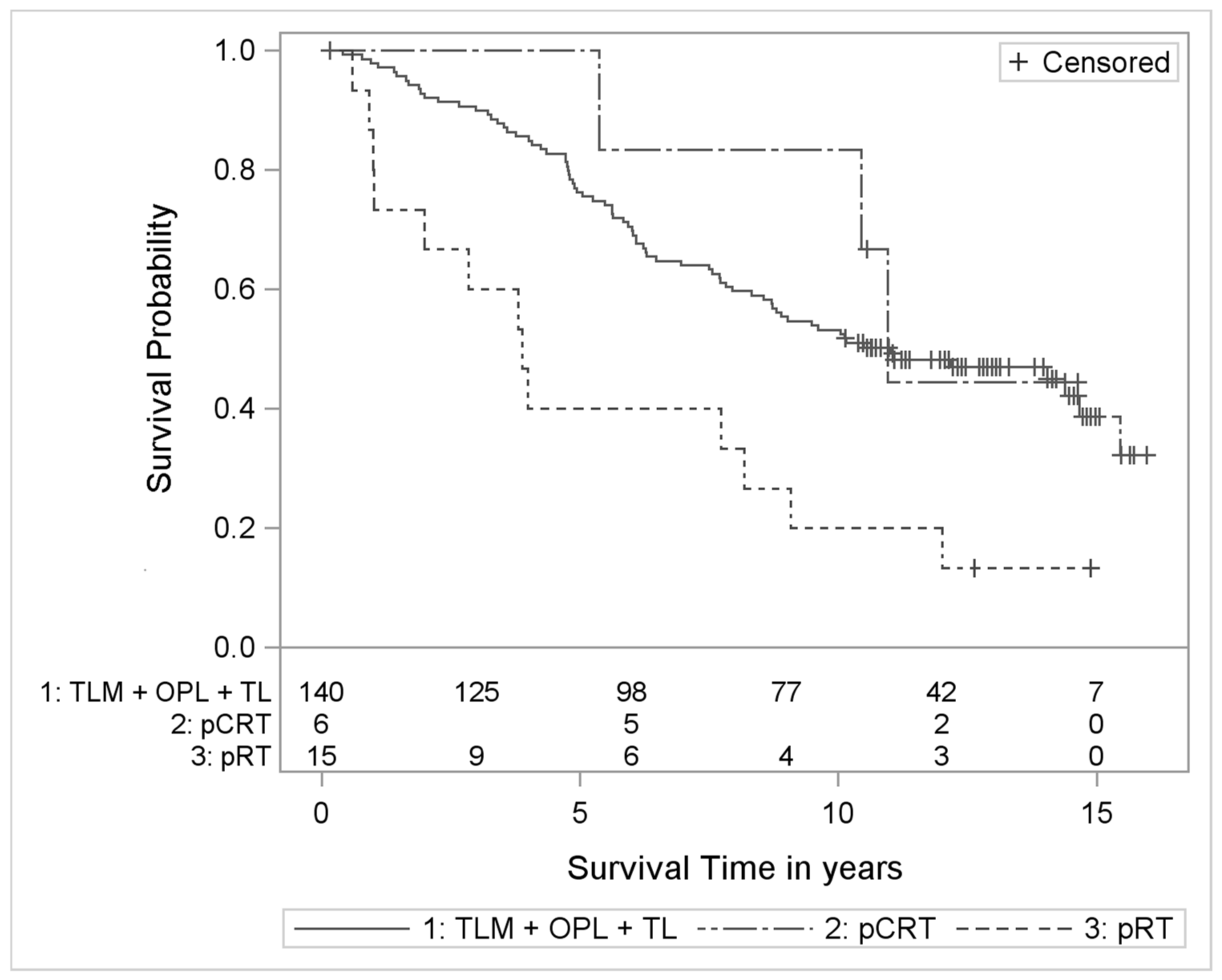
3. Results
4. Discussion
4.1. Could pCRT in T2 Be Superior to Surgery in T1?
4.2. After pRT and TLM: No Differences in LC, but TLM Better in OS
4.3. T2 Compared to T1: Significantly Poorer Outcomes after TLM and pRT
4.4. In T2: Can the Effectiveness of RT Alone Be Further Improved?
4.5. After pRT: Significantly Poorer LP
4.6. After pCRT: LP Rates Comparable to Those after Surgery
4.7. pCRT for T2 Glottic Cancer in the Literature
4.8. Concerns of Toxicity
4.9. Patients’ Preferences
4.10. For Which T2 Glottic Cancer Patients Could pCRT Be Considered?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aCT | adjuvant chemotherapy |
| aCRT | adjuvant chemoradiotherapy |
| aRT | adjuvant radiotherapy |
| AUC | area under the blood concentration-time curve |
| Carbo | Carboplatin |
| CDDP = cis | Cisplatin |
| CI | Confidence interval |
| DSS | Disease-specific survival |
| EORTC | European Organization for Research and Treatment of Cancer |
| 5-FU | 5-fluorouracil |
| HFX | hyerfractionation |
| HR | hazard ratio |
| KM | Kaplan-Meier |
| LC | local control |
| LP | larynx preservation |
| LFS | laryngectomy-free survival |
| n.a. | not available |
| NCDB | National Cancer Data Base |
| n pts. | number of patients |
| OPL | open partial laryngectomy |
| OR | odds ratio |
| OS | overall survival |
| PCM | pharyngeal constrictor muscles |
| pCRT | primary chemoradiotherapy |
| p(C)RT | primary radiotherapy or primary chemoradiotherapy |
| pRT | primary radiotherapy |
| SFX | standard fractionation |
| surg +/- a(C)RT | surgery with or without adjuvant (chemo)radiotherapy |
| TLM | transoral laser microsurgery |
| TL | total laryngectomy |
| TNM | staging according to T = primary tumor, n = lymphnode metastases, and M = distant metastases |
| rTNM | TNM of recurrent tumors |
| UICC | Union International Contre le Cancer (International Union against Cancer |
| VA = VALCSG | The Veterans Affairs Laryngeal Cancer Study Group |
References
- Deng, Y.; Wang, M.; Zhou, L.; Zheng, Y.; Li, N.; Tian, T.; Zhai, Z.; Yang, S.; Hao, Q.; Wu, Y.; et al. Global burden of larynx cancer, 1990–2017: Estimates from the global burden of disease 2017 study. Aging 2020, 12, 2545–2583. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ramroth, H.; Dietz, A.; Becher, H. Interaction effects and population-attributable risks for smoking and alcohol on laryngeal cancer and its subsites. A case-control study from Germany. Methods Inf. Med. 2004, 43, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Hashibe, M.; Boffetta, P.; Zaridze, D.; Shangina, O.; Szeszenia-Dabrowska, N.; Mates, D.; Janout, V.; Fabiánová, E.; Bencko, V.; Moullan, N.; et al. Evidence for an Important Role of Alcohol- and Aldehyde-Metabolizing Genes in Cancers of the Upper Aerodigestive Tract. Cancer Epidemiol. Biomark. Prev. 2006, 15, 696–703. [Google Scholar] [CrossRef]
- Talamini, R.; Bosetti, C.; La Vecchia, C.; Maso, L.D.; Levi, F.; Bidoli, E.; Negri, E.; Pasche, C.; Vaccarella, S.; Barzan, L.; et al. Combined effect of tobacco and alcohol on laryngeal cancer risk: A case–control study. Cancer Causes Control. 2002, 13, 957–964. [Google Scholar] [CrossRef]
- Kaatsch, P.; Spix, C.; Katalinic, A.; Hentschel, S. Contributions to Federal Health Reporting. In Cancer in Germany 2007/2008, 8th ed.; Robert Koch Institute and the Association of Population-based Cancer Registries in Germany, Ed.; Robert-Koch-Institut: Berlin, Germany, 2012. [Google Scholar]
- Ramroth, H.; Ahrens, W.; Dietz, A.; Becher, H. Occupational asbestos exposure as a risk factor for laryngeal carcinoma in a population-based case-control study from Germany. Am. J. Ind. Med. 2011, 54, 510–514. [Google Scholar] [CrossRef]
- Becher, H.; Ramroth, H.; Ahrens, W.; Risch, A.; Schmezer, P.; Dietz, A. Occupation, exposure to polycyclic aromatic hydrocarbons and laryngeal cancer risk. Int. J. Cancer 2005, 116, 451–457. [Google Scholar] [CrossRef]
- Bayer, O.; Cámara, R.; Zeissig, S.R.; Ressing, M.; Dietz, A.; Locati, L.D.; Ramroth, H.; Singer, S. Occupation and cancer of the larynx: A systematic review and meta-analysis. Eur. Arch. Oto-Rhino-Laryngol. 2014, 273, 9–20. [Google Scholar] [CrossRef]
- Halec, G.; Holzinger, D.; Schmitt, M.; Flechtenmacher, C.; Dyckhoff, G.; Lloveras, B.; Hofler, D.; Bosch, F.X.; Pawlita, M. Biological evidence for a causal role of HPV16 in a small fraction of laryngeal squamous cell carcinoma. Br. J. Cancer 2013, 109, 172–183. [Google Scholar] [CrossRef]
- Gamez, M.E.; Blakaj, A.; Zoller, W.; Bonomi, M.; Blakaj, D.M. Emerging Concepts and Novel Strategies in Radiation Therapy for Laryngeal Cancer Management. Cancers 2020, 12, 1651. [Google Scholar] [CrossRef] [PubMed]
- Strong, M.S.; Jako, G.J. Laser Surgery in the Larynx Early Clinical Experience with Continuous Co2 Laser. Ann. Otol. Rhinol. Laryngol. 1972, 81, 791–798. [Google Scholar] [CrossRef]
- Silver, C.E.; Beitler, J.J.; Shaha, A.R.; Rinaldo, A.; Ferlito, A. Current trends in initial management of laryngeal cancer: The declining use of open surgery. Eur. Arch. Oto-Rhino-Laryngol. 2009, 266, 1333–1352. [Google Scholar] [CrossRef]
- Pfister, D.G. NCCN Clinical Practice Guidelines in Oncology in Head and Neck Cancers (version 1.2021). Available online: https://www.nccn.org/professionals/physician_gls/default.aspx (accessed on 29 January 2021).
- Department of Veterans Affairs Laryngeal Cancer Study Group; Wolf, G.T.; Fisher, S.G.; Hong, W.K.; Hillman, R.; Spaulding, M.; E Laramore, G.; Endicott, J.W.; McClatchey, K.; Henderson, W.G. Induction Chemotherapy plus Radiation Compared with Surgery plus Radiation in Patients with Advanced Laryngeal Cancer. N. Engl. J. Med. 1991, 324, 1685–1690. [Google Scholar] [CrossRef]
- Lefebvre, J.-L.; Chevalier, D.; Luboinski, B.; Kirkpatrick, A.; Collette, L.; Sahmoud, T. Larynx Preservation in Pyriform Sinus Cancer: Preliminary Results of a European Organization for Research and Treatment of Cancer Phase III Trial. J. Natl. Cancer Inst. 1996, 88, 890–899. [Google Scholar] [CrossRef]
- Baird, B.J.; Sung, C.K.; Beadle, B.M.; Divi, V. Treatment of early-stage laryngeal cancer: A comparison of treatment options. Oral Oncol. 2018, 87, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.-L.; Li, J.; Yang, X.; Zhang, F.; Xiong, J.-W.; Yang, Z.-L.; Tan, J.; Li, B. Transoral laser microsurgery versus radiotherapy for T1 glottic carcinoma: A systematic review and meta-analysis. Lasers Med. Sci. 2016, 32, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.V.; Dedivitis, R.A.; Matos, L.L.; Aires, F.T.; Cernea, C.R. Comparison between transoral laser surgery and radiotherapy in the treatment of early glottic cancer: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, B. Efficacy of laser surgery versus radiotherapy for treatment of glottic carcinoma: A systematic review and meta-analysis. Lasers Med. Sci. 2018, 34, 847–854. [Google Scholar] [CrossRef]
- Vaculik, M.F.; Mackay, C.A.; Taylor, S.M.; Trites, J.R.B.; Hart, R.D.; Rigby, M.H. Systematic review and meta-analysis of T1 glottic cancer outcomes comparing CO2 transoral laser microsurgery and radiotherapy. J. Otolaryngol. Head Neck Surg. 2019, 48, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hendriksma, M.; Heijnen, B.J.; Sjögren, E.V. Oncologic and functional outcomes of patients treated with transoral CO2 laser microsurgery or radiotherapy for T2 glottic carcinoma: A systematic review of the literature. Curr. Opin. Otolaryngol. Head Neck Surg. 2018, 26, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Warde, P.; O’Sullivan, B.; Bristow, R.G.; Panzarella, T.; Keane, T.J.; Gullane, P.J.; Witterick, I.P.; Payne, D.; Liu, F.-F.; McLean, M.; et al. T1/T2 Glottic Cancer Managed by External Beam Radiotherapy: The Influence of Pretreatment Hemoglobin on Local Control. Int. J. Radiat. Oncol. 1998, 41, 347–353. [Google Scholar] [CrossRef]
- Frata, P.; Cellai, E.; Magrini, S.M.; Bonetti, B.; Vitali, E.; Tonoli, S.; Buglione, M.; Paiar, F.; Barca, R.; Fondelli, S.; et al. Radical radiotherapy for early glottic cancer: Results in a series of 1087 patients from two Italian radiation oncology centers. II. The case of T2N0 disease. Int. J. Radiat. Oncol. 2005, 63, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Cellai, E.; Frata, P.; Magrini, S.M.; Paiar, F.; Barca, R.; Fondelli, S.; Polli, C.; Livi, L.; Bonetti, B.; Vitali, E.; et al. Radical radiotherapy for early glottic cancer: Results in a series of 1087 patients from two Italian radiation oncology centers. I. The case of T1N0 disease. Int. J. Radiat. Oncol. 2005, 63, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Groome, P.A.; O’Sullivan, B.; MacKillop, W.J.; Jackson, L.D.; Schulze, K.; Irish, J.C.; Warde, P.R.; Schneider, K.M.; MacKenzie, R.G.; Hodson, D.I.; et al. Compromised local control due to treatment interruptions and late treatment breaks in early glottic cancer: Population-based outcomes study supporting need for intensified treatment schedules. Int. J. Radiat. Oncol. 2006, 64, 1002–1012. [Google Scholar] [CrossRef]
- Chera, B.S.; Amdur, R.J.; Morris, C.G.; Kirwan, J.M.; Mendenhall, W.M. T1N0 to T2N0 Squamous Cell Carcinoma of the Glottic Larynx Treated With Definitive Radiotherapy. Int. J. Radiat. Oncol. 2010, 78, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.-C.; Au, K.-H.; Ngan, R.K.-C.; Cheung, F.-Y.; Chow, S.-M.; Fu, Y.-T.; Au, J.S.-K.; Law, S.C.-K. Definitive radiotherapy for early stage glottic cancer by 6 MV photons. Head Neck Oncol. 2012, 4, 23. [Google Scholar] [CrossRef][Green Version]
- Al-Mamgani, A.; Van Rooij, P.; Woutersen, D.; Mehilal, R.; Tans, L.; Monserez, D.; De Jong, R.B. Radiotherapy for T1-2N0 glottic cancer: A multivariate analysis of predictive factors for the long-term outcome in 1050 patients and a prospective assessment of quality of life and voice handicap index in a subset of 233 patients. Clin. Otolaryngol. 2013, 38, 306–312. [Google Scholar] [CrossRef]
- Sapienza, L.G.; Ning, M.S.; Taguchi, S.; Calsavara, V.F.; Pellizzon, A.C.D.A.; Gomes, M.J.L.; Kowalski, L.P.; Baiocchi, G. Altered-fractionation radiotherapy improves local control in early-stage glottic carcinoma: A systematic review and meta-analysis of 1762 patients. Oral Oncol. 2019, 93, 8–14. [Google Scholar] [CrossRef]
- Dyckhoff, G.; Plinkert, P.K.; Ramroth, H. A change in the study evaluation paradigm reveals that larynx preservation compromises survival in T4 laryngeal cancer patients. BMC Cancer 2017, 17, 609. [Google Scholar] [CrossRef]
- Dyckhoff, G.; Warta, R.; Herold-Mende, C.; Rudolph, E.; Plinkert, P.; Ramroth, H. An Observational Cohort Study on 194 Supraglottic Cancer Patients: Implications for Laser Surgery and Adjuvant Treatment. Cancers 2021, 13, 568. [Google Scholar] [CrossRef]
- Allison, P. Survival Analysis Using SAS: A Practical Guide, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2010. [Google Scholar]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Lyhne, N.M.; Johansen, J.; Kristensen, C.A.; Andersen, E.; Primdahl, H.; Andersen, L.J.; Bøje, C.R.; Jensen, A.R.; Overgaard, J. Pattern of failure in 5001 patients treated for glottic squamous cell carcinoma with curative intent – A population based study from the DAHANCA group. Radiother. Oncol. 2016, 118, 257–266. [Google Scholar] [CrossRef] [PubMed]
- E Epstein, B.; Lee, D.J.; Kashima, H.; E Johns, M. Stage T1 glottic carcinoma: Results of radiation therapy or laser excision. Radiology 1990, 175, 567–570. [Google Scholar] [CrossRef]
- Stoeckli, S.J.; Schnieper, I.; Huguenin, P.; Schmid, S. Early glottic carcinoma: Treatment according patient’s preference? Head Neck 2003, 25, 1051–1056. [Google Scholar] [CrossRef]
- Schrijvers, M.L.; Van Riel, E.L.; Langendijk, J.A.; Dikkers, F.G.; Schuuring, E.; Van Der Wal, J.E.; Van Der Laan, B.F.A.M. Higher laryngeal preservation rate after CO2laser surgery compared with radiotherapy in T1a glottic laryngeal carcinoma. Head Neck 2009, 31, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, B.; Wen, S. Laser Surgery versus Radiotherapy for T1–T2N0 Glottic Cancer: A Meta-Analysis. ORL 2011, 73, 336–342. [Google Scholar] [CrossRef]
- Abdurehim, Y.; Hua, Z.; Yasin, Y.; Xukurhan, A.; Imam, I.; Yuqin, F. Transoral laser surgery versus radiotherapy: Systematic review and meta-analysis for treatment options of T1a glottic cancer. Head Neck 2011, 34, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Higgins, K.M.; Shah, M.D.; Ogaick, M.J.; Enepekides, D. Treatment of early-stage glottic cancer: Meta-analysis comparison of laser excision versus radiotherapy. J. Otolaryngol. Head Neck Surg. 2009, 38, 603–612. [Google Scholar]
- Spector, J.G.; Sessions, D.G.; Chao, K.S.; Haughey, B.H.; Hanson, J.M.; Simpson, J.R.; Perez, C.A. Stage I (T1 N0 M0) squamous cell carcinoma of the laryngeal glottis: Therapeutic results and voice preservation. Head Neck. 1999, 21, 707–717. [Google Scholar] [CrossRef]
- Warner, L.; Lee, K.; Homer, J. Transoral laser microsurgery versus radiotherapy for T2 glottic squamous cell carcinoma: A systematic review of local control outcomes. Clin. Otolaryngol. 2017, 42, 629–636. [Google Scholar] [CrossRef]
- Peretti, G.; Piazza, C.; Cocco, D.; De Benedetto, L.; Del Bon, F.; De Zinis, L.O.R.; Nicolai, P. Transoral CO2 laser treatment for Tis-T3 glottic cancer: The University of Brescia experience on 595 patients. Head Neck 2009, 32, 977–983. [Google Scholar] [CrossRef]
- Mahler, V.; Boysen, M.; Brøndbo, K. Radiotherapy or CO2 laser surgery as treatment of T1a glottic carcinoma? Eur. Arch. Oto-Rhino-Laryngol. 2009, 267, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Harwood, A.R.; Hawkins, N.V.; Rider, W.D.; Bryce, D.P.; Ch, B. Radiotherapy of early glottic cancer—I. Int. J. Radiat. Oncol. 1979, 5, 473–476. [Google Scholar] [CrossRef]
- Chung, S.Y.; Kim, K.H.; Keum, K.C.; Koh, Y.W.; Kim, S.-H.; Choi, E.C.; Lee, C.G. Radiotherapy Versus Cordectomy in the Management of Early Glottic Cancer. Cancer Res. Treat. 2018, 50, 156–163. [Google Scholar] [CrossRef]
- Sakata, K.-I.; Aoki, Y.; Karasawa, K.; Hasezawa, K.; Muta, N.; Nakagawa, K.; Terahara, A.; Onogi, Y.; Sasaki, Y.; Akanuma, A. Radiation therapy in early glottic carcinoma: Uni- and multivariate analysis of prognostic factors affecting local control. Int. J. Radiat. Oncol. 1994, 30, 1059–1064. [Google Scholar] [CrossRef]
- Trotti, A.; Zhang, Q.; Bentzen, S.M.; Emami, B.; Hammond, M.E.; Jones, C.U.; Morrison, W.H.; Sagar, S.M.; Ridge, J.A.; Fu, K.K.; et al. Randomized Trial of Hyperfractionation Versus Conventional Fractionation in T2 Squamous Cell Carcinoma of the Vocal Cord (RTOG 9512). Int. J. Radiat. Oncol. 2014, 89, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Iro, H.; Waldfahrer, F.; Altendorf-Hofmann, A.; Weidenbecher, M.; Sauer, R.; Steiner, W. Transoral Laser Surgery of Supraglottic Cancer. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 1245–1250. [Google Scholar] [CrossRef]
- Iro, H.; Mantsopoulos, K.; Zenk, J.; Waldfahrer, F.; Psychogios, G. Ergebnisse der transoralen Laserresektion bei T1-2 Karzinomen von Oropharynx, Hypopharynx und Larynx. Laryngo Rhino Otol. 2011, 90, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Lefebvre, J.L. Laryngeal Preservation Strategies in Locally Advanced Laryngeal and Hypopharyngeal Cancers. Front. Oncol. 2019, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, J.-L.; Ang, K.K.; Larynx Preservation Consensus Panel. Larynx preservation clinical trial design: Key issues and recommendations-A consensus panel summary. Head Neck 2009, 31, 429–441. [Google Scholar] [CrossRef]
- Jenckel, F.; Knecht, R. State of the art in the treatment of laryngeal cancer. Anticancer Res. 2013, 33, 4701–4710. [Google Scholar]
- Peretti, G.; Bolzoni, A.; Parrinello, G.; Mensi, M.C.; Shapshay, S.M.; Piazza, C.; Rossini, M.; Antonelli, A.R. Analysis of Recurrences in 322 TIS, T1, or T2 Glottic Carcinomas Treated by Carbon Dioxide Laser. Ann. Otol. Rhinol. Laryngol. 2004, 113, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Peretti, G.; Piazza, C.; Mensi, M.C.; Magnoni, L.; Bolzoni, A. Endoscopic Treatment of cT2 Glottic Carcinoma: Prognostic Impact of Different pT Subcategories. Ann. Otol. Rhinol. Laryngol. 2005, 114, 579–586. [Google Scholar] [CrossRef]
- Mendenhall, W.M.; Amdur, R.J.; Morris, C.G.; Hinerman, R.W. T1-T2N0 Squamous Cell Carcinoma of the Glottic Larynx Treated With Radiation Therapy. J. Clin. Oncol. 2001, 19, 4029–4036. [Google Scholar] [CrossRef]
- Hartl, D.M.; Ferlito, A.; Brasnu, D.F.; Langendijk, J.A.; Rinaldo, A.; Silver, C.E.; Wolf, G.T. Evidence-based review of treatment options for patients with glottic cancer. Head Neck 2011, 33, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, W.M.; Dagan, R.; Bryant, C.M.; Amdur, R.J.; Mancuso, A.A. Definitive Radiotherapy for Squamous Cell Carcinoma of the Glottic Larynx. Cancer Control. 2016, 23, 208–212. [Google Scholar] [CrossRef]
- Hamauchi, S.; Yokota, T.; Onozawa, Y.; Ogawa, H.; Onoe, T.; Kamijo, T.; Iida, Y.; Onitsuka, T.; Yasui, H. Chemoradiotherapy for high-risk stage II laryngeal cancer. Int. J. Clin. Oncol. 2020, 25, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Furusaka, T.; Matsuda, H.; Saito, T.; Katsura, Y.; Ikeda, M. Long-term follow-up and salvage surgery in patients with T2N0M0 squamous cell carcinoma of the glottic larynx who received concurrent chemoradiation therapy with carboplatin (CBDCA) – AUC 1.5 vs. AUC 2.0. Acta Oto-Laryngol. 2012, 132, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Furusaka, T.; Matuda, H.; Saito, T.; Katsura, Y.; Ikeda, M. Long-term observations and salvage operations on patients with T2N0M0 squamous cell carcinoma of the glottic larynx treated with radiation therapy alone. Acta Oto-Laryngol. 2012, 132, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Furusaka, T.; Susaki, Y.; Saito, T.; Katsura, Y.; Ikeda, M. Long-term follow-up and salvage surgery in patients with T2N0M0 squamous cell carcinoma of the glottic larynx following concurrent chemoradiation therapy with cisplatin and 5-fluorouracil for laryngeal preservation. Acta Oto-Laryngol. 2012, 133, 91–98. [Google Scholar] [CrossRef]
- Al Feghali, K.A.; Youssef, B.Y.; Mohamed, A.S.R.; Hilal, L.; Smith, B.D.; Abu-Gheida, I.; Farha, G.; Gunn, G.B.; Phan, J.; Lewin, J.; et al. Outcomes after radiation therapy for T2N0 /stage II glottic squamous cell carcinoma. Head Neck 2020, 42, 2791–2800. [Google Scholar] [CrossRef]
- Kitani, Y.; Kubota, A.; Furukawa, M.; Hori, Y.; Nakayama, Y.; Nonaka, T.; Mizoguchi, N.; Kitani, Y.; Hatakeyama, H.; Oridate, N. Impact of combined modality treatment with radiotherapy and S-1 on T2N0 laryngeal cancer: Possible improvement in survival through the prevention of second primary cancer and distant metastasis. Oral Oncol. 2017, 71, 54–59. [Google Scholar] [CrossRef]
- Rudat, V.; Eckel, H.; Volling, P.; Schröder, M.; Staar, S.; Wallner, F.; Wannenmacher, M.; Dietz, A. Long-term results of a prospective multicenter phase II study to preserve the larynx function using concomitant boost radiochemotherapy with Carboplatin. Radiother. Oncol. 2008, 89, 33–37. [Google Scholar] [CrossRef]
- Forastiere, A.A.; Zhang, Q.; Weber, R.S.; Maor, M.H.; Goepfert, H.; Pajak, T.F.; Morrison, W.H.; Glisson, B.S.; Trotti, A.; Ridge, J.A.; et al. Long-Term Results of RTOG 91-11: A Comparison of Three Nonsurgical Treatment Strategies to Preserve the Larynx in Patients With Locally Advanced Larynx Cancer. J. Clin. Oncol. 2013, 31, 845–852. [Google Scholar] [CrossRef]
- Forastiere, A.A.; Goepfert, H.; Maor, M.; Pajak, T.F.; Weber, R.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; Chao, C.; et al. Concurrent Chemotherapy and Radiotherapy for Organ Preservation in Advanced Laryngeal Cancer. N. Engl. J. Med. 2003, 349, 2091–2098. [Google Scholar] [CrossRef]
- Machtay, M.; Moughan, J.; Trotti, A.; Garden, A.S.; Weber, R.S.; Cooper, J.S.; A Forastiere, A.; Ang, K.K. Factors Associated With Severe Late Toxicity After Concurrent Chemoradiation for Locally Advanced Head and Neck Cancer: An RTOG Analysis. J. Clin. Oncol. 2008, 26, 3582–3589. [Google Scholar] [CrossRef] [PubMed]
- Langendijk, J.A.; Doornaert, P.; Rietveld, D.H.F.; Leeuw, I.M.V.-D.; Leemans, C.R.; Slotman, B.J. A predictive model for swallowing dysfunction after curative radiotherapy in head and neck cancer. Radiother. Oncol. 2009, 90, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, H.R.; Jensen, K.; Aksglæde, K.; Behrens, M.; Grau, C. Late dysphagia after IMRT for head and neck cancer and correlation with dose–volume parameters. Radiother. Oncol. 2013, 107, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Eisbruch, A.; Kim, H.M.; Feng, F.Y.; Lyden, T.H.; Haxer, M.J.; Feng, M.; Worden, F.P.; Bradford, C.R.; Prince, M.E.; Moyer, J.S.; et al. Chemo-IMRT of Oropharyngeal Cancer Aiming to Reduce Dysphagia: Swallowing Organs Late Complication Probabilities and Dosimetric Correlates. Int. J. Radiat. Oncol. 2011, 81, e93–e99. [Google Scholar] [CrossRef] [PubMed]
- Vlacich, G.; Spratt, D.E.; Diaz, R.; Phillips, J.G.; Crass, J.; Li, C.-I.; Shyr, Y.; Cmelak, A.J. Dose to the inferior pharyngeal constrictor predicts prolonged gastrostomy tube dependence with concurrent intensity-modulated radiation therapy and chemotherapy for locally-advanced head and neck cancer. Radiother. Oncol. 2014, 110, 435–440. [Google Scholar] [CrossRef]
- Moreno, A.C.; Frank, S.J.; Garden, A.S.; Rosenthal, D.I.; Fuller, C.D.; Gunn, G.B.; Reddy, J.P.; Morrison, W.H.; Williamson, T.D.; Holliday, E.B.; et al. Intensity modulated proton therapy (IMPT) – The future of IMRT for head and neck cancer. Oral Oncol. 2019, 88, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Bues, M.; Gamez, M.E.; Stefan, C.; Patel, S.H. Individual Field Simultaneous Optimization (IFSO) in spot scanning proton therapy of head and neck cancers. Med. Dosim. 2019, 44, 375–378. [Google Scholar] [CrossRef]
- Kato, T.; Fuwa, N.; Murakami, M. Dose-Volume Comparison of IMRT and PSPT Treatment Plans for Early-Stage Glottic Cancer. Int. J. Part. Ther. 2020, 7, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, P.; Volk, R.J.; Ringash, J.; Peterson, S.K.; Hutcheson, K.A.; Frank, S.J. Assessing head and neck cancer patient preferences and expectations: A systematic review. Oral Oncol. 2016, 62, 44–53. [Google Scholar] [CrossRef]
- List, M.A.; Rutherford, J.L.; Stracks, J.; Pauloski, B.R.; Logemann, J.A.; Lundy, D.; Sullivan, P.; Goodwin, W.; Kies, M.; Vokes, E.E. Prioritizing treatment outcomes: Head and neck cancer patients versus nonpatients. Head Neck 2004, 26, 163–170. [Google Scholar] [CrossRef] [PubMed]
- List, M.A.; Stracks, J.; Colangelo, L.; Butler, P.; Ganzenko, N.; Lundy, D.; Sullivan, P.; Haraf, D.; Kies, M.; Goodwin, W.; et al. How Do Head and Neck Cancer Patients Prioritize Treatment Outcomes Before Initiating Treatment? J. Clin. Oncol. 2000, 18, 877. [Google Scholar] [CrossRef]
- Brotherston, D.C.; Poon, I.; Le, T.; Leung, M.; Kiss, A.; Ringash, J.; Balogh, J.; Lee, J.; Wright, J.R. Patient preferences for oropharyngeal cancer treatment de-escalation. Head Neck 2012, 35, 151–159. [Google Scholar] [CrossRef]
- Hanna, J.; Brauer, P.R.; Morse, E.; Mehra, S. Margins in Laryngeal Squamous Cell Carcinoma Treated with Transoral Laser Microsurgery: A National Database Study. Otolaryngol. Neck Surg. 2019, 161, 986–992. [Google Scholar] [CrossRef]
| Variable | Category | TLM | OPL | TL | pCRT | pRT | Total |
|---|---|---|---|---|---|---|---|
| Total | - | 443 | 59 | 172 | 38 | 45 | 757 |
| Age (cont) a | - | 62.5 (37–91) | 62.1 (34–84) | 61.9 (40–83) | 61.4 (41–81) | 64.9 (40–85) | 62.4 (34–91) |
| Sex | Males | 402 (90.7) | 57 (96.6) | 158 (91.9) | 32 (84.2) | 36 (80.0) | 685 (90.5) |
| - | Females | 41 (9.3) | 2 (3.4) | 14 (8.1) | 6 (15.8) | 9 (20.0) | 72 (9.5) |
| CCI | 0 | 331 (74.7) | 45 (76.3) | 114 (66.3) | 31 (81.6) | 22 (48.9) | 543 (71.7) |
| - | 1 | 112 (25.3) | 14 (23.7) | 58 (33.7) | 7 (18.4) | 23 (51.1) | 214 (28.3) |
| Localization | Glottic | 336 (75.8) | 49 (83.1) | 49 (28.5) | 8 (21.1) | 23 (51.1) | 465 (61.4) |
| - | Supraglottic | 96 (21.7) | 7 (11.9) | 57 (33.1) | 20 (52.6) | 14 (31.1) | 194 (25.6) |
| - | Subglottic | 4 (0.9) | 0 (0.0) | 8 (4.7) | 1 (2.6) | 1 (2.2) | 14 (1.8) |
| - | Transglottic | 4 (0.9) | 0 (0.0) | 38 (22.1) | 6 (15.8) | 3 (6.7) | 51 (6.7) |
| - | Unknown | 3 (0.7) | 3 (5.1) | 20 (11.6) | 3 (7.9) | 4 (8.9) | 33 (4.4) |
| T-Stage | 1 | 277 (62.5) | 32 (54.2) | 5 (2.9) | 5 (13.2) | 12 (26.7) | 331 (43.7) |
| - | 2 | 122 (27.5) | 17 (28.8) | 34 (19.8) | 9 (23.7) | 18 (40.0) | 200 (26.4) |
| - | 3 | 31 (7.0) | 7 (11.9) | 65 (37.8) | 11 (28.9) | 7 (15.6) | 121 (16.0) |
| - | 4 | 13 (2.9) | 3 (5.1) | 68 (39.5) | 13 (34.2) | 8 (17.8) | 105 (13.9) |
| n-Stage | 0 | 363 (81.9) | 54 (91.5) | 105 (61.0) | 19 (50.0) | 30 (66.7) | 571 (75.4) |
| - | 1 | 18 (4.1) | 0 (0.0) | 21 (12.2) | 3 (7.9) | 4 (8.9) | 46 (6.1) |
| - | 2 | 31 (7.0) | 2 (3.4) | 41 (23.8) | 11 (28.9) | 8 (17.8) | 93 (12.3) |
| - | 3 | 1 (0.2) | 0 (0.0) | 1 (0.6) | 3 (7.9) | 2 (4.4) | 7 (0.9) |
| - | X | 30 (6.8) | 3 (5.1) | 4 (2.3) | 2 (5.3) | 1 (2.2) | 40 (5.3) |
| UICC stage | I | 265 (59.8) | 31 (52.5) | 3 (1.7) | 3 (7.9) | 10 (22.2) | 312 (41.2) |
| - | II | 98 (22.1) | 17 (28.8) | 25 (14.5) | 6 (15.8) | 15 (33.3) | 161 (21.3) |
| - | III | 39 (8.8) | 6 (10.2) | 57 (33.1) | 10 (26.3) | 6 (13.3) | 118 (15.6) |
| - | IV | 41 (9.3) | 5 (8.5) | 87 (50.6) | 19 (50.0) | 14 (31.1) | 166 (21.9) |
| Adj. treat. | None * | 360 (81.3) | 52 (88.1) | 93 (54.1) | 38 (100) | 45 (100) | 588 (77.7) |
| - | aRT | 74 (16.7) | 7 (11.9) | 62 (36.0) | 0 (0.0) | 0 (0.0) | 143 (18.9) |
| - | aCRT | 5 (1.1) | 0 (0.0) | 17 (9.9) | 0 (0.0) | 0 (0.0) | 22 (2.9) |
| - | aCT | 4 (0.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (0.5) |
| T Stage, Therapy (n, DSS/OS *) | 5-Year DSS [%] (95%-CI) | 10-Year DSS [%] (95%-CI) | 5-Year OS [%] (95%-CI) | 10-Year OS [%] (95%-CI) |
|---|---|---|---|---|
| T1 | - | - | - | - |
| OP (307/314 *) | 95 (92–97) | 93 (89–95) | 80 (75–84) | 59 (53–64) |
| pCRT (5/5) | 100 (100–100) | 100 (100–100) | 100 (100–100) | 80 (20–97) |
| pRT (12/12) | 100 (100–100) | 75 (31–93) | 67 (34–86) | 42 (15–67) |
| T2 | - | - | - | - |
| OP (172/173 *) | 82 (76–88) | 73 (65–79) | 69 (62–76) | 48 (40–55) |
| pCRT (9/9) | 100 (100–100) | 100 (100–100) | 89 (43–98) | 67 (28–88) |
| pRT (18/18) | 53 (28–73) | 53 (28–73) | 33 (14–55) | 17 (4–37) |
| T3 | - | - | - | - |
| OP (99/103 *) | 76 (65–83) | 65 (53–74) | 61 (50–69) | 35 (26–45) |
| pCRT (11/10) | 70 (33–89) | 26 (1–66) | 55 (23–78) | 9 (1–33) |
| pRT (5/7 *) | 20 (1–58) | 20 (1–58) | 14 (1–46) | 14 (1–46) |
| T4 | - | - | - | - |
| OP (79/84 *) | 52 (39–63) | 40 (27–52) | 38 (28–48) | 20 (12–29) |
| pCRT (13/13) | 8 (0–29) | 8 (0–29) | 8 (0–29) | 8 (0–29) |
| pRT (8/8) | 13 (1–42) | 13 (1–42) | 13 (1–42) | 13 (1–42) |
| Variable | Category | p-Value | HR | 95%-CI | |
|---|---|---|---|---|---|
| Age | 10 years units | <0.0001 | 1.573 | 1.368 | 1.810 |
| Sex | Female vs. male | 0.0670 | 0.665 | 0.429 | 1.029 |
| CCI | 1 vs. 0 | 0.0006 | 1.564 | 1.212 | 2.018 |
| Therapy | pCRT | 0.3685 | 0.704 | 0.327 | 1.514 |
| - | pRT | 0.0094 | 1.793 | 1.154 | 2.785 |
| T category | T2 vs. T1 | 0.0675 | 1.278 | 0.982 | 1.662 |
| Localization | supraglottic | <0.0001 | 1.925 | 1.432 | 2.588 |
| - | subglottic | 0.3721 | 1.690 | 0.534 | 5.348 |
| - | transglottic | 0.0921 | 2.070 | 0.888 | 4.825 |
| - | unknown | 0.4939 | 1.368 | 0.558 | 3.354 |
| Variable | T Category | Patients n (%) | 5-Year [%] (95%-CI) | 10-Year [%] (95%-CI) |
|---|---|---|---|---|
| DSS | T1 | 270 * (62.6) | 96 (92–98) | 93 (89–96) |
| - | T2 | 122 * (28.3) | 85 (77–90) | 78 (68–84) |
| - | T1 + T2 | 392 * (91.0) | 92 (89–95) | 88 (84–91) |
| OS | T1 | 277 (62.5) | 80 (75–84) | 58 (52–63) |
| - | T2 | 122 (27.5) | 73 (64–80) | 50 (41–59) |
| - | T1 + T2 | 399 (90.1) | 78 (73–82) | 55 (50–60) |
| LP | T1 | 277 (62.5) | 93 (89–96) | 93 (89–96) |
| - | T2 | 122 (27.5) | 82 (73–88) | 82 (73–88) |
| - | T1 + T2 | 399 (90.1) | 90 (86–93) | 90 (86–92) |
| LFS | T1 | 277 (62.5) | 74 (68–79) | 54 (48–60) |
| - | T2 | 122 (27.5) | 65 (56–73) | 46 (36–54) |
| - | T1 + 2 | 399 (90.1) | 71 (67–76) | 52 (47.57) |
| Meta-Analysis T Categories Patients (n)/Studies (n) | LC [HR (95-CI)] | DSS [HR (95%-CI)] | OS [HR (95%-CI)] | LP [HR (95%-CI)] |
|---|---|---|---|---|
| Higgins, 2009 [41], Tis-T2 7676/26 | 0.81 (0.51–1.35) | n.a. | 1.48 (1.19–1.85) | TL-free survial 0.73 0.39–1.35 *2 |
| Mo, 2017 [18], T1 1238-1452/11 | 0.98 (0.7–1.38) | n.a. | 1.35 (1.02–1.79) | 5.81 (3.36–10.05) |
| Guimaraes, 2018 [19] *1 Tis-T1a 1034-1481/6-10 | WMD TLM vs. pRT −0.01 [−0.07, 0.04] Z = 0.45; p = 0.65 | WMD TLM vs. pRT −0.02 [−0.04, −0.00] Z = 2.03; p = 0.04 | WMD −0.05 [−0.09, −0.00) Z = 1.97; p = 0.05 | WMD −0.10 [−0.13, −0.07] Z = 6.53; p < 0.00001 |
| Ding, 2019 [20], T1-T2 2480/18 | 1.19 (0.76–1.85) | 1.60 (0.89–2.88) | 1.39 1.06–1.81) | 3.85 1.92–7.72 |
| Vaculik, 2019 [21], T1 1987/17 | 1.19 0.79–1.81 | 2.70 1.32–5.54 | 1.52 1.07–2.14 | 6.31 3.77–10.56 |
| Study Stage (n pts.) | 5-Year-LC [%] | 5-Year DSS [%] | 5-Year OS [%] | 5-Year-LP [%] |
|---|---|---|---|---|
| Warde, 1998 [23] | - | - | - | - |
| T1a (403)/T1b (46) | 91/82 | n.a. | 75.8 (T1+T2) | n.a. |
| T2 (286) | 69 | n.a. | - | n.a. |
| Frata/Cellai 2005 [24,25] | - | - | - | - |
| T1a (660)/T1b (171) | 84/81 | 95 | 77 | n.a. |
| T2 (256) | 73 | 86 | 59 | - |
| Groome, 2006 [26] | - | - | - | - |
| T1 (491) | 82 | 93 | 77 | n.a. |
| T2 (213) | 63 | 81 | 70 | n.a. |
| Chera, 2010 [27] | - | - | - | - |
| T1a (253)/T1b (72) | 94/93 | 97/99 | 82/83 | n.a. |
| T2a (163)/T2b (95) | 80/70 | 94/90 | 76/78 | n.a. |
| Tong, 2012 [28] | - | - | - | - |
| T1a (324) /T1b (109) | 92/89 | 98 | 89 | 87 (T1+2) |
| T2 (262) | 79 | 98 | 89 | - |
| Al-Mamgani, 2013 [29] | - | - | - | - |
| T1 (719) | 92 | n.a. | n.a. | n.a. |
| T2 (331) | 78 | n.a. | n.a. | n.a. |
| Study Treatment (n pts.) | 5/10-Year OS [%] | 5/10-Year-LP [%] |
|---|---|---|
| Furusaka, 2012 [62] | - | - |
| pRT alone (57) | 88.5/73.5 | 60.4/50.1 |
| Furusaka, 2012 [61] | - | - |
| pCRT (Carbo AUC 1.5) (25) | 83.4/77.0 | 79.0/73.0 |
| pCRT (Carbo AUC 2.0) (25) | 95.7/91.1 | 79.0/73.0 |
| Furusaka, 2013 [63] | - | - |
| pCRT (Cis/5-FU) (32) | 95.3/95.3 | 75.1/75.1 |
| Category | Variable | Risk Points | |||
|---|---|---|---|---|---|
| T classifaction | T1–T2 | 0 | |||
| T3–T4 | 4 | ||||
| Neck irradiation | Primary alone ± ipsilateral | 0 | |||
| Primary ± bilateral | 9 | ||||
| Weight loss | none | 0 | |||
| 1–10% | 5 | ||||
| >10% | 7 | ||||
| Primary tumor site | Larynx | 0 | |||
| Hypopharynx | 5 | ||||
| Oropharynx | 7 | ||||
| Nasopharynx | 9 | ||||
| Treatment modality | Conventional RT | 0 | |||
| Accelerated RT | 6 | ||||
| Concomitant CRT | 5 | ||||
| - | |||||
| Sum RP = TDRS: | 0–9 RP | 10–18 RP | >18 RP | ||
| Low risk | Interm. risk | High risk | |||
| ≤10% | 10–30% | >30% | |||
| Individual risk group: |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyckhoff, G.; Warta, R.; Herold-Mende, C.; Rudolph, E.; Plinkert, P.K.; Ramroth, H. Could Primary Chemoradiotherapy in T2 Glottic Cancers Yield Results Comparable to Primary Radiotherapy in T1? Considerations from 531 German Early Stage Patients. Cancers 2021, 13, 1601. https://doi.org/10.3390/cancers13071601
Dyckhoff G, Warta R, Herold-Mende C, Rudolph E, Plinkert PK, Ramroth H. Could Primary Chemoradiotherapy in T2 Glottic Cancers Yield Results Comparable to Primary Radiotherapy in T1? Considerations from 531 German Early Stage Patients. Cancers. 2021; 13(7):1601. https://doi.org/10.3390/cancers13071601
Chicago/Turabian StyleDyckhoff, Gerhard, Rolf Warta, Christel Herold-Mende, Elisabeth Rudolph, Peter K. Plinkert, and Heribert Ramroth. 2021. "Could Primary Chemoradiotherapy in T2 Glottic Cancers Yield Results Comparable to Primary Radiotherapy in T1? Considerations from 531 German Early Stage Patients" Cancers 13, no. 7: 1601. https://doi.org/10.3390/cancers13071601
APA StyleDyckhoff, G., Warta, R., Herold-Mende, C., Rudolph, E., Plinkert, P. K., & Ramroth, H. (2021). Could Primary Chemoradiotherapy in T2 Glottic Cancers Yield Results Comparable to Primary Radiotherapy in T1? Considerations from 531 German Early Stage Patients. Cancers, 13(7), 1601. https://doi.org/10.3390/cancers13071601






