Simple Summary
Intracellular Ca2+ signaling is a critical factor in breast cancer metastasis. In the proliferation stage, increases in intracellular Ca2+ concentration through voltage-dependent Ca2+ channels, P2Y2 channels, transient receptor potential (TRP) channels, store-operated Ca2+ channels (SOCCs), and IP3 receptors and a decrease in intracellular Ca2+ concentration through plasma membrane Ca2+ ATPases and secretory pathway Ca2+ ATPases (SPCA) activate breast cancer cell proliferation. TRPM7, SOCC, inositol trisphosphate receptor (IP3R), ryanodine receptor (RyR), and sarco-/endo-plasmic reticulum Ca2+-ATPase (SERCA) increase the expression of epithelial-to-mesenchymal transition (EMT)-related proteins; meanwhile, SPCA and the Na+/Ca2+ exchanger (NCX) control the activation of EMT-related proteins. Increased Ca2+ through TRPC1, TRPM7/8, P2X7, and SOCC enhances breast cancer cell migration. The stromal interaction molecule (STIM)-Orai complex, P2X7, and Ca2+ sensing receptors are involved in invadopodia. Various pharmacological agents for Ca2+ channels have been proposed against breast cancer and have provided potential strategies for treating metastatic processes.
Abstract
Metastatic features of breast cancer in the brain are considered a common pathology in female patients with late-stage breast cancer. Ca2+ signaling and the overexpression pattern of Ca2+ channels have been regarded as oncogenic markers of breast cancer. In other words, breast tumor development can be mediated by inhibiting Ca2+ channels. Although the therapeutic potential of inhibiting Ca2+ channels against breast cancer has been demonstrated, the relationship between breast cancer metastasis and Ca2+ channels is not yet understood. Thus, we focused on the metastatic features of breast cancer and summarized the basic mechanisms of Ca2+-related proteins and channels during the stages of metastatic breast cancer by evaluating Ca2+ signaling. In particular, we highlighted the metastasis of breast tumors to the brain. Thus, modulating Ca2+ channels with Ca2+ channel inhibitors and combined applications will advance treatment strategies for breast cancer metastasis to the brain.
1. Introduction
Cancer metastasis occurs in several stages, including proliferation, epithelial-to-mesenchymal transition (EMT), invasion, transport, colonization, and angiogenesis (Figure 1) [1]. In fully developed tumorigenesis stages, circulating tumor cells move into another tissue and transform into mesenchymal stem cell-like cells as a result of EMT [2,3]. EMT is the initiation step in cancer metastasis [4]. Tumor cells are transported through the bloodstream after invading blood vessels [5,6,7] in a process called intravasation [8]. The metastasized tumor cells attach and grow via colonization; then, the blood vessels that supply nutrients are generated during angiogenesis, leading to cancer development [2,9,10]. In many stages of metastasis, the proteins and factors related to metastasis are intricate [11]. Therefore, messenger signaling to block metastasis and tumorigenesis is necessary for the fundamental processes that regulate initial signaling factors, but protein signaling is not. Breast cancer is the most common cancer type, and it has been considered one of the most malignant cancers in women worldwide [12,13]. Breast cancer subtypes include triple-negative and triple-positive breast cancer resulting from the existence and nonexistence of estrogen receptors, progesterone receptors, or human epidermal growth factor receptor-2 (HER2) [14,15]. Each subtype has the following cell lines: triple-negative (MDA-MB-231, MDA-MB-486, and MCF-10A [16,17]), triple-positive (BSMZ, BT474, and EFM192A [16]), and hormone receptor-positive cell lines that express estrogen receptors and progesterone receptors in the absence of HER2 (MCF-7 and T47D [16]). Genotypic or phenotypic heterogeneity of breast cancer is diverse. While triple-negative breast cancer generally has the most aggressive behavior and poor clinical outcomes [18,19,20], triple-positive breast cancer has also been found to exhibit aggressive behavior, despite the availability of antibody-targeted therapy or chemotherapy [21].
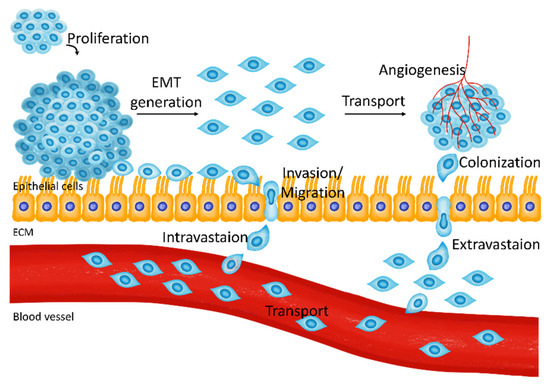
Figure 1.
The metastatic pathway of breast cancer cells. Proliferated breast cancer cells are transformed into mesenchymal-like cells and undergo invasion and intravasation to blood vessels. Transporting tumor cells perform extravasation from blood vessels and generate a cancerous environment through colonization and angiogenesis.
In this review, we elucidate the essential processes of metastasis in breast cancer. In particular, Ca2+ signaling molecules are introduced, and the processes involved in the fundamental modulation of Ca2+ signaling modules and potential strategies against breast cancer are addressed.
1.1. Ca2+ Signaling-Associated Molecules
The physiological role of Ca2+ signaling is commonly known to include muscle contraction to crosslink actin, myosin, and muscle fibers [22]. In addition, Ca2+ signaling regulates physiological and pathological cellular pathways, including cell proliferation, differentiation, migration, muscle contraction, neurotransmitter release, and fluid secretion [23,24,25]. The regulation of cellular Ca2+ as a key signaling messenger is precisely modulated by numerous Ca2+ channels and transporters associated with the membrane of intracellular compartments or plasma membranes [26]. The concentration of intracellular Ca2+ ([Ca2+]i) in the resting state is sustained up to 100 nM, allowing the use of evoked Ca2+ for the signaling pathways involved in cellular functions [26]. Generally, evoked intracellular Ca2+ passes through the endoplasmic reticulum (ER) membrane via two mechanisms: the outward movement to the cytoplasm through the inositol trisphosphate receptor (IP3R) [27,28,29] and the ryanodine receptor (RyR) from intracellular Ca2+ stores [30,31,32]. Elicited Ca2+ is attenuated by movement into the ER via sarco-/endoplasmic reticulum Ca2+-ATPase (SERCA) or movement to the extracellular matrix via plasma membrane Ca2+-ATPase (PMCA) [33,34]. The types of Ca2+ channels in the plasma membrane are discussed in the following section. RyR is activated by several molecules or drugs such as cADP ribose [35,36], 4-chloro-m-cresol [37], and suramin [38], and SERCA is stimulated by adenosine triphosphate (ATP) [34]. IP3R is activated by IP3 via extracellular signaling through the hydrolysis of phosphatidylinositol 4,5-bisphosphate by phospholipase C (PLC) [27,29,39].
1.2. Types of Ca2+ Channels
Plasma membrane-localized Ca2+ channels are classified into three types: voltage-gated Ca2+ channels (VGCCs), ligand-gated Ca2+ channels (LGCCs), and store-operated Ca2+ channels (SOCCs) [26]. VGCCs are stimulated by depolarization of the plasma membrane through a concentration gradient of [Ca2+]i [40]. Exceptionally, the Na+/Ca2+ exchanger (NCX) is activated by changes in Ca2+ concentration to control [Ca2+]i by exchanging Ca2+ and Na+ [41]. LGCCs in the plasma membrane are composed of numerous types of channels, such as ATP receptors and ionotropic glutamate receptors (e.g., α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors or N-methyl-d-aspartate receptors) [42,43,44]. LGCCs in the plasma membrane, which are structurally classified as G protein-coupled receptors (GPCRs), consist of seven transmembrane domains and have the largest number of subtypes in cell-surface receptor groups [45]. IP3R, RyR, and two-pore Ca2+ channels are also present in LGCCs. As there are numerous GPCR subfamilies, these receptors are targets of various therapeutic drugs [46]. In particular, GPCRs are stimulated by neurotransmitters and neurotransmitter-like agonists, including noradrenaline and cholinergic compounds and molecules such as carbamylcholine and vasopressin [47]. Stimulation of GPCRs is initiated by the phosphorylation of guanidine diphosphate for guanidine triphosphate through the α-subunit of G-proteins (Gα) [48]. Activated G-proteins dissociate Gα to deliver several intracellular signals according to each Gα subunit: Gs (adenylyl cyclase increases), Gi (adenylyl cyclase decreases), and Gq (PLC increases) [49]. Each signal mediates a tremendous number of cellular functions by increasing or decreasing Ca2+ signaling. Lastly, SOCCs are affected by decreasing the concentration of Ca2+ in the ER by delivering signals from the oligomerized stromal interaction molecule (STIM), which senses Ca2+ depletion on the ER membrane and subsequently activates Orai channels [50,51]. In addition, transient receptor potential (TRP) channels play various important roles in the cell life cycle, including the transduction of neurotransmitters and immunization, and are activated by temperature, pH, and specific compounds [52,53]. Furthermore, SOCC-related proteins, including Orais and STIMs, show different characteristics against the estrogen receptor [54]. In the estrogen receptor-positive cell line MCF-7, store-operated Ca2+ entry (SOCE) is mediated by the combination of STIM1/2 and Orai3; meanwhile, in the estrogen receptor-negative cell line MDA-MB-231, SOCE is mediated by the combination of STIM1 and Orai1 in activated SOCCs [54].
Recent studies have demonstrated that Ca2+ plays a crucial role not only in malignant proliferation but also in cancer metastasis [55,56,57,58,59,60]. Breast cancer is considered a metastatic cancer due to its aggressive features [61]. Thus, in this review, we focus on the characteristics of breast cancer in the context of Ca2+ signaling and cancer metastasis, especially from breast to brain. Furthermore, we discuss potential strategies to overcome the disadvantages of breast cancer-targeted therapy, taking Ca2+ signaling into consideration.
2. The Relationship between Breast Cancer Metastasis and Ca2+ Channels
The large range of physiological, pharmacological, and clinical roles of Ca2+ signaling in breast cancer are well known, including the expression patterns of the channels, the effect of channel activity on tumorigenesis, and therapeutic targets [62,63,64]. Several Ca2+-related proteins and channels are overexpressed in breast cancer cells, including IP3R [65], Orais [66,67,68], PMCA [69,70], and TRP channels [71,72,73,74]. Each channel plays a critical role in cancer cell viability. In this section, we summarize the effects of Ca2+ channels on the growth and metastatic stages of breast cancer.
2.1. Proliferation in the Initial Metastatic Stage
Cancer metastasis is initiated from an excessively developed primary tumor and its subsequent transport to the bloodstream [1]. To treat metastatic tumors, their vigorous proliferation and immoderately active cell cycle must be controlled to block cancer growth. One of the Ca2+-ATPases, secretory pathway Ca2+-ATPase (SPCA, localized on Golgi and transports Ca2+ into the Golgi), has been shown to be activated in breast cancer. This pathway induces tumorigenesis by activating extracellular signal-regulated kinase (ERK)1/2 activity and increasing tumor proliferation [75,76]. SPCA1-silenced MDA-MB-231 (triple-negative) cells with SPCA1 siRNA exhibit a lower rate of cell growth and decreased insulin-like growth factor receptor expression [75]. SPCA2 shows different expression patterns according to the presence of the estrogen receptor [76]. SPCA2 is overexpressed in estrogen receptor-positive MCF-7 cells, but is barely expressed in estrogen receptor-negative MCF-10A cells [76]. SPCA2 silencing with shRNA decreases MCF-7 cell proliferation; however, SPCA2 overexpression increases MCF-10A cell proliferation [76]. SPCA2 can induce store-independent Orai1 Ca2+ influx [77]. The increased proliferation of breast cancer cells (MCF-7 wild-type and SPCA2-overexpressed MCF-10A) is induced by Ca2+ influx from SPCA2-stimulated Orai1 [76]. Inhibition of PMCA2 decreases cancer proliferation [78] and leads to cancer cell death [79] in MDA-MB-231 cells. Additionally, downregulation of PMCA2, which is overexpressed in MDA-MB-231 cells, enhances the anticancer effect of doxorubicin [78]. T-type VGCC, CaV3.1, and CaV3.2 are blocked by NNC-55-0396, a T-type Ca2+ channel blocker, and each relevant siRNA attenuates breast cancer proliferation regardless of estrogen receptor-positive or -negative cell lines (MCF-7, MDA-MB-231, and MCF-10A) [80]. Increased [Ca2+]i through IP3R3 induces MCF-7 breast cancer cell growth [81]. Additionally, TNF-αinduces the release of ATP, and the ATP-stimulated Ca2+ channel P2Y2 receptor induces tumor growth and invasion of MDA-MB-231 cells (estrogen receptor-negative), but MCF-7 (estrogen receptor-positive) cells, which has a low metastatic feature, induces less release of ATP and reveals low P2Y2 receptor activation [82].
TRP channels play critical roles in cell viability by increasing channel activities, including TRP canonical (C), TRP melastatin (M), and TRP vanilloid (V) in breast cancer cells and tissues [74,83,84,85]. TRP channels are classified into six subfamilies, including TRPC, TRPM, TRPV, TRP ankyrin (A), TRP canonical (C), and TRP mucolipin (ML), which are composed of six transmembrane domains [52,53]. These nonselective Ca2+-permeable channels have a superfamily of at least 20 subtypes that function in various ways [26,52] and can regulate breast cancer tumorigenesis. TRPC channels, including TRPC3, TRPC5, and TRPC6, are typical oncogenic proteins that can be used to diagnose breast cancer [72,84,86]. Increases in extracellular Ca2+ concentration ([Ca2+]ex) induce overexpression of the TRPC1 channel and increase proliferation through epidermal growth factor receptor (EGFR) signals with ERK1/2 phosphorylation in MCF-7 cells [83]. TRPC6 is more highly expressed than other TRPC channels in human breast cancer MCF-7/MDA-MB-231 cell lines (regardless of breast cancer subtypes) and tissues, but TRPC3 is highly expressed only in the estrogen receptor-negative MDA-MB-231 cells and tissues [84]. In particular, activated TRPC6 increases cellular proliferation [84]. TRPM7 is overexpressed in human breast adenocarcinoma tissue, whereas TRPM7 silencing attenuates MCF-7 cell proliferation [85]. Increased [Ca2+]i induces cell proliferation through TRPV6-mediated Ca2+ influx [87] and TRPV6 inhibition, which are upregulated by sex hormones leading to T47D (hormone receptor-positive) cancer cell death [74]. A schematic illustration of Ca2+ channels that increase breast cancer proliferation is shown in Figure 2.
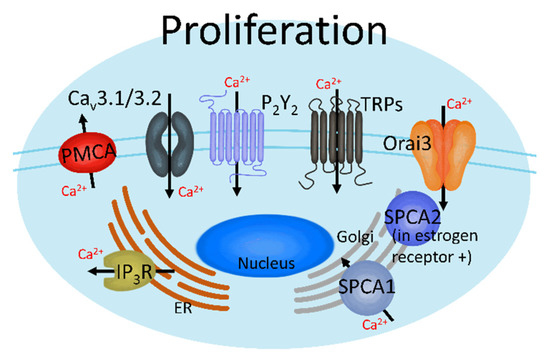
Figure 2.
Ca2+ channels induce breast cancer proliferation. These channels can regulate breast cancer cell proliferation by activating each Ca2+ transporter, which increases or decreases intracellular Ca2+ ([Ca2+]i).
2.2. EMT
To invade blood vessels, primary tissue cells must undergo cellular shape and construction transformation, which is known as EMT. In other words, epithelial tumor cells that reside in the primary tumor tissue turn into mesenchymal tumor cells that surround the bloodstream and invade the extracellular matrix (ECM) [3,88]. During EMT, the expression of several Ca2+ channels and transporters is modulated by transforming growth factor (TGF-β) [89] and epidermal growth factor (EGF) [90] in human breast cancer cells (Figure 3). In TGF-β-induced MCF-7 EMT, IP3R, and SERCA3 proteins are overexpressed, while NCX1 is downregulated [89]. TGF-β stimulates store-operated Ca2+ entry (SOCE) by upregulating STIM1 and Orai1 expression through the overexpression of the transcription factor Oct4 in MCF-7 cells (estrogen receptor-positive), but not in MDA-MB-231 cells (estrogen receptor-negative) [91]. SERCA2, IP3R1/3, RyR2, and Orai1 are overexpressed during EGF-induced EMT in MDA-MB-468 [90,92]. Additionally, EGF-stimulated EMT, which transforms breast cancer MDA-MB-468 cells into a mesenchymal-like shape, increases the expression of ATP-binding cassette subfamily C member 3 (ABCC3) after Ca2+ signaling of TRPC1 [93].
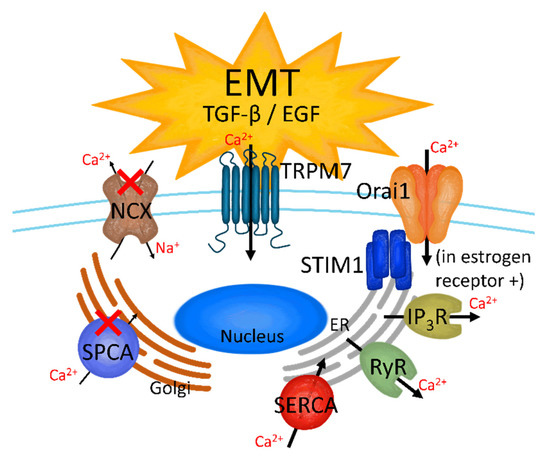
Figure 3.
The effect on Ca2+ channels in TGF-β- and EGF-induced EMT breast cancer cells. TRM7, store-operated Ca2+ channels (SOCC), inositol trisphosphate receptor (IP3R), ryanodine receptor (RyR), and sarco-/endo-plasmic reticulum Ca2+-ATPase (SERCA) are activated when the breast cancer cells are stimulated by TGF-β or EGF; on the other hand, SPCA and NCX are downregulated in the TGF-β- and EGF-induced EMT stage.
The Ca2+-ATPase SPCA2, which is encoded by the ATP2C2 gene, is an epithelial marker that inhibits cell-adhesion protein E-cadherin biogenesis in breast cancer cells regardless of estrogen receptor existence (MCF-7 and MDA-MB-231) [94]. Activation of SPCA induces cell-to-cell contacts and continuously stimulates E-cadherin-induced Hippo-YAP signaling to inhibit EMT formation in MCF-7 cells [94]. Meanwhile, SPCA2-silenced MCF-7 cells show EGF-induced expression of EMT-related proteins, including zinc finger E-box-binding homeobox 1, N-cadherin, snail family transcription repressor 2, fibronectin, and vimentin [94]. Interestingly, SPCA2 upregulation in MDA-MB-231 cells decreases EMT-related protein expression and attenuates metastasis in the MDA-MB-231-injected breast cancer mouse model [94]. SOCE intensifies TGF-β-induced EMT in MDA-MB-231 and MCF-10A cells by upregulating STIM1 [95] and TRPC1 [96]. Additionally, TRPC1 is involved in the regulation of hypoxia-induced EMT in MDA-MB-468 cells [97]. As EMT regulatory factors, signal transducer and activator of transcription 3 (STAT3) phosphorylation and vimentin expression are induced by Ca2+ signaling through TRPM7 [98]. To initiate EMT, the binding among primary cells must collapse, and there must also be a reduction in the expression of the binding protein E-cadherin [99]. Thus, Ca2+ signaling can attenuate E-cadherin expression [100]. EMT is also activated by hypoxia and vascular endothelial growth factor (VEGF) [101]. Furthermore, ATP-mediated Ca2+ increase-stimulated EMT is induced by the stimulation of EGF and hypoxia in MDA-MB-468 and MDA-MB-231 cells [102,103]. Treatment with a Ca2+ chelating agent such as 1,2-bis (o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) or ethylene glycol-bis (β-aminoethyl ether)-N,N,N′N′-tetraacetic acid (EGTA) also attenuates EGF- or hypoxia-induced EMT, which is stimulated by TRPM7 Ca2+ signaling in MDA-MB-468 cells [98].
2.3. Migration and Intravasation
Breast cancer cells are transported to target tissues by migrating through blood vessels and invading the bloodstream. Intracellular Ca2+ signaling regulates cellular movement with proteins that induce migration (Figure 4), including myosin light chain kinase [104], myosin II [105,106], calpain [107], Ca2+/calmodulin-dependent protein kinase II (CaMKII) [108,109], and focal adhesion kinase (FAK) [110]. In breast cancer cells (MDA-MB-231), SOCE induces cellular migration [111]. Knockdown of Orai1 mRNA expression attenuates breast cancer cell migration, and Orai1 overexpression induces migration through increased Ras and Rac levels, using defects in focal adhesion to move the cells [111]. Migratory cells are needed to alter cytoskeletal structures with the formation of invadopodia to adhere to the forward region in the direction of progress and degrade the ECM [112,113]. STIM1 knockdown in MDA-MB-231 cells attenuates invadopodia formation and cancer invasion [114]. Additionally, elevated [Ca2+]i, which is induced by phospholipase C, activates the ERK 1/2 signaling pathway and subsequently stimulates MDA-MB-231 breast cancer cell migration [115]. The expression of each TRP subtype can be distinguished according to the histologic grade and invasive degree of cancer [72]. TRPM8 and TRPC1 are generally expressed in tissues smaller than 2 cm and in grade I tissue, and TRPM7 is expressed in tissue larger than 2 cm and in grade II tissue [72]. In addition, TRPM7 induces breast cancer migration regardless of the cellular subtypes (MDA-MB-231 and MCF-7) [116]. Although TRPC6 and TRPV6 have no relationship with histologic grade, TRPV6 is found in the invasive area, and silencing of TRPV6 expression reduces breast cancer cell migration [72].
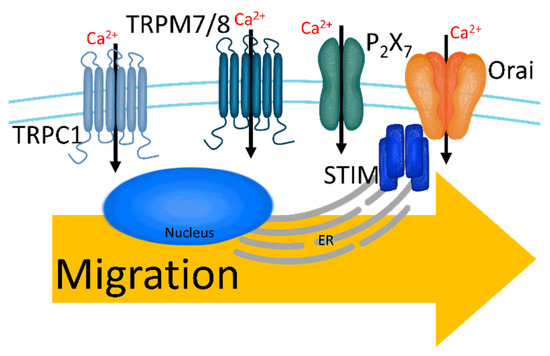
Figure 4.
The upregulated Ca2+ channels in migratory breast cancer cells. Increased [Ca2+]i through TRPC1, TRPM7/8, P2X7, and SOCC induces migration and intravasation.
For intravasation, invadopodia must be established [112]. Invadopodia formation is initiated by the activation of the nonreceptor tyrosine kinase Src (proto-oncogene tyrosine-protein kinase) [117,118,119]. STIM1-Orai1 stimulation induces Src activation and recruits metalloproteinases that degrade the ECM, leading to invadopodia formation in MCF-7 cells [120]. The ATP-gated channel P2X7 induces the release of gelatinolytic cysteine cathepsins from invadopodia to degrade ECM in MDA-MB-435 cells (triple-negative breast cancer) [121]. [Ca2+]ex and [Ca2+]i also regulate the intravasation of breast cancer cells through the extracellular Ca2+-sensing receptor (CaSR) with mitogen-activated protein kinases [122]. Increased [Ca2+]ex activates CaSR and subsequently stimulates EGFR to induce invasion, whereas CaSR knockdown attenuates the intravasation of MDA-MB-231 cells [122].
2.4. Colonization and Angiogenesis
Tumor cells invading other tissues colonize by clustering their cells to form tumor tissue and carry out angiogenesis, thus creating an appropriate environment for tumorigenesis. Although it has a significant role in brain metastasis, the relationship between colonization and Ca2+ signaling has not been clearly elucidated. The mitochondrial Ca2+ uniporter is located at the mitochondrial membrane of breast cancer cells and regulates tumor proliferation [123,124,125], and triple-negative breast cancer cells (MDA-MB-231 and BT-549) express this transporter to a greater degree than non-triple-negative breast cancer cells (T47D, BT-474, and MCF-7) [126]. Downregulation of the mitochondrial Ca2+ uniporter through extracellular vesicles suppresses MDA-MB-231 cell colonization [126]. Additionally, the Ca2+-binding protein S100A4 promotes MDA-MB-231 cell colonization [127,128]. S100A4 is an agonist of GPCR signaling, including the PLCβ-IP3 pathway [129], which is known to promote metastasis in breast cancer [130]. S100A4 knockdown reduces the brain colonization of MDA-MB-231 cells [127], and S100A4 expression is increased in colonized MDA-MB-231 cells [128].
IP3R is located within the ER membrane and is also expressed in the nuclear envelope [131]. Ca2+ signaling through IP3R in the nucleus plays a critical role in inducing angiogenesis in breast cancer cells (MDA-MB-468) and regulates angiogenesis-related genes, including early growth response-1, C-X-C motif chemokine ligand 10 (CXCL10), C-C motif chemokine ligand (CCL)-2, and dentin matrix acidic phosphoprotein 1 (DMP1) [132]. S100A4 plays critical roles in various angiogenic pathways in MDA-MB-231 cells through upregulation of matrix metalloproteinase-13 [133], TGF-β1-induced ERK1/2 signaling [134], and osterix, which is a transcription factor for bone formation [135]. In addition, a recent study showed that SOCE activity is elevated by angiotensin-converting enzyme (ACE)2/angiotensin-1(1–7) according to breast cancer cell subtypes [136]. (ACE)2/angiotensin-1(1–7), which is more highly expressed in MCF-7 cells than in MDA-MB-231 cells, increases SOCE to inhibit migration [136]. (ACE)2/angiotensin-1(1–7)-silenced MCF-7 cells show decreased migration, while (ACE)2/angiotensin-1(1–7)-overexpressing MDA-MB-231 cells show increased migration [136].
3. Metastasis of Breast Tumor to the Brain
Brain metastasis from breast cancer is the most common, and secondary brain tumors from breast cancer are diagnosed more often than primary brain tumors [137]. Metastatic features of cancer cells toward specific organs were demonstrated in Stephen Paget’s seed and soil theory, which was analyzed using autopsy records of breast cancer patients [138]. The microenvironment of cancer cells facilitates metastasis (seed growth) under favorable circumstances. Paget postulated that various cytokines secreted from cancer cells, including CCL2, CCL5, and interleukin-6, and a specific microenvironment communicates to attract cancer cells toward specific organs [138]. Accordingly, roughly 20% of breast cancer patients show central nervous system metastases, and these cases are increasing [139].
Ca2+ signaling in breast cancer is prominent in breast cancer metastasis. Therefore, controlling the activity of Ca2+ channels in breast tumor cells can lead to new therapeutic methods for brain metastases resulting from breast cancer. Several studies have suggested Ca2+ channels as new therapeutic targets for metastasis. Additional investigations have addressed the attenuation of Ca2+ signaling, which modulates the adhesive function and permeability. For example, MDA-MB-231 cells can cross the human brain microvascular endothelial cell (HBMEC) monolayer by stimulating vascular permeability factor (VPF) [140] and stromal cell-derived factor-1α (SDF-1α) [141]. VEGF/VPF stimulation also increases MDA-MB-231 cell adhesion onto the HBMEC monolayer and induces the redistribution of F-actin and disruption of vascular endothelial cadherin, which increases migration [140]. Treatment with the Ca2+-chelating agent BAPTA attenuates VEGF/VPF-induced cell adhesion, F-actin redistribution, and cadherin disruption [140]. Treatment with SDF-1α increases FAK phosphorylation by stimulating PI-3K signaling, which increases MDA-MB-231 cell migration, and SDF-1α is overexpressed in breast tissues compared to in normal tissues [141]. BAPTA attenuates SDF-1α-induced cellular permeability in a co-culture of MDA-MB-231 cells and HBMECs [141]. Beyond the role of Ca2+ signaling in adhesion and permeability, additional mechanisms of cell-cell crosstalk present a challenge in the metastatic process.
Additionally, Sharma et al. showed that regulating CaV3.2 through specific radiofrequencies with an amplitude of 27.12 MHz attenuated brain metastatic breast cancer cells in vivo [142]. The study demonstrated that specific frequencies modulate several cancers in patients receiving noninvasive cancer therapy. The mouths of the patients were used to deliver frequencies via antenna, and it was found that a frequency of 27.12 MHz was breast cancer-specific [142]. These frequencies revealed antitumor effects in a xenograft mouse model and in brain tumor patients, suppressing brain metastases from breast cancer [142]. As previously mentioned, activation of CaV3.2 through 27.12 MHz frequencies increases Ca2+ influx to the activated p38 pathway, which attenuates tumor progression [142].
Mechanosensitive Ca2+ channels are also involved in the metastasis of cells. Piezo channels, expressed in MCF-7 cells, regulate intracellular functions such as integrin activity in HeLa cells [143], regulation of neuronal-glial specification in human neuronal stem cells [144], maintenance of homeostatic cell numbers in epithelia [145], and sensing confinement of Chinese hamster ovary (CHO) cells [146]. Piezo2 is involved in mechanotransduction and force transmission in MDA-MB-231 cells [147]. Piezo2 activation is required for actin cytoskeletal reorganization and FAK phosphorylation through Fyn kinase [147]. Piezo2 activates the RhoA signaling cascade to promote brain metastasis in breast cancer. Piezo2 knockdown decreases the invasion of MDA-MB-231-BrM2 cells, which metastasize cells from breast cancer to the brain [147]. Additionally, when triple-negative breast cancer cells migrate to the brain, astrocytes activate the S100A4-related pathway (protocadherin 7 (PCDH7)-PLCβ-Ca2+-CaMKII/S100A4) [148]. In brain metastasis tissues from patients, PCDH7 expression is higher than that in lung metastasis tissue and mediates cellular interaction between astrocytes and cancer cells [148]. Furthermore, PCDH7 expression induces the penetration of tumor cells over the blood-brain barrier, which then increases tumor cell intravasation in the brain [148]. The cell-to-cell interaction between mouse astrocytes and MDA-MB-231 cells in the PCDH7-stimulated mouse model activated PLCβ-Ca2+-CaMKII/S100A4 signaling in MDA-MB-231 cells [148]. Moreover, the brain is highly responsive to estrogen [149], and brain metastasis is revealed in estrogen receptor-positive areas. Interestingly, estrogen receptor-negative breast cancer cells can be affected by estrogen through the involvement of astrocytes [127]. When astrocytes are stimulated by estrogen, the migratory ability of MDA-MB-231 cells cocultured with astrocytes reportedly increases, according to a wound-healing migration assay [127]. In this case, silencing S100A4 expression attenuates astrocyte-induced migration and colonization of MDA-MB-231 cells [127]. As mentioned previously, Ca2+ signaling is crucial for cancer development and progression. Therefore, more studies focusing on the relationship between cancer and Ca2+ should be conducted.
4. The Pharmacological Application of Ca2+ Signaling Blockers to Breast Cancer
Various attempts to use antagonists of Ca2+ channels have been proposed to control breast cancer tumorigenesis. The mediation of [Ca2+]i signaling is critical for cellular functions regardless of the cellular type (tumor vs. nontumor). In other words, the application of Ca2+ signaling blockers for anticancer drugs requires in-depth studies of the basic mechanisms underlying Ca2+ signaling and cancer cells. Thus, we summarized the studies that have used Ca2+ channel blockers for breast cancer medication to understand the associated mechanisms (Table 1). Recent studies have shown that the L-type Ca2+ channel blockers amlodipine, diltiazem, and verapamil have been used to modulate high blood pressure [150,151,152] and attenuate HT39-transplanted breast cancer growth [153]. Mice with increased Ca2+ concentration in serum exhibit a larger amount of HT39 tumor tissue, while treatment with amlodipine attenuates Ca2+ signaling in HT39 cells with a decrease in tumor size [153]. The T-type Ca2+ channel blockers mibefradil (another hypertension drug [154]) and pimozide (chronic psychosis drug [155]) inhibit MCF-7 breast cancer cell growth by inhibiting T-type Ca2+ current; furthermore, combined treatment with pimozide and mibefradil shows synergistic effects on cell growth in MCF-7 cells, decreasing cell growth [156].
As mentioned in Section 2.1, TRP channels are prominent in breast cancer. Among these, TRPM channels are considered therapeutic targets for antagonists. The TRPM7 inhibitor 2-aminoethyl diphenylborinate (2-APB [157]) attenuates MDA-MB-231, AU565, and T47D cell proliferation, increasing S phase and decreasing G0/G1 phase in the breast cancer cell cycle [158]. Moreover, TRPM7-silenced MDA-MB-231 cells have no antitumor effects when 2-APB is administered [158]. Treatment with the antifungal agent clotrimazole, which inhibits TRPM2 activity [159], decreases MDA-MB-231 cell invasion, which is accompanied by apoptosis and G1-phase arrest [160]. Clotrimazole increases cleaved poly (ADP-ribose) polymerase (PARP), cleaved caspase-3, and B-cell lymphoma-2 (Bcl-2)-associated X expression, which induces apoptotic signaling in MDA-MB-231 cells [160]. Inhibition of Ca2+ signaling with the voltage-independent Ca2+ channel inhibitor carboxyamidotriazole reduces MCF-7 proliferation by arresting G2/M phase cell cycle, decreasing BCL-2 (which blocks apoptotic signaling) expression, and increasing p21 expression, which induces apoptotic signaling [161]. Furthermore, treatment with carboxyamidotriazole reduces mitochondrial membrane potential [161], which is highly activated in cancer stem cells to produce reactive oxygen species (ROS) [162]. In addition, administration of the SERCA inhibitor thapsigargin inhibits S100A4 protein expression in MDA-MB-231 breast cancer cells [163].

Table 1.
The Ca2+ channel blockers with potential anticancer effects.
Table 1.
The Ca2+ channel blockers with potential anticancer effects.
| Reagents | Description | Effect | Ref. |
|---|---|---|---|
| Amlodipine | Medication for high blood pressure and L-type Ca2+ channel inhibitor | Decrease of HT39-transplanted breast cancer growth | [153] |
| Diltiazem | |||
| Verapamil | |||
| Mibefradil | Hypertension drug | Decrease of MCF-7 growth through inhibition of T-type Ca2+ current | [154] |
| Pimozide | Chronic psychosis drug | [155] | |
| 2-APB | TRPM7 inhibitor | Decrease of MDA-MB-231, AU565, and T47D cell growth through pausing cell cycle | [158] |
| Clotrimazole | TRPM2 inhibitor | Decrease of MDA-MB-231 cell growth through G1-phase arrest | [160] |
| Carboxyamidotriazole | Reduce mitochondrial membrane potential | Attenuation of ROS | [162] |
| Thapsigargin | SERCA inhibitor | Inhibition of S100A4 expression in MDA-MB-231 | [163] |
In addition, Ca2+ channel blockers enhance the therapeutic effect of traditional drugs or overcome resistance to insignificant drugs. In an attempt to improve their therapeutic effect on breast cancer, mibefradil enhanced the apoptotic effect of the anticancer drug 2-deoxy-D-glucose (2-DG) by arresting the cell cycle in MDA-MB-231 cells [164]. Furthermore, clotrimazole increases the inhibitory effect of imatinib mesylate on T74D cells to mediate kinase inhibition [165]. Mibefradil is a T-type Ca2+ channel blocker that arrests the cell cycle at the G1 phase and evaluates glucose metabolism [164]. The application of only 2-DG also inhibits MDA-MB-231 cell growth. Although the inhibition rate is very low (approximately 10%), the combination of mibefradil and 2-DG leads to a synergistic antitumor effect (approximately 30% of inhibition rate) [164]. The combination of imatinib mesylate and clotrimazole synergistically decreases T74D cell growth by increasing lactate dehydrogenase and nitric oxide leakage [164], which induces membrane damage and apoptosis in cancer cells [166,167]. Doxorubicin and daunorubicin are the most well-known anthracycline antibiotics and are also first-line drugs for malignancies [168]. They have structural features that can be intercalated into DNA bases and inhibit topo ii/DNA ternary complexes [169]. Additionally, the quinone ring, a common structure for anthracyclines such as doxorubicin and daunorubicin, induces ROS production [170,171,172,173]. Doxorubicin and daunorubicin are typical anticancer reagents; however, they are hindered by multidrug resistance in breast cancer [174,175]. The addition of diltiazem to doxorubicin-treated MCF-7 cells increases the expression of apoptosis-related p53 genes [174]. The combination of daunorubicin and amlodipine reportedly predominantly attenuates tumor volume in the MCF-7 xenograft tumor model via mitochondrial destruction [175]. Despite these applicable combinations, more studies on effective combinations of Ca2+ channel blockers and traditional anticancer drugs should be conducted. These combined treatments are suggested as novel therapeutic strategies against breast cancer and breast-to-brain metastatic cancer.
As mentioned above, Ca2+ channel blockers have pharmacological potential. However, the therapeutic application of Ca2+ channel blockers is challenging, as each reagent does not act on a single channel or transporter. The TRPM7 inhibitor 2-APB inhibits IP3R [176], Orai1/2-induced SOCE [177], and other TRP channels [178]. In contrast, 2-APB induces Orai3-induced Ca2+ influx [177]. Additionally, the Ca2+ channel blocker clotrimazole can inhibit Ca2+-activated potassium channel 3.1 [179], which drives Ca2+ through SOCE [180]. Although several Ca2+ channel blockers are pharmacologically complicated to use as therapeutic strategies, the specific mechanisms of Ca2+ channel blockers need to be clarified.
5. Future Perspective
The relationship between Ca2+ channels and breast cancer has been assessed for several decades; however, the effect of Ca2+ channels on the metastasis of breast cancer to the brain requires further investigation. The treatment of breast cancer by modulating Ca2+ channel expression and its activity has been considered a cancer therapeutic strategy using various Ca2+ channel blockers. Although Ca2+ signaling is closely related to cancer metastasis in various organs, the application of Ca2+ channel modulation for breast cancer metastasis has not been sufficiently studied. Based on the scope of metastatic breast cancer in this review, several studies have shown that Ca2+ channels have the potential to control metastatic stages and the movement of metastatic breast cancer cells to the brain by modulating adhesive function and permeability. Over the past several years, the number of cases of brain metastases from breast cancer has increased, and the entire metastatic process has not been fully elucidated. In addition, other metastatic processes should be highlighted beyond adhesive and invasive processes. For example, cellular-secreted processes and gene transcription activities are associated with Ca2+ signaling. In other words, communication between cancer cells and other tissues will commence with the untact mode, such as cytokine release. This mode builds up prior to the contact mode, which includes adhesion. As mentioned at the beginning of this article, Ca2+ is an attractive source of the untact mode for transferring the on-mode of metastatic signals through simple mobilization from abundant sources. Therefore, blocking Ca2+ channels as gatekeepers and modulating Ca2+ signaling can be attractive candidates for therapeutic approaches, and suitable combination therapies are suggested as relevant options for metastatic breast cancer therapy.
Author Contributions
D.L. and J.H.H. contributed to the conception and design of the manuscript; D.L. produced all figures, drafted the article, and revised the article for important intellectual content; J.H.H. supervised the manuscript and contributed to the final approval of the version to be published. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT; [NRF-2019R1F1A1046785]). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
2-APB: 2-aminoethyl diphenylborinate; 2-DG: 2-deoxy-D-glucose; ABCC3: ATP-binding cassette, subfamily C, member 3; ATP: adenosine triphosphate; ACE: angiotensin-converting enzyme; Bcl-2: B-cell lymphoma-2; BAPTA: 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; CXCL10: C-X-C motif chemokine ligand 10; CCL: C-C motif chemokine ligand; CaSR: Ca2+-sensing receptor; CaMKII: Ca2+/calmodulin-dependent protein kinase II; CHO: chinese hamster ovary; DMP1: dentin matrix acidic phosphoprotein 1; ER: endoplasmic reticulum; EGF: epidermal growth factor; EGFR: epidermal growth factor receptor; EGTA: ethylene glycol-bis(β-aminoethyl ether)-N,N,N′N′-tetraacetic acid EMT: epithelial-to-mesenchymal transition; [Ca2+]ex: extracellular Ca2+; ECM: extracellular matrix; ERK: extracellular signal-regulated kinases; FAK: focal adhesion kinase; GPCR: G protein-coupled receptor; HBMEC: human brain microvascular endothelial cell; HER2: human epidermal growth factor receptor 2; human IP3R: inositol trisphosphate receptor; [Ca2+]i: intracellular Ca2+; LGCC: ligand-gated Ca2+ channels, NCX: Na+/Ca2+ exchanger; PARP: poly (ADP-ribose) polymerase; PLC: phospholipase C; PMCA: plasma membrane Ca2+-ATPase; PCDH7: protocadherin 7; ROS: reactive oxygen species; RyR: ryanodine receptor; SERCA: sarco-/endo-plasmic reticulum Ca2+-ATPase;: secretory pathway Ca2+ ATPase; SOCC: store-operated Ca2+ channel; SOCE: store-operated Ca2+ entry; SDF-1α: stromal cell-derived factor-1α; STIM: stromal interaction molecule; STAT3: signal transducer and activator of transcription 3; TGF: transforming growth factor; TRP: transient receptor potential (TRP canonical (C), TRP melastatin (M), and TRP vanilloid (V), TRP ankyrin (A), TRP canonical (C), and TRP mucolipin (ML)); VEGF: vascular endothelial growth factor; VPF: vascular permeability factor; VGCC: voltage-gated Ca2+ channel; Gα: α-subunit of G-proteins.
References
- Gupta, G.P.; Massague, J. Cancer metastasis: Building a framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Ksiazkiewicz, M.; Markiewicz, A.; Zaczek, A.J. Epithelial-mesenchymal transition: A hallmark in metastasis formation linking circulating tumor cells and cancer stem cells. Pathobiology 2012, 79, 195–208. [Google Scholar] [CrossRef]
- Skovierova, H.; Okajcekova, T.; Strnadel, J.; Vidomanova, E.; Halasova, E. Molecular regulation of epithelial-to-mesenchymal transition in tumorigenesis (Review). Int. J. Mol. Med. 2018, 41, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Bockhorn, M.; Jain, R.K.; Munn, L.L. Active versus passive mechanisms in metastasis: Do cancer cells crawl into vessels, or are they pushed? Lancet Oncol. 2007, 8, 444–448. [Google Scholar] [CrossRef]
- Dua, R.S.; Gui, G.P.H.; Isacke, C.M. Endothelial adhesion molecules in breast cancer invasion into the vascular and lymphatic systems. Ejso-Eur. J. Surg. Oncol. 2005, 31, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, I. Matrix metalloproteinases in tumor invasion and metastasis. Semin. Cancer Biol. 2000, 10, 415–433. [Google Scholar] [CrossRef]
- Chiang, S.P.; Cabrera, R.M.; Segall, J.E. Tumor cell intravasation. Am. J. Physiol. Cell Physiol. 2016, 311, C1–C14. [Google Scholar] [CrossRef]
- Fidler, I.J. The organ microenvironment and cancer metastasis. Differentiation 2002, 70, 498–505. [Google Scholar] [CrossRef]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef]
- Neophytou, C.; Boutsikos, P.; Papageorgis, P. Molecular Mechanisms and Emerging Therapeutic Targets of Triple-Negative Breast Cancer Metastasis. Front. Oncol. 2018, 8, 31. [Google Scholar] [CrossRef]
- Rubens, R.D. Metastatic breast cancer. Curr. Opin. Oncol. 1993, 5, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Rojas, K.; Stuckey, A. Breast Cancer Epidemiology and Risk Factors. Clin. Obstet. Gynecol. 2016, 59, 651–672. [Google Scholar] [CrossRef]
- Nwabo Kamdje, A.H.; Seke Etet, P.F.; Vecchio, L.; Muller, J.M.; Krampera, M.; Lukong, K.E. Signaling pathways in breast cancer: Therapeutic targeting of the microenvironment. Cell. Signal 2014, 26, 2843–2856. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas, N. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef] [PubMed]
- Soule, H.D.; Maloney, T.M.; Wolman, S.R.; Peterson, W.D., Jr.; Brenz, R.; McGrath, C.M.; Russo, J.; Pauley, R.J.; Jones, R.F.; Brooks, S.C. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990, 50, 6075–6086. [Google Scholar]
- Shin, H.Y.; Kim, S.H.; Lee, Y.J.; Kim, D.K. The effect of mechanical ventilation tidal volume during pneumoperitoneum on shoulder pain after a laparoscopic appendectomy. Surg. Endosc. 2010, 24, 2002–2007. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Koo, J.S.; Kim, M.S.; Park, H.S.; Lee, J.S.; Lee, J.S.; Kim, S.I.; Park, B.W. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast 2012, 21, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Baeg, S.; Park, I.; Kim, J.; Park, C.; Cho, H.; Yang, K.; Kim, J.; Shin, Y.; Park, K.; Gwak, G. Comparative Study for Clinical Outcomes of Triple-Positive and Triple-Negative Breast Cancer: Long-term Results in 161 Patients Followed in a Single Center. J. Breast Dis. 2020, 8, 78–84. [Google Scholar] [CrossRef]
- Eroles, P.; Bosch, A.; Perez-Fidalgo, J.A.; Lluch, A. Molecular biology in breast cancer: Intrinsic subtypes and signaling pathways. Cancer Treat. Rev. 2012, 38, 698–707. [Google Scholar] [CrossRef]
- Szent-Gyorgyi, A.G. Calcium regulation of muscle contraction. Biophys. J. 1975, 15, 707–723. [Google Scholar] [CrossRef]
- Brini, M.; Cali, T.; Ottolini, D.; Carafoli, E. Neuronal calcium signaling: Function and dysfunction. Cell. Mol. Life Sci. 2014, 71, 2787–2814. [Google Scholar] [CrossRef]
- Vig, M.; Kinet, J.P. Calcium signaling in immune cells. Nat. Immunol. 2009, 10, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Ambudkar, I.S. Calcium signalling in salivary gland physiology and dysfunction. J. Physiol. 2016, 594, 2813–2824. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Parys, J.B.; De Smedt, H. Inositol 1,4,5-trisphosphate and its receptors. Adv. Exp. Med. Biol. 2012, 740, 255–279. [Google Scholar] [CrossRef] [PubMed]
- Kania, E.; Roest, G.; Vervliet, T.; Parys, J.B.; Bultynck, G. IP3 Receptor-Mediated Calcium Signaling and Its Role in Autophagy in Cancer. Front. Oncol. 2017, 7, 140. [Google Scholar] [CrossRef]
- Fedorenko, O.A.; Popugaeva, E.; Enomoto, M.; Stathopulos, P.B.; Ikura, M.; Bezprozvanny, I. Intracellular calcium channels: Inositol-1,4,5-trisphosphate receptors. Eur. J. Pharmacol. 2014, 739, 39–48. [Google Scholar] [CrossRef]
- Amador, F.J.; Stathopulos, P.B.; Enomoto, M.; Ikura, M. Ryanodine receptor calcium release channels: Lessons from structure-function studies. FEBS J. 2013, 280, 5456–5470. [Google Scholar] [CrossRef]
- Van Petegem, F. Ryanodine receptors: Allosteric ion channel giants. J. Mol. Biol. 2015, 427, 31–53. [Google Scholar] [CrossRef]
- Lanner, J.T.; Georgiou, D.K.; Joshi, A.D.; Hamilton, S.L. Ryanodine receptors: Structure, expression, molecular details, and function in calcium release. Cold Spring Harb. Perspect. Biol. 2010, 2, a003996. [Google Scholar] [CrossRef]
- Wuytack, F.; Raeymaekers, L.; Missiaen, L. Molecular physiology of the SERCA and SPCA pumps. Cell Calcium 2002, 32, 279–305. [Google Scholar] [CrossRef]
- Strehler, E.E.; Treiman, M. Calcium pumps of plasma membrane and cell interior. Curr. Mol. Med. 2004, 4, 323–335. [Google Scholar] [CrossRef]
- Lee, H.C.; Walseth, T.F.; Bratt, G.T.; Hayes, R.N.; Clapper, D.L. Structural determination of a cyclic metabolite of NAD+ with intracellular Ca2+-mobilizing activity. J. Biol. Chem. 1989, 264, 1608–1615. [Google Scholar] [CrossRef]
- Lee, H.C.; Aarhus, R.; Graeff, R.; Gurnack, M.E.; Walseth, T.F. Cyclic Adp Ribose Activation of the Ryanodine Receptor Is Mediated by Calmodulin. Nature 1994, 370, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, D.; Hori, T.; Saitoh, N.; Takahashi, T. 4-Chloro-m-cresol, an activator of ryanodine receptors, inhibits voltage-gated K+ channels at the rat calyx of Held. Eur. J. Neurosci. 2007, 26, 1530–1536. [Google Scholar] [CrossRef]
- Hill, A.P.; Kingston, O.; Sitsapesan, R. Functional regulation of the cardiac ryanodine receptor by suramin and calmodulin involves multiple binding sites. Mol. Pharmacol. 2004, 65, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Mikoshiba, K. IP3 receptor/Ca2+ channel: From discovery to new signaling concepts. J. Neurochem. 2007, 102, 1426–1446. [Google Scholar] [CrossRef]
- Catterall, W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011, 3, a003947. [Google Scholar] [CrossRef]
- Khananshvili, D. The SLC8 gene family of sodium-calcium exchangers (NCX)—Structure, function, and regulation in health and disease. Mol. Aspects Med. 2013, 34, 220–235. [Google Scholar] [CrossRef]
- McBain, C.J.; Mayer, M.L. N-methyl-D-aspartic acid receptor structure and function. Physiol. Rev. 1994, 74, 723–760. [Google Scholar] [CrossRef] [PubMed]
- Jonas, P.; Burnashev, N. Molecular Mechanisms Controlling Calcium-Entry through Ampa-Type Glutamate-Receptor Channels. Neuron 1995, 15, 987–990. [Google Scholar] [CrossRef]
- Reyes, E.P.; Cerpa, V.; Corvalan, L.; Retamal, M.A. Cxs and Panx- hemichannels in peripheral and central chemosensing in mammals. Front. Cell. Neurosci. 2014, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, R.; Lagerstrom, M.C.; Lundin, L.G.; Schioth, H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003, 63, 1256–1272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xie, X. Tools for GPCR drug discovery. Acta Pharmacol. Sin. 2012, 33, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Dhyani, V.; Gare, S.; Gupta, R.K.; Swain, S.; Venkatesh, K.V.; Giri, L. GPCR mediated control of calcium dynamics: A systems perspective. Cell. Signal 2020, 74, 109717. [Google Scholar] [CrossRef]
- Bourne, H.R.; Sanders, D.A.; McCormick, F. The GTPase superfamily: Conserved structure and molecular mechanism. Nature 1991, 349, 117–127. [Google Scholar] [CrossRef]
- Miyano, K.; Sudo, Y.; Yokoyama, A.; Hisaoka-Nakashima, K.; Morioka, N.; Takebayashi, M.; Nakata, Y.; Higami, Y.; Uezono, Y. History of the G protein-coupled receptor (GPCR) assays from traditional to a state-of-the-art biosensor assay. J. Pharmacol. Sci. 2014, 126, 302–309. [Google Scholar] [CrossRef]
- Prakriya, M.; Lewis, R.S. Store-Operated Calcium Channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef]
- Parekh, A.B.; Putney, J.W., Jr. Store-operated calcium channels. Physiol. Rev. 2005, 85, 757–810. [Google Scholar] [CrossRef]
- Ramsey, I.S.; Delling, M.; Clapham, D.E. An introduction to TRP channels. Annu. Rev. Physiol. 2006, 68, 619–647. [Google Scholar] [CrossRef]
- Clapham, D.E.; Runnels, L.W.; Strubing, C. The TRP ion channel family. Nat. Rev. Neurosci. 2001, 2, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Motiani, R.K.; Abdullaev, I.F.; Trebak, M. A novel native store-operated calcium channel encoded by Orai3: Selective requirement of Orai3 versus Orai1 in estrogen receptor-positive versus estrogen receptor-negative breast cancer cells. J. Biol. Chem. 2010, 285, 19173–19183. [Google Scholar] [CrossRef]
- Cui, C.; Merritt, R.; Fu, L.; Pan, Z. Targeting calcium signaling in cancer therapy. Acta Pharm. Sin. B 2017, 7, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Stock, C.; Schwab, A. Ion channels and transporters in metastasis. Biochim. Biophys. Acta 2015, 1848, 2638–2646. [Google Scholar] [CrossRef]
- Tsai, F.C.; Seki, A.; Yang, H.W.; Hayer, A.; Carrasco, S.; Malmersjo, S.; Meyer, T. A polarized Ca2+, diacylglycerol and STIM1 signalling system regulates directed cell migration. Nat. Cell Biol. 2014, 16, 133–144. [Google Scholar] [CrossRef]
- Dingsdale, H.; Okeke, E.; Awais, M.; Haynes, L.; Criddle, D.N.; Sutton, R.; Tepikin, A.V. Saltatory formation, sliding and dissolution of ER-PM junctions in migrating cancer cells. Biochem. J. 2013, 451, 25–32. [Google Scholar] [CrossRef]
- Cuddapah, V.A.; Turner, K.L.; Sontheimer, H. Calcium entry via TRPC1 channels activates chloride currents in human glioma cells. Cell Calcium 2013, 53, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, A.; Toader, D.O.; Cretoiu, S.M.; Cretoiu, D.; Suciu, N.; Radu, B.M. Alterations in Calcium Signaling Pathways in Breast Cancer. Calcium Signal Transduct. 2018, 24, 165. [Google Scholar] [CrossRef][Green Version]
- Tungsukruthai, S.; Petpiroon, N.; Chanvorachote, P. Molecular Mechanisms of Breast Cancer Metastasis and Potential Anti-metastatic Compounds. AntiCancer Res. 2018, 38, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- So, C.L.; Saunus, J.M.; Roberts-Thomson, S.J.; Monteith, G.R. Calcium signalling and breast cancer. Semin. Cell Dev. Biol. 2019, 94, 74–83. [Google Scholar] [CrossRef]
- Tajbakhsh, A.; Pasdar, A.; Rezaee, M.; Fazeli, M.; Soleimanpour, S.; Hassanian, S.M.; FarshchiyanYazdi, Z.; Rad, T.Y.; Ferns, G.A.; Avan, A. The current status and perspectives regarding the clinical implication of intracellular calcium in breast cancer. J. Cell. Physiol. 2018, 233, 5623–5641. [Google Scholar] [CrossRef] [PubMed]
- Azimi, I.; Roberts-Thomson, S.J.; Monteith, G.R. Calcium influx pathways in breast cancer: Opportunities for pharmacological intervention. Br. J. Pharmacol. 2014, 171, 945–960. [Google Scholar] [CrossRef]
- Singh, A.; Chagtoo, M.; Tiwari, S.; George, N.; Chakravarti, B.; Khan, S.; Lakshmi, S.; Godbole, M.M. Inhibition of Inositol 1, 4, 5-Trisphosphate Receptor Induce Breast Cancer Cell Death through Deregulated Autophagy and Cellular Bioenergetics. J. Cell. Biochem. 2017, 118, 2333–2346. [Google Scholar] [CrossRef] [PubMed]
- McAndrew, D.; Grice, D.M.; Peters, A.A.; Davis, F.M.; Stewart, T.; Rice, M.; Smart, C.E.; Brown, M.A.; Kenny, P.A.; Roberts-Thomson, S.J.; et al. ORAI1-mediated calcium influx in lactation and in breast cancer. Mol. Cancer Ther. 2011, 10, 448–460. [Google Scholar] [CrossRef]
- Faouzi, M.; Hague, F.; Potier, M.; Ahidouch, A.; Sevestre, H.; Ouadid-Ahidouch, H. Down-regulation of Orai3 arrests cell-cycle progression and induces apoptosis in breast cancer cells but not in normal breast epithelial cells. J. Cell. Physiol. 2011, 226, 542–551. [Google Scholar] [CrossRef]
- Faouzi, M.; Kischel, P.; Hague, F.; Ahidouch, A.; Benzerdjeb, N.; Sevestre, H.; Penner, R.; Ouadid-Ahidouch, H. ORAI3 silencing alters cell proliferation and cell cycle progression via c-myc pathway in breast cancer cells. Biochim. Biophys. Acta 2013, 1833, 752–760. [Google Scholar] [CrossRef]
- Lee, W.J.; Roberts-Thomson, S.J.; Holman, N.A.; May, F.J.; Lehrbach, G.M.; Monteith, G.R. Expression of plasma membrane calcium pump isoform mRNAs in breast cancer cell lines. Cell. Signal 2002, 14, 1015–1022. [Google Scholar] [CrossRef]
- Lee, W.J.; Roberts-Thomson, S.J.; Monteith, G.R. Plasma membrane calcium-ATPase 2 and 4 in human breast cancer cell lines. Biochem. Biophys. Res. Commun. 2005, 337, 779–783. [Google Scholar] [CrossRef]
- Takahashi, N.; Chen, H.Y.; Harris, I.S.; Stover, D.G.; Selfors, L.M.; Bronson, R.T.; Deraedt, T.; Cichowski, K.; Welm, A.L.; Mori, Y.; et al. Cancer Cells Co-opt the Neuronal Redox-Sensing Channel TRPA1 to Promote Oxidative-Stress Tolerance. Cancer Cell 2018, 33, 985–1003.e1007. [Google Scholar] [CrossRef] [PubMed]
- Dhennin-Duthille, I.; Gautier, M.; Faouzi, M.; Guilbert, A.; Brevet, M.; Vaudry, D.; Ahidouch, A.; Sevestre, H.; Ouadid-Ahidouch, H. High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: Correlation with pathological parameters. Cell. Physiol. Biochem. 2011, 28, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.V.; Al-Refae, K.; Wolk, G.; Bonatz, G.; Altmuller, J.; Becker, C.; Gisselmann, G.; Hatt, H. Expression and functionality of TRPV1 in breast cancer cells. Breast Cancer 2016, 8, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Bolanz, K.A.; Hediger, M.A.; Landowski, C.P. The role of TRPV6 in breast carcinogenesis. Mol. Cancer Ther. 2008, 7, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Grice, D.M.; Vetter, I.; Faddy, H.M.; Kenny, P.A.; Roberts-Thomson, S.J.; Monteith, G.R. Golgi Calcium Pump Secretory Pathway Calcium ATPase 1 (SPCA1) Is a Key Regulator of Insulin-like Growth Factor Receptor (IGF1R) Processing in the Basal-like Breast Cancer Cell Line MDA-MB-231. J. Biol. Chem. 2010, 285, 37458–37466. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Grice, D.M.; Faddy, H.M.; Nguyen, N.; Leitch, S.; Wang, Y.; Muend, S.; Kenny, P.A.; Sukumar, S.; Roberts-Thomson, S.J.; et al. Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell 2010, 143, 84–98. [Google Scholar] [CrossRef]
- Cross, B.M.; Hack, A.; Reinhardt, T.A.; Rao, R. SPCA2 regulates Orai1 trafficking and store independent Ca2+ entry in a model of lactation. PLoS ONE 2013, 8, e67348. [Google Scholar] [CrossRef]
- Peters, A.A.; Milevskiy, M.J.; Lee, W.C.; Curry, M.C.; Smart, C.E.; Saunus, J.M.; Reid, L.; da Silva, L.; Marcial, D.L.; Dray, E.; et al. The calcium pump plasma membrane Ca2+-ATPase 2 (PMCA2) regulates breast cancer cell proliferation and sensitivity to doxorubicin. Sci. Rep. 2016, 6, 25505. [Google Scholar] [CrossRef]
- Curry, M.; Roberts-Thomson, S.J.; Monteith, G.R. PMCA2 silencing potentiates MDA-MB-231 breast cancer cell death initiated with the Bcl-2 inhibitor ABT-263. Biochem. Biophys. Res. Commun. 2016, 478, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.T.; Huang, L.; Pottle, J.E.; Liu, K.; Yang, Y.; Zeng, X.; Keyser, B.M.; Agrawal, K.C.; Hansen, J.B.; Li, M. Selective blockade of T-type Ca2+ channels suppresses human breast cancer cell proliferation. Cancer Lett. 2008, 267, 116–124. [Google Scholar] [CrossRef]
- Szatkowski, C.; Parys, J.B.; Ouadid-Ahidouch, H.; Matifat, F. Inositol 1,4,5-trisphosphate-induced Ca2+ signalling is involved in estradiol-induced breast cancer epithelial cell growth. Mol. Cancer 2010, 9. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Eun, S.Y.; Lee, J.S.; Park, S.W.; Lee, J.H.; Chang, K.C.; Kim, H.J. P2Y2 receptor activation by nucleotides released from highly metastatic breast cancer cells increases tumor growth and invasion via crosstalk with endothelial cells. Breast Cancer Res. 2014, 16, R77. [Google Scholar] [CrossRef] [PubMed]
- El Hiani, Y.; Lehen’kyi, V.; Ouadid-Ahidouch, H.; Ahidouch, A. Activation of the calcium-sensing receptor by high calcium induced breast cancer cell proliferation and TRPC1 cation channel over-expression potentially through EGFR pathways. Arch. Biochem. Biophys. 2009, 486, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Aydar, E.; Yeo, S.; Djamgoz, M.; Palmer, C. Abnormal expression, localization and interaction of canonical transient receptor potential ion channels in human breast cancer cell lines and tissues: A potential target for breast cancer diagnosis and therapy. Cancer Cell Int. 2009, 9, 23. [Google Scholar] [CrossRef]
- Guilbert, A.; Gautier, M.; Dhennin-Duthille, I.; Haren, N.; Sevestre, H.; Ouadid-Ahidouch, H. Evidence that TRPM7 is required for breast cancer cell proliferation. Am. J. Physiol. Cell Physiol. 2009, 297, C493–C502. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Pan, Q.; Meng, H.; Jiang, Y.; Mao, A.; Wang, T.; Hua, D.; Yao, X.; Jin, J.; Ma, X. Enhancement of vascular endothelial growth factor release in long-term drug-treated breast cancer via transient receptor potential channel 5-Ca2+-hypoxia-inducible factor 1alpha pathway. Pharmacol. Res. 2015, 93, 36–42. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yang, D.; Myeong, J.; Ha, K.; Kim, S.H.; Park, E.J.; Kim, I.G.; Cho, N.H.; Lee, K.P.; Jeon, J.H.; et al. Regulation of calcium influx and signaling pathway in cancer cells via TRPV6-Numb1 interaction. Cell Calcium 2013, 53, 102–111. [Google Scholar] [CrossRef]
- Samatov, T.R.; Tonevitsky, A.G.; Schumacher, U. Epithelial-mesenchymal transition: Focus on metastatic cascade, alternative splicing, non-coding RNAs and modulating compounds. Mol. Cancer 2013, 12, 107. [Google Scholar] [CrossRef]
- Mahdi, S.H.; Cheng, H.; Li, J.; Feng, R. The effect of TGF-beta-induced epithelial-mesenchymal transition on the expression of intracellular calcium-handling proteins in T47D and MCF-7 human breast cancer cells. Arch. Biochem. Biophys. 2015, 583, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.M.; Parsonage, M.T.; Cabot, P.J.; Parat, M.O.; Thompson, E.W.; Roberts-Thomson, S.J.; Monteith, G.R. Assessment of gene expression of intracellular calcium channels, pumps and exchangers with epidermal growth factor-induced epithelial-mesenchymal transition in a breast cancer cell line. Cancer Cell Int. 2013, 13, 76. [Google Scholar] [CrossRef]
- Hu, J.; Qin, K.; Zhang, Y.; Gong, J.; Li, N.; Lv, D.; Xiang, R.; Tan, X. Downregulation of transcription factor Oct4 induces an epithelial-to-mesenchymal transition via enhancement of Ca2+ influx in breast cancer cells. Biochem. Biophys. Res. Commun. 2011, 411, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.M.; Peters, A.A.; Grice, D.M.; Cabot, P.J.; Parat, M.O.; Roberts-Thomson, S.J.; Monteith, G.R. Non-stimulated, agonist-stimulated and store-operated Ca2+ influx in MDA-MB-468 breast cancer cells and the effect of EGF-induced EMT on calcium entry. PLoS ONE 2012, 7, e36923. [Google Scholar] [CrossRef] [PubMed]
- Stewart, T.A.; Azimi, I.; Thompson, E.W.; Roberts-Thomson, S.J.; Monteith, G.R. A role for calcium in the regulation of ATP-binding cassette, sub-family C, member 3 (ABCC3) gene expression in a model of epidermal growth factor-mediated breast cancer epithelial-mesenchymal transition. Biochem. Biophys. Res. Commun. 2015, 458, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.K.; Makena, M.R.; Llongueras, J.P.; Prasad, H.; Ko, M.; Bandral, M.; Rao, R. A Ca2+-ATPase Regulates E-cadherin Biogenesis and Epithelial-Mesenchymal Transition in Breast Cancer Cells. Mol. Cancer Res. 2019, 17, 1735–1747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Miao, Y.; Zheng, X.; Gong, Y.; Zhang, J.; Zou, F.; Cai, C. STIM1 and STIM2 differently regulate endogenous Ca2+ entry and promote TGF-beta-induced EMT in breast cancer cells. Biochem. Biophys. Res. Commun. 2017, 488, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Schaar, A.; Sukumaran, P.; Sun, Y.; Dhasarathy, A.; Singh, B.B. TRPC1-STIM1 activation modulates transforming growth factor beta-induced epithelial-to-mesenchymal transition. Oncotarget 2016, 7, 80554–80567. [Google Scholar] [CrossRef] [PubMed]
- Azimi, I.; Milevskiy, M.J.G.; Kaemmerer, E.; Turner, D.; Yapa, K.T.D.S.; Brown, M.A.; Thompson, E.W.; Roberts-Thomson, S.J.; Monteith, G.R. TRPC1 is a differential regulator of hypoxia-mediated events and Akt signalling in PTEN-deficient breast cancer cells. J. Cell Sci. 2017, 130, 2292–2305. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.M.; Azimi, I.; Faville, R.A.; Peters, A.A.; Jalink, K.; Putney, J.W.; Goodhill, G.J.; Thompson, E.W.; Roberts-Thomson, S.J.; Monteith, G.R. Induction of epithelial-mesenchymal transition (EMT) in breast cancer cells is calcium signal dependent. Oncogene 2014, 33, 2307–2316. [Google Scholar] [CrossRef]
- Zhang, J.T.; Jiang, X.H.; Xie, C.; Cheng, H.; Dong, J.D.; Wang, Y.; Fok, K.L.; Zhang, X.H.; Sun, T.T.; Tsang, L.L.; et al. Downregulation of CFTR promotes epithelial-to-mesenchymal transition and is associated with poor prognosis of breast cancer. Bba-Mol. Cell Res. 2013, 1833, 2961–2969. [Google Scholar] [CrossRef]
- Iamshanova, O.; Fiorio Pla, A.; Prevarskaya, N. Molecular mechanisms of tumour invasion: Regulation by calcium signals. J. Physiol. 2017, 595, 3063–3075. [Google Scholar] [CrossRef]
- Dragoni, S.; Laforenza, U.; Bonetti, E.; Lodola, F.; Bottino, C.; Berra-Romani, R.; Carlo Bongio, G.; Cinelli, M.P.; Guerra, G.; Pedrazzoli, P.; et al. Vascular endothelial growth factor stimulates endothelial colony forming cells proliferation and tubulogenesis by inducing oscillations in intracellular Ca2+ concentration. Stem Cells 2011, 29, 1898–1907. [Google Scholar] [CrossRef]
- Davis, F.M.; Kenny, P.A.; Soo, E.T.L.; van Denderen, B.J.W.; Thompson, E.W.; Cabot, P.J.; Parat, M.O.; Roberts-Thomson, S.J.; Monteith, G.R. Remodeling of Purinergic Receptor-Mediated Ca2+ Signaling as a Consequence of EGF-Induced Epithelial-Mesenchymal Transition in Breast Cancer Cells. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Azimi, I.; Beilby, H.; Davis, F.M.; Marcial, D.L.; Kenny, P.A.; Thompson, E.W.; Roberts-Thomson, S.J.; Monteith, G.R. Altered purinergic receptor-Ca2+ signaling associated with hypoxia-induced epithelial-mesenchymal transition in breast cancer cells. Mol. Oncol. 2016, 10, 166–178. [Google Scholar] [CrossRef]
- Tsai, F.C.; Meyer, T. Ca2+ pulses control local cycles of lamellipodia retraction and adhesion along the front of migrating cells. Curr. Biol. 2012, 22, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Betapudi, V. Life without double-headed non-muscle myosin II motor proteins. Front. Chem. 2014, 2, 45. [Google Scholar] [CrossRef]
- Betapudi, V.; Rai, V.; Beach, J.R.; Egelhoff, T. Novel regulation and dynamics of myosin II activation during epidermal wound responses. Exp. Cell Res. 2010, 316, 980–991. [Google Scholar] [CrossRef]
- Campbell, R.L.; Davies, P.L. Structure-function relationships in calpains. Biochem. J. 2012, 447, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Cuddapah, V.A.; Sontheimer, H. Molecular interaction and functional regulation of ClC-3 by Ca2+/calmodulin-dependent protein kinase II (CaMKII) in human malignant glioma. J. Biol. Chem. 2010, 285, 11188–11196. [Google Scholar] [CrossRef] [PubMed]
- Daft, P.G.; Yuan, K.; Warram, J.M.; Klein, M.J.; Siegal, G.P.; Zayzafoon, M. Alpha-CaMKII plays a critical role in determining the aggressive behavior of human osteosarcoma. Mol. Cancer Res. 2013, 11, 349–359. [Google Scholar] [CrossRef]
- Giannone, G.; Ronde, P.; Gaire, M.; Haiech, J.; Takeda, K. Calcium oscillations trigger focal adhesion disassembly in human U87 astrocytoma cells. J. Biol. Chem. 2002, 277, 26364–26371. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J.J.; Huang, X.Y. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell 2009, 15, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.C.; Chen, W.T. Cellular invasion into matrix beads: Localization of beta 1 integrins and fibronectin to the invadopodia. J. Cell Sci. 1991, 99 Pt 2, 213–225. [Google Scholar]
- Caldieri, G.; Buccione, R. Aiming for invadopodia: Organizing polarized delivery at sites of invasion. Trends Cell Biol. 2010, 20, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Lai, C.S.; Chen, Y.F.; Chiu, W.T.; Chen, H.C.; Shen, M.R. STIM1-dependent Ca2+ signaling regulates podosome formation to facilitate cancer cell invasion. Sci. Rep. 2017, 7, 11523. [Google Scholar] [CrossRef]
- Di, J.; Huang, H.; Qu, D.; Tang, J.; Cao, W.; Lu, Z.; Cheng, Q.; Yang, J.; Bai, J.; Zhang, Y.; et al. Rap2B promotes proliferation, migration, and invasion of human breast cancer through calcium-related ERK1/2 signaling pathway. Sci. Rep. 2015, 5, 12363. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, A.; Gautier, M.; Dhennin-Duthille, I.; Rybarczyk, P.; Sahni, J.; Sevestre, H.; Scharenberg, A.M.; Ouadid-Ahidouch, H. Transient receptor potential melastatin 7 is involved in oestrogen receptor-negative metastatic breast cancer cells migration through its kinase domain. Eur. J. Cancer 2013, 49, 3694–3707. [Google Scholar] [CrossRef] [PubMed]
- Mader, C.C.; Oser, M.; Magalhaes, M.A.; Bravo-Cordero, J.J.; Condeelis, J.; Koleske, A.J.; Gil-Henn, H. An EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. Cancer Res. 2011, 71, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.R.; Chen, C.L.; Chen, H.C. FAK is required for the assembly of podosome rosettes. J. Cell Biol. 2011, 195, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Yoshida, S.; Muroi, E.; Yoshida, N.; Kawamura, M.; Kouchi, Z.; Nakamura, Y.; Sakai, R.; Fukami, K. Phosphoinositide 3-kinase signaling pathway mediated by p110alpha regulates invadopodia formation. J. Cell Biol. 2011, 193, 1275–1288. [Google Scholar] [CrossRef]
- Sun, J.; Lu, F.; He, H.; Shen, J.; Messina, J.; Mathew, R.; Wang, D.; Sarnaik, A.A.; Chang, W.C.; Kim, M.; et al. STIM1- and Orai1-mediated Ca2+ oscillation orchestrates invadopodium formation and melanoma invasion. J. Cell Biol. 2014, 207, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Jelassi, B.; Chantome, A.; Alcaraz-Perez, F.; Baroja-Mazo, A.; Cayuela, M.L.; Pelegrin, P.; Surprenant, A.; Roger, S. P2X(7) receptor activation enhances SK3 channels- and cystein cathepsin-dependent cancer cells invasiveness. Oncogene 2011, 30, 2108–2122. [Google Scholar] [CrossRef]
- Parkash, J.A.I.; Asotra, K. Combinatorial intervention of prostaglandin E2 receptor and calcium sensing receptor to attenuate breast cancer cell proliferation, migration and bone metastasis. Exp. Ther. Med. 2010, 1, 227–231. [Google Scholar] [CrossRef]
- De Stefani, D.; Raffaello, A.; Teardo, E.; Szabo, I.; Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 2011, 476, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Baughman, J.M.; Perocchi, F.; Girgis, H.S.; Plovanich, M.; Belcher-Timme, C.A.; Sancak, Y.; Bao, X.R.; Strittmatter, L.; Goldberger, O.; Bogorad, R.L.; et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 2011, 476, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Curry, M.C.; Peters, A.A.; Kenny, P.A.; Roberts-Thomson, S.J.; Monteith, G.R. Mitochondrial calcium uniporter silencing potentiates caspase-independent cell death in MDA-MB-231 breast cancer cells. Biochem. Biophys. Res. Commun. 2013, 434, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Lu, S.; He, Z.; Huang, H.; Yao, Z.; Miao, Y.; Cai, C.; Zou, F. MCU-dependent negative sorting of miR-4488 to extracellular vesicles enhances angiogenesis and promotes breast cancer metastatic colonization. Oncogene 2020. [Google Scholar] [CrossRef]
- Sartorius, C.A.; Hanna, C.T.; Gril, B.; Cruz, H.; Serkova, N.J.; Huber, K.M.; Kabos, P.; Schedin, T.B.; Borges, V.F.; Steeg, P.S.; et al. Estrogen promotes the brain metastatic colonization of triple negative breast cancer cells via an astrocyte-mediated paracrine mechanism. Oncogene 2016, 35, 2881–2892. [Google Scholar] [CrossRef] [PubMed]
- Nutter, F.; Holen, I.; Brown, H.K.; Cross, S.S.; Evans, C.A.; Walker, M.; Coleman, R.E.; Westbrook, J.A.; Selby, P.J.; Brown, J.E.; et al. Different molecular profiles are associated with breast cancer cell homing compared with colonisation of bone: Evidence using a novel bone-seeking cell line. Endocr. Relat. Cancer 2014, 21, 327–341. [Google Scholar] [CrossRef]
- Kiryushko, D.; Novitskaya, V.; Soroka, V.; Klingelhofer, J.; Lukanidin, E.; Berezin, V.; Bock, E. Molecular mechanisms of Ca2+ signaling in neurons induced by the S100A4 protein. Mol. Cell. Biol. 2006, 26, 3625–3638. [Google Scholar] [CrossRef]
- Sherbet, G.V. Metastasis promoter S100A4 is a potentially valuable molecular target for cancer therapy. Cancer Lett. 2009, 280, 15–30. [Google Scholar] [CrossRef]
- Resende, R.R.; Andrade, L.M.; Oliveira, A.G.; Guimaraes, E.S.; Guatimosim, S.; Leite, M.F. Nucleoplasmic calcium signaling and cell proliferation: Calcium signaling in the nucleus. Cell Commun. Signal. 2013, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, E.; Machado, R.; Fonseca, M.C.; Franca, A.; Carvalho, C.; Araujo, E.S.A.C.; Almeida, B.; Cassini, P.; Hissa, B.; Drumond, L.; et al. Inositol 1, 4, 5-trisphosphate-dependent nuclear calcium signals regulate angiogenesis and cell motility in triple negative breast cancer. PLoS ONE 2017, 12, e0175041. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Liang, Y.; Diao, X.; Chen, Q. S100A4 promotes invasion and angiogenesis in breast cancer MDA-MB-231 cells by upregulating matrix metalloproteinase-13. Acta Biochim. Polocy 2012, 59, 593–598. [Google Scholar] [CrossRef]
- Wang, X.G.; Meng, Q.; Qi, F.M.; Yang, Q.F. Blocking TGF-beta inhibits breast cancer cell invasiveness via ERK/S100A4 signal. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3844–3853. [Google Scholar] [PubMed]
- Qu, S.; Wu, J.; Bao, Q.; Yao, B.; Duan, R.; Chen, X.; Li, L.; Yuan, H.; Jin, Y.; Ma, C. Osterix promotes the migration and angiogenesis of breast cancer by upregulation of S100A4 expression. J. Cell. Mol. Med. 2019, 23, 1116–1127. [Google Scholar] [CrossRef]
- Yu, C.; Tang, W.; Wang, Y.; Shen, Q.; Wang, B.; Cai, C.; Meng, X.; Zou, F. Downregulation of ACE2/Ang-(1-7)/Mas axis promotes breast cancer metastasis by enhancing store-operated calcium entry. Cancer Lett. 2016, 376, 268–277. [Google Scholar] [CrossRef]
- Witzel, I.; Oliveira-Ferrer, L.; Pantel, K.; Muller, V.; Wikman, H. Breast cancer brain metastases: Biology and new clinical perspectives. Breast Cancer Res. 2016, 18, 8. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, H.; Jiang, X.; Qian, C.; Liu, Z.; Luo, D. Factors involved in cancer metastasis: A better understanding to “seed and soil” hypothesis. Mol. Cancer 2017, 16, 176. [Google Scholar] [CrossRef]
- Custodio-Santos, T.; Videira, M.; Brito, M.A. Brain metastasization of breast cancer. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 132–147. [Google Scholar] [CrossRef]
- Lee, T.H.; Avraham, H.K.; Jiang, S.X.; Avraham, S. Vascular endothelial growth factor modulates the transendothelial migration of MDA-MB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. J. Biol. Chem. 2003, 278, 5277–5284. [Google Scholar] [CrossRef]
- Lee, B.C.; Lee, T.H.; Avraham, S.; Avraham, H.K. Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1alpha in breast cancer cell migration through human brain microvascular endothelial cells. Mol. Cancer Res. 2004, 2, 327–338. [Google Scholar]
- Sharma, S.; Wu, S.Y.; Jimenez, H.; Xing, F.; Zhu, D.; Liu, Y.; Wu, K.; Tyagi, A.; Zhao, D.; Lo, H.W.; et al. Ca2+ and CACNA1H mediate targeted suppression of breast cancer brain metastasis by AM RF EMF. EBioMedicine 2019, 44, 194–208. [Google Scholar] [CrossRef] [PubMed]
- McHugh, B.J.; Buttery, R.; Lad, Y.; Banks, S.; Haslett, C.; Sethi, T. Integrin activation by Fam38A uses a novel mechanism of R-Ras targeting to the endoplasmic reticulum. J. Cell Sci. 2010, 123, 51–61. [Google Scholar] [CrossRef]
- Pathak, M.M.; Nourse, J.L.; Tran, T.; Hwe, J.; Arulmoli, J.; Le, D.T.; Bernardis, E.; Flanagan, L.A.; Tombola, F. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, 16148–16153. [Google Scholar] [CrossRef] [PubMed]
- Eisenhoffer, G.T.; Loftus, P.D.; Yoshigi, M.; Otsuna, H.; Chien, C.B.; Morcos, P.A.; Rosenblatt, J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 2012, 484, 546–549. [Google Scholar] [CrossRef]
- Hung, W.C.; Yang, J.R.; Yankaskas, C.L.; Wong, B.S.; Wu, P.H.; Pardo-Pastor, C.; Serra, S.A.; Chiang, M.J.; Gu, Z.; Wirtz, D.; et al. Confinement Sensing and Signal Optimization via Piezo1/PKA and Myosin II Pathways. Cell Rep. 2016, 15, 1430–1441. [Google Scholar] [CrossRef]
- Pardo-Pastor, C.; Rubio-Moscardo, F.; Vogel-Gonzalez, M.; Serra, S.A.; Afthinos, A.; Mrkonjic, S.; Destaing, O.; Abenza, J.F.; Fernandez-Fernandez, J.M.; Trepat, X.; et al. Piezo2 channel regulates RhoA and actin cytoskeleton to promote cell mechanobiological responses. Proc. Natl. Acad. Sci. USA 2018, 115, 1925–1930. [Google Scholar] [CrossRef]
- Ren, D.; Zhu, X.; Kong, R.; Zhao, Z.; Sheng, J.; Wang, J.; Xu, X.; Liu, J.; Cui, K.; Zhang, X.H.; et al. Targeting Brain-Adaptive Cancer Stem Cells Prohibits Brain Metastatic Colonization of Triple-Negative Breast Cancer. Cancer Res. 2018, 78, 2052–2064. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ovejero, D.; Veiga, S.; Garcia-Segura, L.M.; Doncarlos, L.L. Glial expression of estrogen and androgen receptors after rat brain injury. J. Comp. Neurol. 2002, 450, 256–271. [Google Scholar] [CrossRef]
- Fares, H.; DiNicolantonio, J.J.; O’Keefe, J.H.; Lavie, C.J. Amlodipine in hypertension: A first-line agent with efficacy for improving blood pressure and patient outcomes. Open Heart 2016, 3, e000473. [Google Scholar] [CrossRef]
- Hansson, L.; Hedner, T.; Lund-Johansen, P.; Kjeldsen, S.E.; Lindholm, L.H.; Syvertsen, J.O.; Lanke, J.; de Faire, U.; Dahlof, B.; Karlberg, B.E. Randomised trial of effects of calcium antagonists compared with diuretics and beta-blockers on cardiovascular morbidity and mortality in hypertension: The Nordic Diltiazem (NORDIL) study. Lancet 2000, 356, 359–365. [Google Scholar] [CrossRef]
- Hornung, R.S.; Jones, R.I.; Gould, B.A.; Sonecha, T.; Raftery, E.B. Twice-daily verapamil for hypertension: A comparison with propranolol. Am. J. Cardiol. 1986, 57, 93D–98D. [Google Scholar] [CrossRef]
- Taylor, J.M.; Simpson, R.U. Inhibition of cancer cell growth by calcium channel antagonists in the athymic mouse. Cancer Res. 1992, 52, 2413–2418. [Google Scholar]
- Brogden, R.N.; Markham, A. Mibefradil. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in the management of hypertension and angina pectoris. Drugs 1997, 54, 774–793. [Google Scholar] [CrossRef] [PubMed]
- Sultana, A.; McMonagle, T. Pimozide for schizophrenia or related psychoses. Cochrane Database Syst. Rev. 2000, 10. [Google Scholar] [CrossRef]
- Bertolesi, G.E.; Shi, C.; Elbaum, L.; Jollimore, C.; Rozenberg, G.; Barnes, S.; Kelly, M.E. The Ca2+ channel antagonists mibefradil and pimozide inhibit cell growth via different cytotoxic mechanisms. Mol. Pharmacol. 2002, 62, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Chokshi, R.; Fruasaha, P.; Kozak, J.A. 2-Aminoethyl diphenyl borinate (2-APB) inhibits TRPM7 channels through an intracellular acidification mechanism. Channels 2012, 6, 362–369. [Google Scholar] [CrossRef]
- Liu, H.; Dilger, J.P.; Lin, J. The Role of Transient Receptor Potential Melastatin 7 (TRPM7) in Cell Viability: A Potential Target to Suppress Breast Cancer Cell Cycle. Cancers 2020, 12, 131. [Google Scholar] [CrossRef]
- Huang, Y.H.; Fliegert, R.; Guse, A.H.; Lu, W.; Du, J. A structural overview of the ion channels of the TRPM family. Cell Calcium 2020, 85. [Google Scholar] [CrossRef]
- Bae, S.H.; Park, J.H.; Choi, H.G.; Kim, H.; Kim, S.H. Imidazole Antifungal Drugs Inhibit the Cell Proliferation and Invasion of Human Breast Cancer Cells. Biomol. Ther. 2018, 26, 494–502. [Google Scholar] [CrossRef]
- Guo, L.; Li, Z.S.; Wang, H.L.; Ye, C.Y.; Zhang, D.C. Carboxyamido-triazole inhibits proliferation of human breast cancer cells via G2/M cell cycle arrest and apoptosis. Eur. J. Pharmacol. 2006, 538, 15–22. [Google Scholar] [CrossRef]
- Zhang, B.B.; Wang, D.G.; Guo, F.F.; Xuan, C. Mitochondrial membrane potential and reactive oxygen species in cancer stem cells. Fam. Cancer 2015, 14, 19–23. [Google Scholar] [CrossRef]
- Kotnova, A.P.; Lyanova, B.M.; Dukhanina, E.A.; Portseva, T.N.; Ilyin, Y.V.; Georgieva, S.G.; Stepchenko, A.G.; Pankratova, E.V. Thapsigargin, Inhibitor of Sarco-Endoplasmic Ca2+-ATPase, Effectively Suppresses the Expression of S100A4 Protein in Human Breast Cancer Cell Line. Dokl. Biochem. Biophys. 2019, 486, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Park, B.N.; Roh, J.H.; An, Y.S.; Hur, H.; Yoon, J.K. Enhancing the Therapeutic Efficacy of 2-Deoxyglucose in Breast Cancer Cells Using Cell-cycle Synchronization. Anticancer Res. 2016, 36, 5975–5980. [Google Scholar] [CrossRef]
- Motawi, T.M.; Sadik, N.A.; Fahim, S.A.; Shouman, S.A. Combination of imatinib and clotrimazole enhances cell growth inhibition in T47D breast cancer cells. Chem. Biol. Interact. 2015, 233, 147–156. [Google Scholar] [CrossRef]
- Kim, P.K.; Zamora, R.; Petrosko, P.; Billiar, T.R. The regulatory role of nitric oxide in apoptosis. Int. Immunopharmacol. 2001, 1, 1421–1441. [Google Scholar] [CrossRef]
- Maes, M.; Vanhaecke, T.; Cogliati, B.; Yanguas, S.C.; Willebrords, J.; Rogiers, V.; Vinken, M. Measurement of Apoptotic and Necrotic Cell Death in Primary Hepatocyte Cultures. Methods Mol. Biol. 2015, 1250, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Lothstein, L.; Israel, M.; Sweatman, T.W. Anthracycline drug targeting: Cytoplasmic versus nuclear--a fork in the road. Drug Resist Updat 2001, 4, 169–177. [Google Scholar] [CrossRef]
- Froelich-Ammon, S.J.; Osheroff, N. Topoisomerase poisons: Harnessing the dark side of enzyme mechanism. J. Biol. Chem. 1995, 270, 21429–21432. [Google Scholar] [CrossRef]
- Frishman, W.H.; Sung, H.M.; Yee, H.C.M.; Liu, L.L.; Einzig, A.I.; Dutcher, J.; Keefe, D. Cardiovascular toxicity with cancer chemotherapy—Introduction. Curr. Prob. Cardiol. 1996, 21, 227–286. [Google Scholar] [CrossRef]
- Olson, R.D.; Mushlin, P.S. Doxorubicin cardiotoxicity: Analysis of prevailing hypotheses. FASEB J. 1990, 4, 3076–3086. [Google Scholar] [CrossRef]
- Minotti, G.; Licata, S.; Saponiero, A.; Menna, P.; Calafiore, A.M.; Di Giammarco, G.; Liberi, G.; Animati, F.; Cipollone, A.; Manzini, S.; et al. Anthracycline metabolism and toxicity in human myocardium: Comparisons between doxorubicin, epirubicin, and a novel disaccharide analogue with a reduced level of formation and [4Fe-4S] reactivity of its secondary alcohol metabolite. Chem. Res. Toxicol. 2000, 13, 1336–1341. [Google Scholar] [CrossRef]
- Ravid, A.; Rocker, D.; Machlenkin, A.; Rotem, C.; Hochman, A.; Kessler-Icekson, G.; Liberman, U.A.; Koren, R. 1,25-Dihydroxyvitamin D3 enhances the susceptibility of breast cancer cells to doxorubicin-induced oxidative damage. Cancer Res. 1999, 59, 862–867. [Google Scholar]
- Al-Malky, H.S.; Osman, A.M.; Damanhouri, Z.A.; Alkreathy, H.M.; Al Aama, J.Y.; Ramadan, W.S.; Al Qahtani, A.A.; Al Mahdi, H.B. Modulation of doxorubicin-induced expression of the multidrug resistance gene in breast cancer cells by diltiazem and protection against cardiotoxicity in experimental animals. Cancer Cell Int. 2019, 19, 191. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, R.J.; Ying, X.; Tian, W.; Yao, H.J.; Men, Y.; Yu, Y.; Zhang, L.A.; Ju, R.J.; Wang, X.X.; et al. Targeting Therapy with Mitosomal Daunorubicin plus Amlodipine Has the Potential To Circumvent Intrinsic Resistant Breast Cancer. Mol. Pharmaceut. 2011, 8, 162–175. [Google Scholar] [CrossRef]
- Diver, J.M.; Sage, S.O.; Rosado, J.A. The inositol trisphosphate receptor antagonist 2-aminoethoxydiphenylborate (2-APB) blocks Ca2+ entry channels in human platelets: Cautions for its use in studying Ca2+ influx. Cell Calcium 2001, 30, 323–329. [Google Scholar] [CrossRef]
- DeHaven, W.I.; Smyth, J.T.; Boyles, R.R.; Bird, G.S.; Putney, J.W., Jr. Complex actions of 2-aminoethyldiphenyl borate on store-operated calcium entry. J. Biol. Chem. 2008, 283, 19265–19273. [Google Scholar] [CrossRef]
- Xu, S.Z.; Zeng, F.; Boulay, G.; Grimm, C.; Harteneck, C.; Beech, D.J. Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: A differential, extracellular and voltage-dependent effect. Br. J. Pharmacol. 2005, 145, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Catacuzzeno, L.; Franciolini, F. Role of KCa3.1 Channels in Modulating Ca2+ Oscillations during Glioblastoma Cell Migration and Invasion. Int. J. Mol. Sci. 2018, 19, 2970. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.D.; Hanley, P.J.; Rinne, S.; Zuzarte, M.; Daut, J. Calcium-activated K+ channel (K(Ca)3.1) activity during Ca2+ store depletion and store-operated Ca2+ entry in human macrophages. Cell Calcium 2010, 48, 19–27. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).