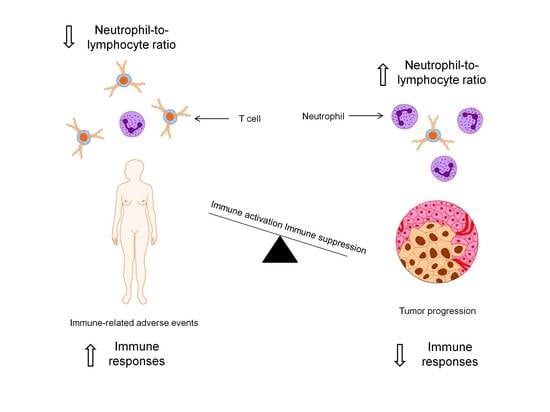
Neutrophil-to-Lymphocyte Ratio Predicts Development of Immune-Related Adverse Events and Outcomes from Immune Checkpoint Blockade: A Case-Control Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
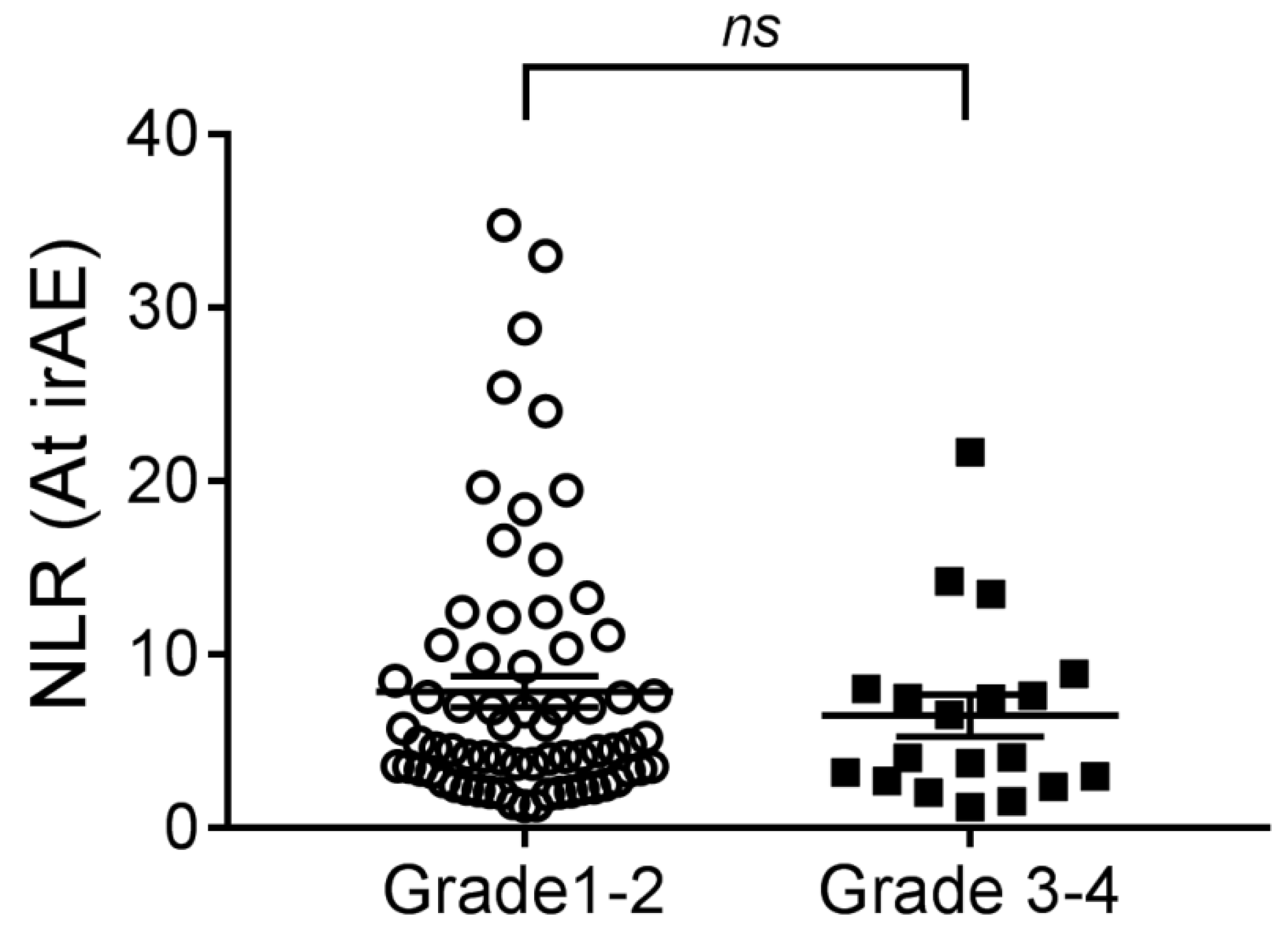
3.2. Prognostic Role of NLR and PLR for the Development of irAEs
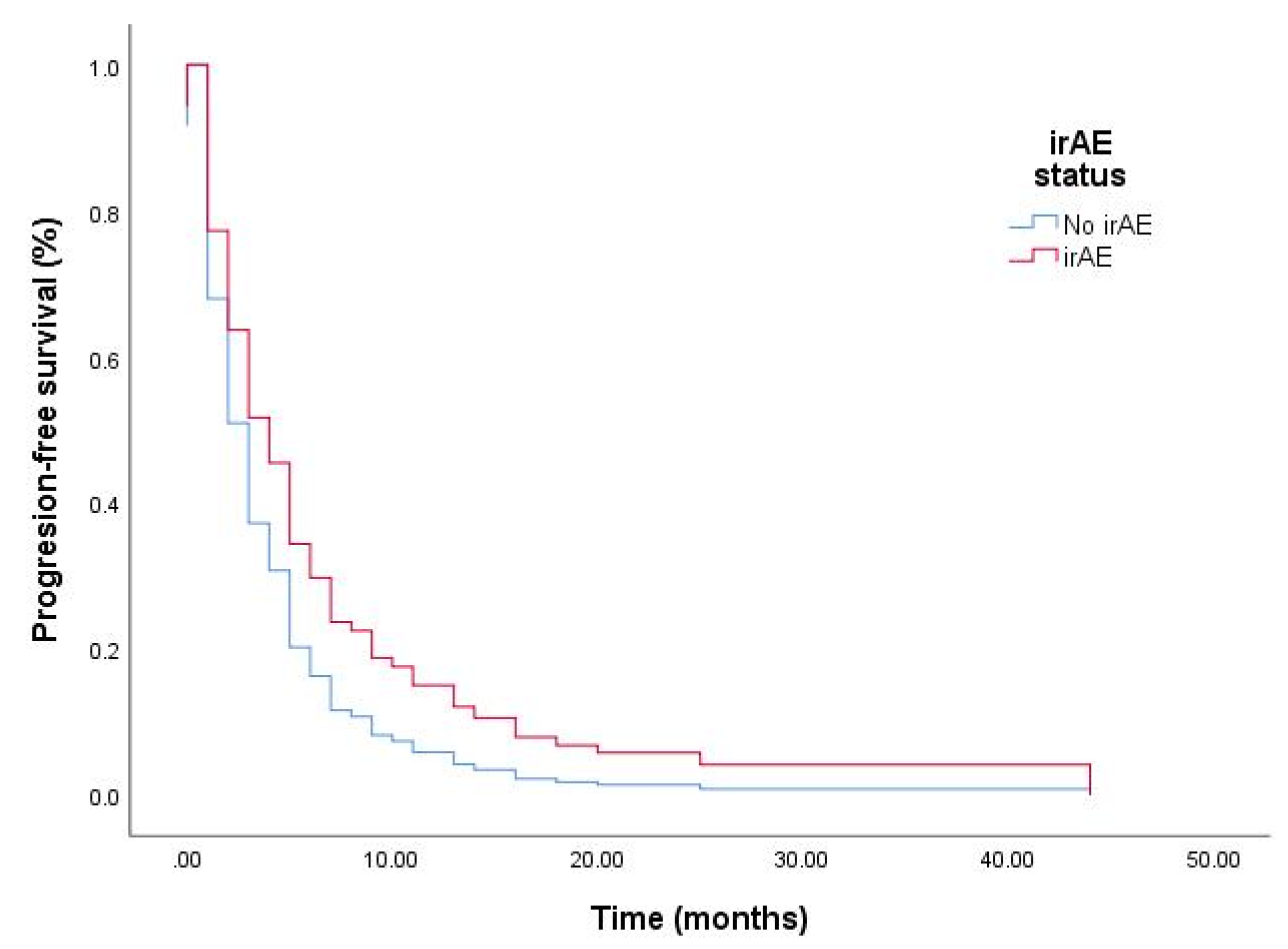
3.3. Univariate Analyses for PFS and OS
3.4. Multivariate Analyses for PFS
3.5. Multivariate Analyses for OS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kohrt, H.E.; Tumeh, P.C.; Benson, D.; Bhardwaj, N.; Brody, J.; Formenti, S.; Fox, B.A.; Galon, J.; June, C.H.; Kalos, M.; et al. Immunodynamics: A cancer immunotherapy trials network review of immune monitoring in immuno-oncology clinical trials. J. Immunother. Cancer 2016, 4, 15. [Google Scholar] [CrossRef]
- Khan, M.; Lin, J.; Liao, G.; Tian, Y.; Liang, Y.; Li, R.; Liu, M.; Yuan, Y. Comparative analysis of immune checkpoint inhibitors and chemotherapy in the treatment of advanced non-small cell lung cancer: A meta-analysis of randomized controlled trials. Medicine 2018, 97, e11936. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Almazor, M.E.; Kim, S.T.; Abdel-Wahab, N.; Diab, A. Review: Immune-Related Adverse Events With Use of Checkpoint Inhibitors for Immunotherapy of Cancer. Arthritis Rheumatol. 2017, 69, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Koelzer, V.H.; Rothschild, S.I.; Zihler, D.; Wicki, A.; Willi, B.; Willi, N.; Voegeli, M.; Cathomas, G.; Zippelius, A.; Mertz, K.D. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors-an autopsy study. J. Immunother. Cancer 2016, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Diem, S.; Schmid, S.; Krapf, M.; Flatz, L.; Born, D.; Jochum, W.; Templeton, A.J.; Früh, M. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017, 111, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, Y.; Liu, F.; Qiu, X.; Zhang, X.; Fang, C.; Qian, X.; Li, Y. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol. Immunother. 2020, 69, 1813–1822. [Google Scholar] [CrossRef]
- Pavan, A.; Calvetti, L.; Dal Maso, A.; Attili, I.; Del Bianco, P.; Pasello, G.; Guarneri, V.; Aprile, G.; Conte, P.; Bonanno, L. Peripheral Blood Markers Identify Risk of Immune-Related Toxicity in Advanced Non-Small Cell Lung Cancer Treated with Immune-Checkpoint Inhibitors. Oncologist 2019, 24, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef]
- Ma, L.; Zeng, A.; Chen, B.; Chen, Y.; Zhou, R. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in patients with systemic lupus erythematosus and their correlation with activity: A meta-analysis. Int. Immunopharmacol. 2019, 76, 105949. [Google Scholar] [CrossRef]
- Zhou, X.; Yao, Z.; Yang, H.; Liang, N.; Zhang, X.; Zhang, F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 2020, 18, 87. [Google Scholar] [CrossRef]
- Zahorec, R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl. Lek. List. 2001, 102, 5–14. [Google Scholar]
- Smith, R.A.; Bosonnet, L.; Ghaneh, P.; Sutton, R.; Evans, J.; Healey, P.; Garvey, C.; Hughes, M.; Raraty, M.; Campbell, F.; et al. The platelet-lymphocyte ratio improves the predictive value of serum CA19-9 levels in determining patient selection for staging laparoscopy in suspected periampullary cancer. Surgery 2008, 143, 658–666. [Google Scholar] [CrossRef]
- Kim, S.; Eliot, M.; Koestler, D.C.; Wu, W.C.; Kelsey, K.T. Association of Neutrophil-to-Lymphocyte Ratio With Mortality and Cardiovascular Disease in the Jackson Heart Study and Modification by the Duffy Antigen Variant. JAMA Cardiol. 2018, 3, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Condamine, T.; Dominguez, G.A.; Youn, J.I.; Kossenkov, A.V.; Mony, S.; Alicea-Torres, K.; Tcyganov, E.; Hashimoto, A.; Nefedova, Y.; Lin, C.; et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci. Immunol. 2016, 1, aaf8943. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef]
- Tay, S.H.; Celhar, T.; Fairhurst, A.M. Low-Density Neutrophils in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2020, 72, 1587–1595. [Google Scholar] [CrossRef]
- Basu, A.; Kollengode, K.A.; Rafatnia, A.; Manoli, H.; Danenberg, G.; Chakravartty, E.; Epstein, A.L.; Pinski, J.K. Relationship between neutrophil lymphocyte ratio (NLR) and MDSC concentration in localized and metastatic castration resistant prostate cancer (mCRPC) patients. J. Clin. Oncol. 2018, 36, 338. [Google Scholar] [CrossRef]
- Gasparyan, A.Y.; Ayvazyan, L.; Mukanova, U.; Yessirkepov, M.; Kitas, G.D. The Platelet-to-Lymphocyte Ratio as an Inflammatory Marker in Rheumatic Diseases. Ann. Lab. Med. 2019, 39, 345–357. [Google Scholar] [CrossRef]
- Eun, Y.; Kim, I.Y.; Sun, J.M.; Lee, J.; Cha, H.S.; Koh, E.M.; Kim, H.; Lee, J. Risk factors for immune-related adverse events associated with anti-PD-1 pembrolizumab. Sci. Rep. 2019, 9, 14039. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tanaka, R.; Maruyama, H.; Ishitsuka, Y.; Okiyama, N.; Watanabe, R.; Fujimoto, M.; Fujisawa, Y. Correlation between blood cell count and outcome of melanoma patients treated with anti-PD-1 antibodies. Jpn. J. Clin. Oncol. 2019, 49, 431–437. [Google Scholar] [CrossRef]
- Giannicola, R.; D’arrigo, G.; Botta, C.; Agostino, R.; Del Medico, P.; Falzea, A.C.; Barbieri, V.; Staropoli, N.; Del Giudice, T.; Pastina, P.; et al. Early blood rise in auto-antibodies to nuclear and smooth muscle antigens is predictive of prolonged survival and autoimmunity in metastatic-non-small cell lung cancer patients treated with PD-1 immune-check point blockade by nivolumab. Mol. Clin. Oncol. 2019, 11, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef]
- Owen, D.H.; Wei, L.; Bertino, E.M.; Edd, T.; Villalona-Calero, M.A.; He, K.; Shields, P.G.; Carbone, D.P.; Otterson, G.A. Incidence, Risk Factors, and Effect on Survival of Immune-related Adverse Events in Patients With Non-Small-cell Lung Cancer. Clin. Lung Cancer 2018, 19, e893–e900. [Google Scholar] [CrossRef]
- Lin, B.D.; Hottenga, J.J.; Abdellaoui, A.; Dolan, C.V.; de Geus, E.J.; Kluft, C.; Boomsma, D.I.; Willemsen, G. Causes of variation in the neutrophil-lymphocyte and platelet-lymphocyte ratios: A twin-family study. Biomark. Med. 2016, 10, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Prabawa, I.P.Y.; Bhargah, A.; Liwang, F.; Tandio, D.A.; Tandio, A.L.; Lestari, A.A.W.; Budiana, I.N.G.; Manuaba, I.B.A.P. Pretreatment Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte Ratio (PLR) as a Predictive Value of Hematological Markers in Cervical Cancer. Asian Pac. J. Cancer Prev. 2019, 20, 863–868. [Google Scholar] [CrossRef]
- Guo, W.; Lu, X.; Liu, Q.; Zhang, T.; Li, P.; Qiao, W.; Deng, M. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for breast cancer patients: An updated meta-analysis of 17079 individuals. Cancer Med. 2019, 8, 4135–4148. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.Y.; Li, J.; Shao, X.Y.; Zhang, C.X. The elevated NLR, PLR and PLT may predict the prognosis of patients with colorectal cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 68837–68846. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.J.; Kim, S.H.; Kim, Y.J.; Kim, M.; Keam, B.; Kim, T.M.; Kim, D.W.; Heo, D.S.; Lee, J.S. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol. Immunother. 2018, 67, 459–470. [Google Scholar] [CrossRef]
- Nakaya, A.; Kurata, T.; Yoshioka, H.; Takeyasu, Y.; Niki, M.; Kibata, K.; Satsutani, N.; Ogata, M.; Miyara, T.; Nomura, S. Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. Int. J. Clin. Oncol. 2018, 23, 634–640. [Google Scholar] [CrossRef]
- Fukui, T.; Okuma, Y.; Nakahara, Y.; Otani, S.; Igawa, S.; Katagiri, M.; Mitsufuji, H.; Kubota, M.; Hiyoshi, Y.; Ishihara, M.; et al. Activity of Nivolumab and Utility of Neutrophil-to-Lymphocyte Ratio as a Predictive Biomarker for Advanced Non-Small-Cell Lung Cancer: A Prospective Observational Study. Clin. Lung Cancer 2019, 20, 208–214.e2. [Google Scholar] [CrossRef]
- Sagiv, J.Y.; Michaeli, J.; Assi, S.; Mishalian, I.; Kisos, H.; Levy, L.; Damti, P.; Lumbroso, D.; Polyansky, L.; Sionov, R.V.; et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015, 10, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Gooden, M.J.; de Bock, G.H.; Leffers, N.; Daemen, T.; Nijman, H.W. The prognostic influence of tumour-infiltrating lymphocytes in cancer: A systematic review with meta-analysis. Br. J. Cancer 2011, 105, 93–103. [Google Scholar] [CrossRef]
- de Coaña, Y.P.; Poschke, I.; Gentilcore, G.; Mao, Y.; Nyström, M.; Hansson, J.; Masucci, G.V.; Kiessling, R. Ipilimumab treatment results in an early decrease in the frequency of circulating granulocytic myeloid-derived suppressor cells as well as their Arginase1 production. Cancer Immunol. Res. 2013, 1, 158–162. [Google Scholar] [CrossRef]
- Kondo, T.; Nomura, M.; Otsuka, A.; Nonomura, Y.; Kaku, Y.; Matsumoto, S.; Muto, M. Predicting marker for early progression in unresectable melanoma treated with nivolumab. Int. J. Clin. Oncol. 2019, 24, 323–327. [Google Scholar] [CrossRef] [PubMed]
| Patients with irAEs (n = 91) | Patients without irAEs (n = 56) | p-Value | |
|---|---|---|---|
| Age (years) | 61.0 (52.0–70.0) | 63.0 (55.5–68.0) | 0.500 |
| Gender (female) | 31 (34.1) | 17 (30.4) | 0.641 |
| Ethnicity | 0.165 | ||
| Chinese | 67 (73.6) | 44 (78.6) | |
| Malay | 11 (12.1) | 2 (3.6) | |
| Indian | 2 (2.2) | 4 (7.1) | |
| Others | 11 (12.1) | 6 (10.7) | |
| ECOG PS | 0.949 | ||
| 0 | 30 (33.0) | 19 (33.9) | |
| 1 | 48 (52.7) | 31 (55.4) | |
| 2 | 2 (5.5) | 4 (7.1) | |
| Smoking status | 0.486 | ||
| Smoker | 40 (44.0) | 23 (41.1) | |
| Non-smoker | 35 (38.5) | 26 (46.4) | |
| BMI | 22.4 (19.2–25.0) | 22.2 (20.6–23.5) | 0.945 |
| Cancer stage | 0.380 | ||
| I | 6 (6.6) | 1 (1.8) | |
| II | 6 (6.6) | 2 (3.6) | |
| III | 13 (14.3) | 9 (16.1) | |
| IV | 54 (59.3) | 41 (73.2) | |
| Cancer type | 0.803 | ||
| Lung cancer | 44 (48.4) | 37 (66.1) | |
| Renal cell carcinoma | 1 (1.1) | 1 (1.8) | |
| Nasopharyngeal carcinoma | 6 (6.6) | 3 (5.4) | |
| Melanoma | 1 (1.1) | 1 (1.8) | |
| Duration of ICI treatment (days) | 72.5 (28.0–215.5) | 68.0 (21.0–143.0) | 0.225 |
| No. of cycles | 4 (2-11) | 4 (2–8) | 0.166 |
| Class of ICI treatment | 0.452 | ||
| Anti-CTLA-4 | 2 (2.2) | 0 (0.0) | |
| Anti-PD-1 | 54 (59.3) | 37 (66.1) | |
| Anti-PD-L1 | 34 (37.4) | 19 (33.9) | |
| Line of treatment | 0.946 | ||
| 1st | 25 (27.5) | 15 (26.8) | |
| 2nd | 25 (27.5) | 18 (32.1) | |
| 3rd | 18 (19.8) | 10 (17.9) | |
| 4th and beyond | 21 (23.1) | 12 (21.4) | |
| Concomitant chemotherapy | 11 (12.1) | 8 (14.3) | 0.700 |
| Concomitant radiotherapy | 9 (9.9) | 5 (8.9) | 0.847 |
| Brain metastases | 9 (9.9) | 8 (14.3) | 0.432 |
| Baseline FBC data (N × 109/L) | |||
| Neutrophil | 4.29 (3.23–5.73) | 5.49 (3.40–7.23) | 0.122 |
| Lymphocyte | 1.30 (0.88–1.74) | 1.25 (0.82–1.77) | 0.603 |
| Platelet | 264 (195–342) | 305 (213–367) | 0.177 |
| NLR | |||
| Baseline | 3.12 (2.22–5.93) | 3.77 (2.92–7.49) | 0.136 |
| Week 6 | 3.20 (2.23–5.08) | 4.21 (2.48–6.83) | 0.063 |
| PLR | |||
| Baseline | 211.84 (131.72–317.09) | 213.00 (144.53–384.15) | 0.582 |
| Week 6 | 196.00 (134.97–337.79) | 236.97 (172.62–383.85) | 0.116 |
| Organ system affected at 1st irAE | |||
| Endocrine | 12 (8.1) | ||
| Hepatic | 11 (7.4) | ||
| Neurological | 11 (7.4) | ||
| Gastrointestinal | 9 (6.1) | ||
| Dermatological | 8 (5.4) | ||
| Pulmonary | 5 (3.4) | ||
| Rheumatic | 3 (2.0) |
| Variable | Category | PFS | |
|---|---|---|---|
| Adjusted HR (95% CI) | p Value | ||
| Model 1 | |||
| ECOG PS | 2 | 1.18 (0.49–2.83) | 0.718 |
| irAE status | irAE case | 0.67 (0.45–0.99) | 0.046 |
| No. of cycles | 0.91 (0.88–0.94) | <0.001 | |
| Concomitant chemotherapy | 0.61 (0.33–1.13) | 0.115 | |
| Baseline NLR | 1.03 (0.98–1.09) | 0.221 | |
| Baseline PLR | 1.00 (1.00–1.00) | 0.764 | |
| Model 2 * | |||
| ECOG PS | 2 | 1.10 (0.37–3.24) | 0.862 |
| irAE status | irAE case | 0.77 (0.50–1.19) | 0.240 |
| No. of cycles | 0.91 (0.88–0.95) | <0.001 | |
| Concomitant chemotherapy | 0.83 (0.45–1.53) | 0.546 | |
| Week 6 NLR | 1.05 (1.00–1.11) | 0.066 | |
| Reduction in PLR | Baseline/Week 6 PLR ratio ≥ 1 | 0.64 (0.40–1.04) | 0.071 |
| Model 3 * | |||
| ECOG PS | 2 | 1.10 (0.37–3.28) | 0.865 |
| irAE status | irAE case | 0.76 (0.49–1.18) | 0.226 |
| No. of cycles | 0.91 (0.88–0.94) | <0.001 | |
| Concomitant chemotherapy | 0.80 (0.43–1.49) | 0.485 | |
| Week 6 NLR | NLR ≥ 3 | 1.27 (0.80–2.00) | 0.307 |
| Reduction in PLR | Baseline/Week 6 PLR ratio ≥ 1 | 0.60 (0.37–0.95) | 0.029 |
| Model 4 * | |||
| ECOG Performance | 2 | 1.23 (0.42–3.61) | 0.705 |
| irAE status | irAE case | 0.77 (0.49–1.18) | 0.229 |
| No. of cycles | 0.91 (0.88–0.95) | <0.001 | |
| Concomitant chemotherapy | 0.77 (0.42–1.42) | 0.399 | |
| Week 6 NLR | NLR ≥ 5 | 1.17 (0.73–1.8) | 0.521 |
| Reduction in PLR | Baseline/Week 6 PLR ratio ≥ 1 | 0.59 (0.37–0.95) | 0.031 |
| Model 5* | |||
| ECOG PS | 2 | 1.37 (0.46–4.03) | 0.574 |
| irAE status | irAE case | 0.72 (0.47–1.11) | 0.141 |
| No. of cycles | 0.92 (0.88–0.95) | <0.001 | |
| Concomitant chemotherapy | 0.93 (0.49–1.76) | 0.811 | |
| Reduction in NLR | Baseline/Week 6 NLR ratio ≥ 1 | 0.56 (0.33–0.95) | 0.031 |
| Reduction in PLR | Baseline/Week 6 PLR ratio ≥ 1 | 0.73 (0.44–1.22) | 0.228 |
| Variable | Category | OS | |
|---|---|---|---|
| Adjusted HR (95% CI) | p Value | ||
| Model 1 | |||
| ECOG PS | 2 | 2.09 (0.82–5.36) | 0.124 |
| BMI | 0.92 (0.86–0.97) | 0.004 | |
| Cancer type | Lung cancer | 0.78 (0.50–1.21) | 0.268 |
| irAE status | irAE case | 0.68 (0.44–1.05) | 0.084 |
| No. of cycles | 0.88 (0.84–0.93) | <0.001 | |
| Concomitant chemotherapy | 0.52 (0.26–1.05) | 0.066 | |
| Baseline NLR | 1.04 (1.02–1.06) | 0.001 | |
| Baseline PLR | PLR ≥ 180 | 1.12 (0.70–1.81) | 0.631 |
| Model 2 | |||
| ECOG PS | 2 | 2.37 (0.96–5.87) | 0.061 |
| BMI | 0.90 (0.85–0.95) | <0.001 | |
| Cancer type | Lung cancer | 0.71 (0.46–1.10) | 0.122 |
| irAE status | irAE case | 0.75 (0.48–1.16) | 0.199 |
| No. of cycles | 0.86 (0.82–0.91) | <0.001 | |
| Concomitant chemotherapy | 0.64 (0.32–1.26) | 0.196 | |
| Baseline NLR | NLR ≥ 3 | 2.64 (1.49–4.69) | 0.001 |
| Baseline PLR | PLR ≥ 180 | 0.72 (0.41–1.27) | 0.257 |
| Model 3 | |||
| ECOG PS | 2 | 2.48 (0.96–6.39) | 0.060 |
| BMI | 0.91 (0.86–0.97) | 0.003 | |
| Cancer type | Lung cancer | 0.75 (0.49–1.17) | 0.207 |
| irAE status | irAE case | 0.67 (0.43–1.04) | 0.074 |
| No. of cycles | 0.88 (0.84–0.93) | <0.001 | |
| Concomitant chemotherapy | 0.56 (0.28–1.12) | 0.102 | |
| Baseline NLR | NLR ≥ 5 | 1.46 (0.87–2.43) | 0.149 |
| Baseline PLR | PLR ≥ 180 | 1.12 (0.68–1.84) | 0.666 |
| Model 4* | |||
| ECOG PS | 2 | 1.45 (0.46–4.61) | 0.527 |
| BMI | 0.90 (0.84–0.97) | 0.004 | |
| Cancer type | Lung cancer | 0.94 (0.58–1.51) | 0.791 |
| irAE status | irAE case | 0.72 (0.45–1.16) | 0.178 |
| No. of cycles | 0.92 (0.88–0.95) | <0.001 | |
| Concomitant chemotherapy | 1.18 (0.56–2.49) | 0.663 | |
| Week 6 NLR | 1.29 (1.17–1.42) | <0.001 | |
| Week 6 PLR | 1.00 (1.00–1.00) | 0.006 | |
| Model 5 * | |||
| ECOG PS | 2 | 2.23 (0.74–6.72) | 0.156 |
| BMI | 0.91 (0.85–0.97) | 0.006 | |
| Cancer type | Lung cancer | 0.72 (0.44–1.18) | 0.197 |
| irAE status | irAE case | 0.67 (0.42–1.08) | 0.101 |
| No. of cycles | 0.89 (0.86–0.93) | <0.001 | |
| Concomitant chemotherapy | 1.00 (0.47–2.12) | 0.997 | |
| Week 6 NLR | NLR ≥ 3 | 3.12 (1.73–5.61) | <0.001 |
| Week 6 PLR | 1.00 (1.00–1.00) | 0.823 | |
| Model 6* | |||
| ECOG PS | 2 | 2.81 (0.91–8.69) | 0.074 |
| BMI | 0.93 (0.86–1.00) | 0.035 | |
| Cancer type | Lung cancer | 0.85 (0.53–1.38) | 0.517 |
| irAE status | irAE case | 0.69 (0.43–1.10) | 0.117 |
| No. of cycles | 0.91 (0.87–0.95) | <0.001 | |
| Concomitant chemotherapy | 0.84 (0.41–1.72) | 0.631 | |
| Week 6 NLR | NLR ≥ 5 | 3.55 (1.84–6.85) | <0.001 |
| Week 6 PLR | 1.00 (1.00–1.00) | 0.395 | |
| Model 7* | |||
| ECOG PS | 2 | 4.06 (1.26–13.05) | 0.019 |
| BMI | 0.94 (0.87–1.01) | 0.071 | |
| Cancer type | Lung cancer | 1.08 (0.68–1.73) | 0.735 |
| irAE status | irAE case | 0.64 (0.40–1.02) | 0.063 |
| No. of cycles | 0.90 (0.86–0.95) | <0.001 | |
| Concomitant chemotherapy | 1.20 (0.56–2.59) | 0.637 | |
| Reduction in NLR | Baseline/Week 6 NLR ratio ≥ 1 | 0.38 (0.20–0.62) | <0.001 |
| Week 6 PLR | 1.00 (1.00–1.00) | 0.222 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, P.Y.; Oen, K.Q.X.; Lim, G.R.S.; Hartono, J.L.; Muthiah, M.; Huang, D.Q.; Teo, F.S.W.; Li, A.Y.; Mak, A.; Chandran, N.S.; et al. Neutrophil-to-Lymphocyte Ratio Predicts Development of Immune-Related Adverse Events and Outcomes from Immune Checkpoint Blockade: A Case-Control Study. Cancers 2021, 13, 1308. https://doi.org/10.3390/cancers13061308
Lee PY, Oen KQX, Lim GRS, Hartono JL, Muthiah M, Huang DQ, Teo FSW, Li AY, Mak A, Chandran NS, et al. Neutrophil-to-Lymphocyte Ratio Predicts Development of Immune-Related Adverse Events and Outcomes from Immune Checkpoint Blockade: A Case-Control Study. Cancers. 2021; 13(6):1308. https://doi.org/10.3390/cancers13061308
Chicago/Turabian StyleLee, Pei Yi, Kellynn Qi Xuan Oen, Grace Rui Si Lim, Juanda Leo Hartono, Mark Muthiah, Daniel Q. Huang, Felicia Su Wei Teo, Andrew Yunkai Li, Anselm Mak, Nisha Suyien Chandran, and et al. 2021. "Neutrophil-to-Lymphocyte Ratio Predicts Development of Immune-Related Adverse Events and Outcomes from Immune Checkpoint Blockade: A Case-Control Study" Cancers 13, no. 6: 1308. https://doi.org/10.3390/cancers13061308
APA StyleLee, P. Y., Oen, K. Q. X., Lim, G. R. S., Hartono, J. L., Muthiah, M., Huang, D. Q., Teo, F. S. W., Li, A. Y., Mak, A., Chandran, N. S., Tan, C. L., Yang, P., Tai, E. S., Ng, K. W. P., Vijayan, J., Chan, Y. C., Tan, L. L., Lee, M. B.-H., Chua, H. R., ... Tay, S. H. (2021). Neutrophil-to-Lymphocyte Ratio Predicts Development of Immune-Related Adverse Events and Outcomes from Immune Checkpoint Blockade: A Case-Control Study. Cancers, 13(6), 1308. https://doi.org/10.3390/cancers13061308