Doxorubicin-Loaded Gold Nanoarchitectures as a Therapeutic Strategy against Diffuse Intrinsic Pontine Glioma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Nanoparticles
2.2. Drug Delivery Studies
2.3. Transmission Electron Microscopy
2.4. Human DIPG Neurosphere-Forming Cultures
2.5. Proliferation Assays
2.6. Soft Agar Colony Assays
2.7. Western Blot Experiments
2.8. High Content Imaging
2.9. Confocal Imaging
2.10. Orthotopic DIPG Animal Model and Drug Treatments
2.11. Detection of Gold in DIPG Xenografted Brains
2.12. Statistical Analysis
3. Results
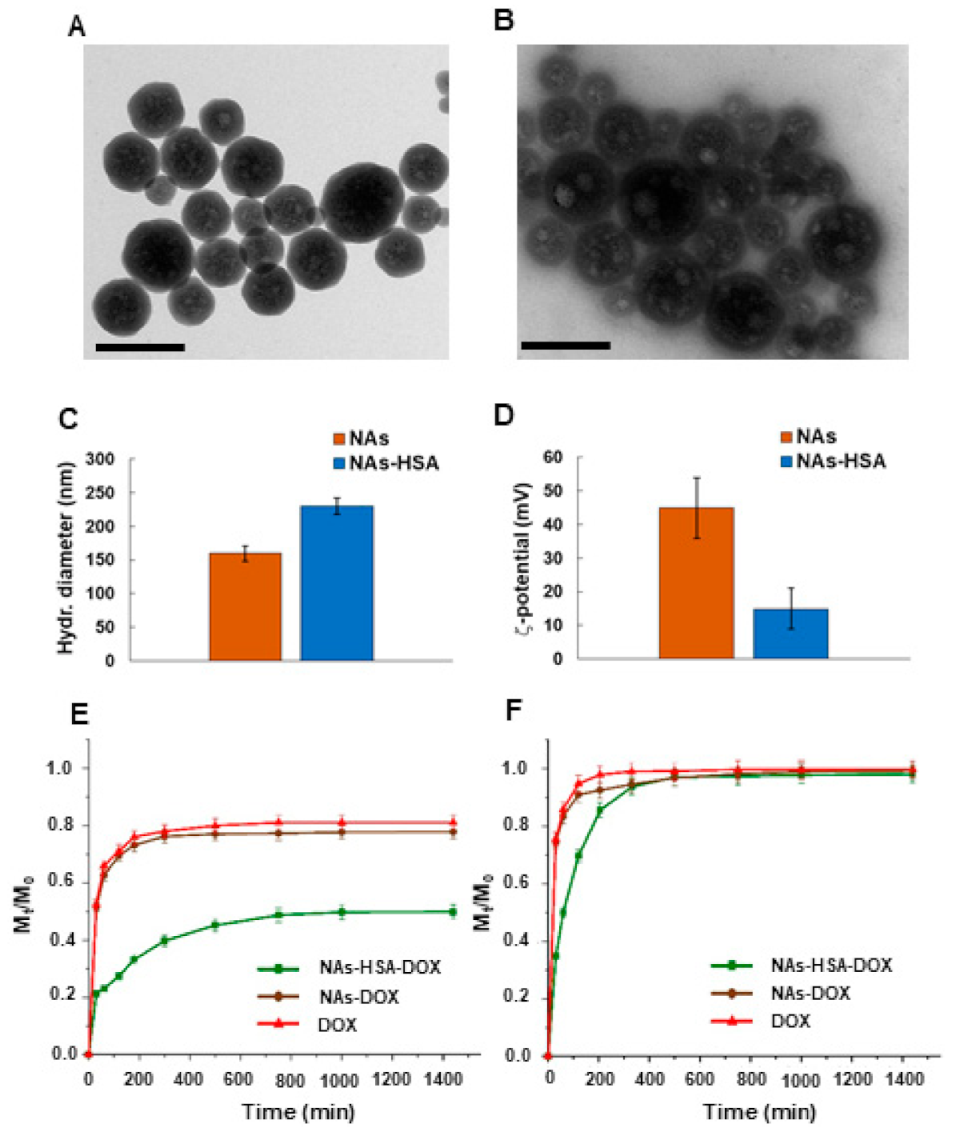
3.1. Preparation and Characterization of NAs-HSA-Dox
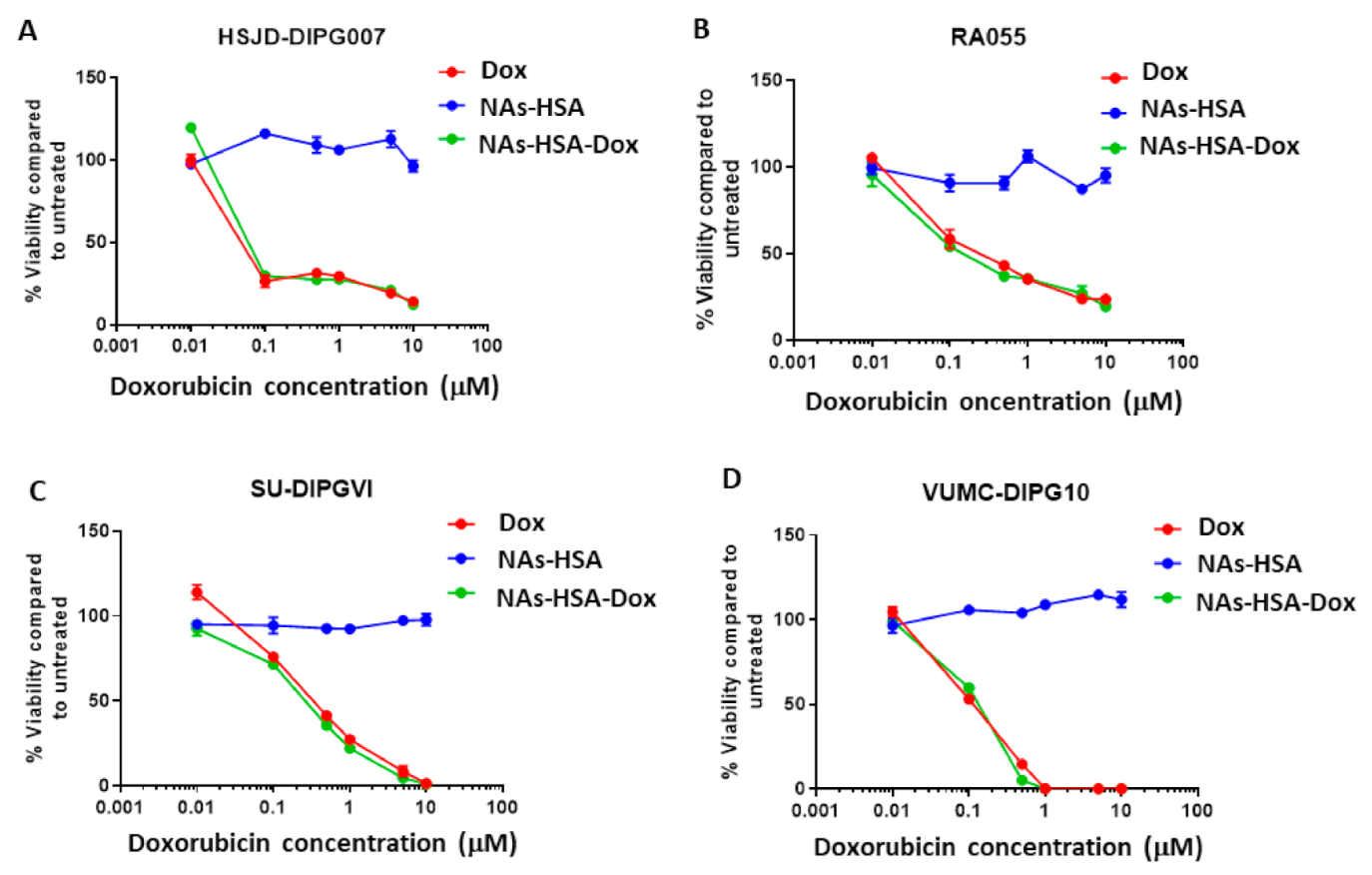
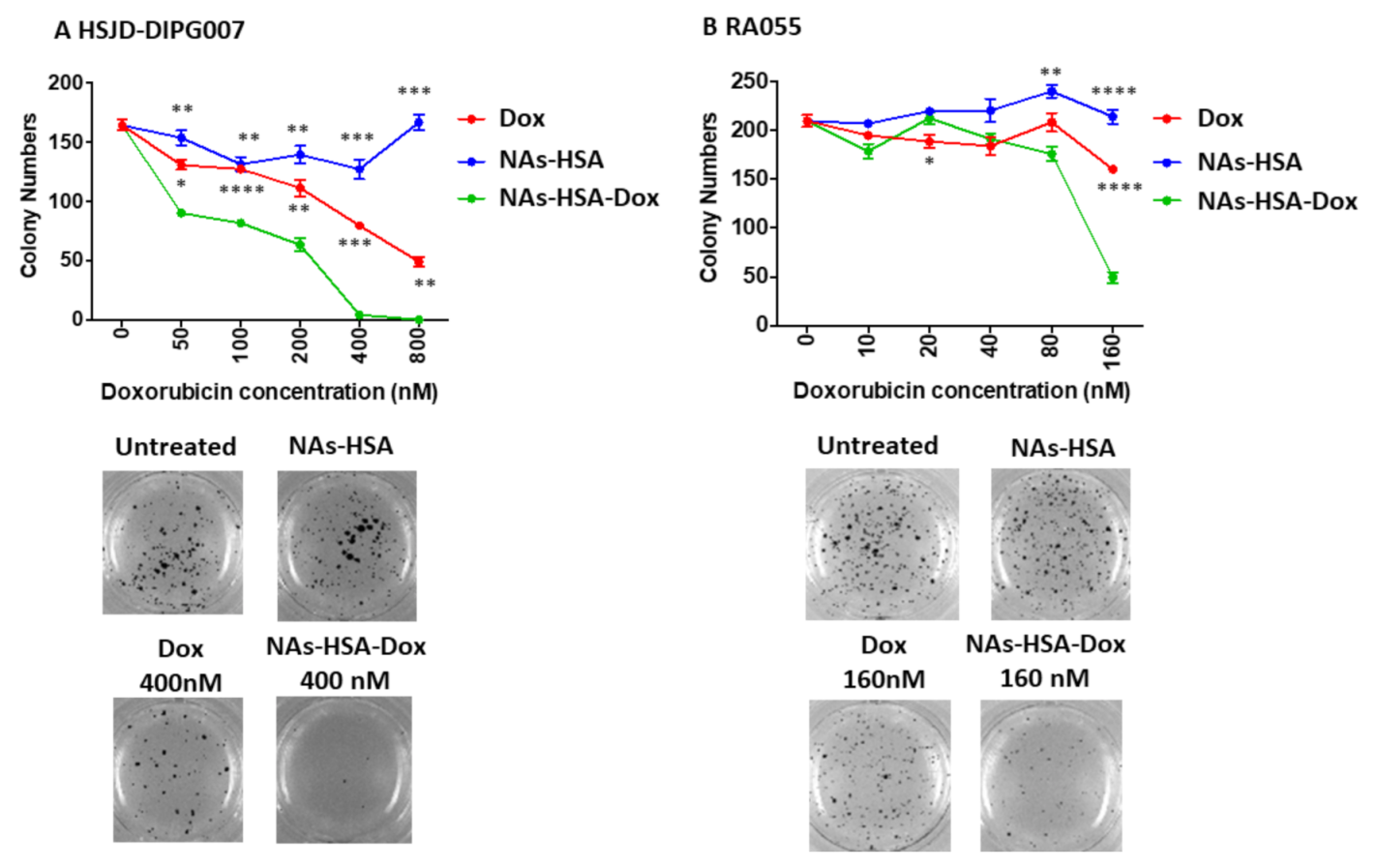
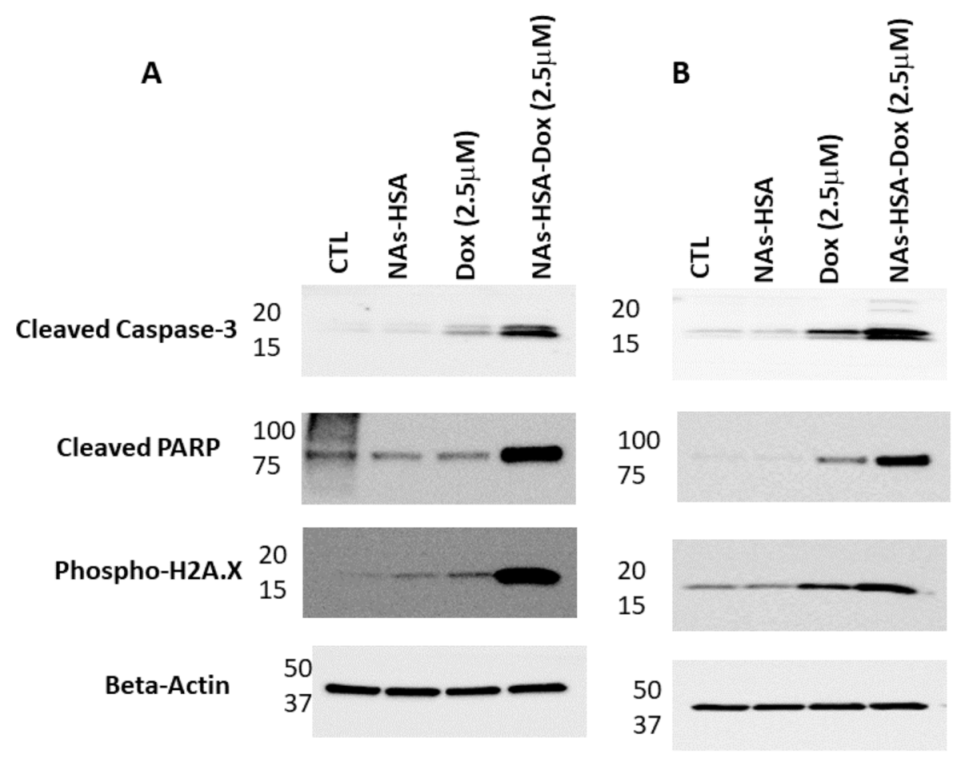
3.2. Cytotoxic Efficacy of Doxorubicin-Loaded Gold Nanoparticles in Neurosphere-Forming DIPG Cultures
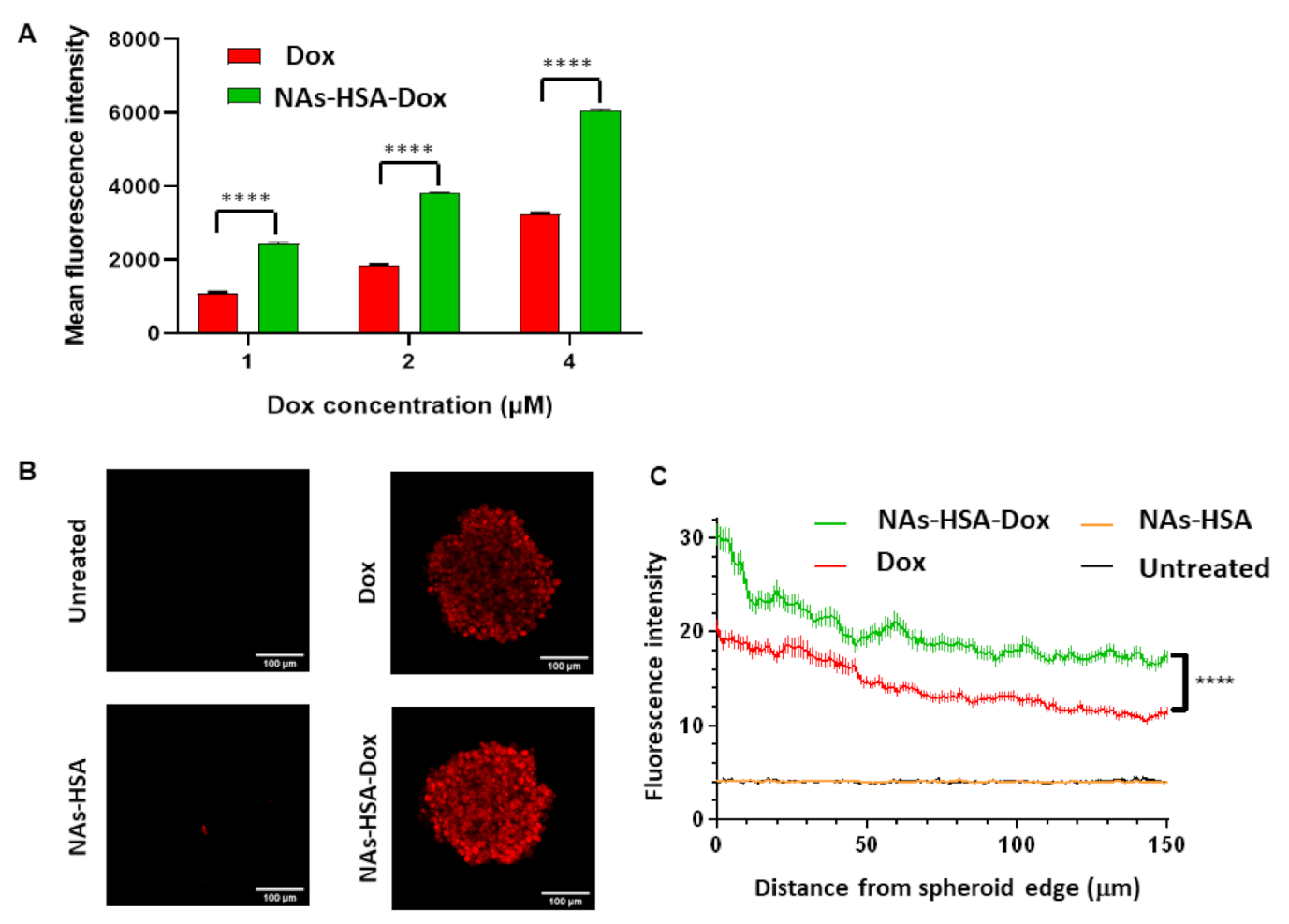
3.3. Penetration of Doxorubicin-Loaded Nanoparticles in DIPG Neurospheres
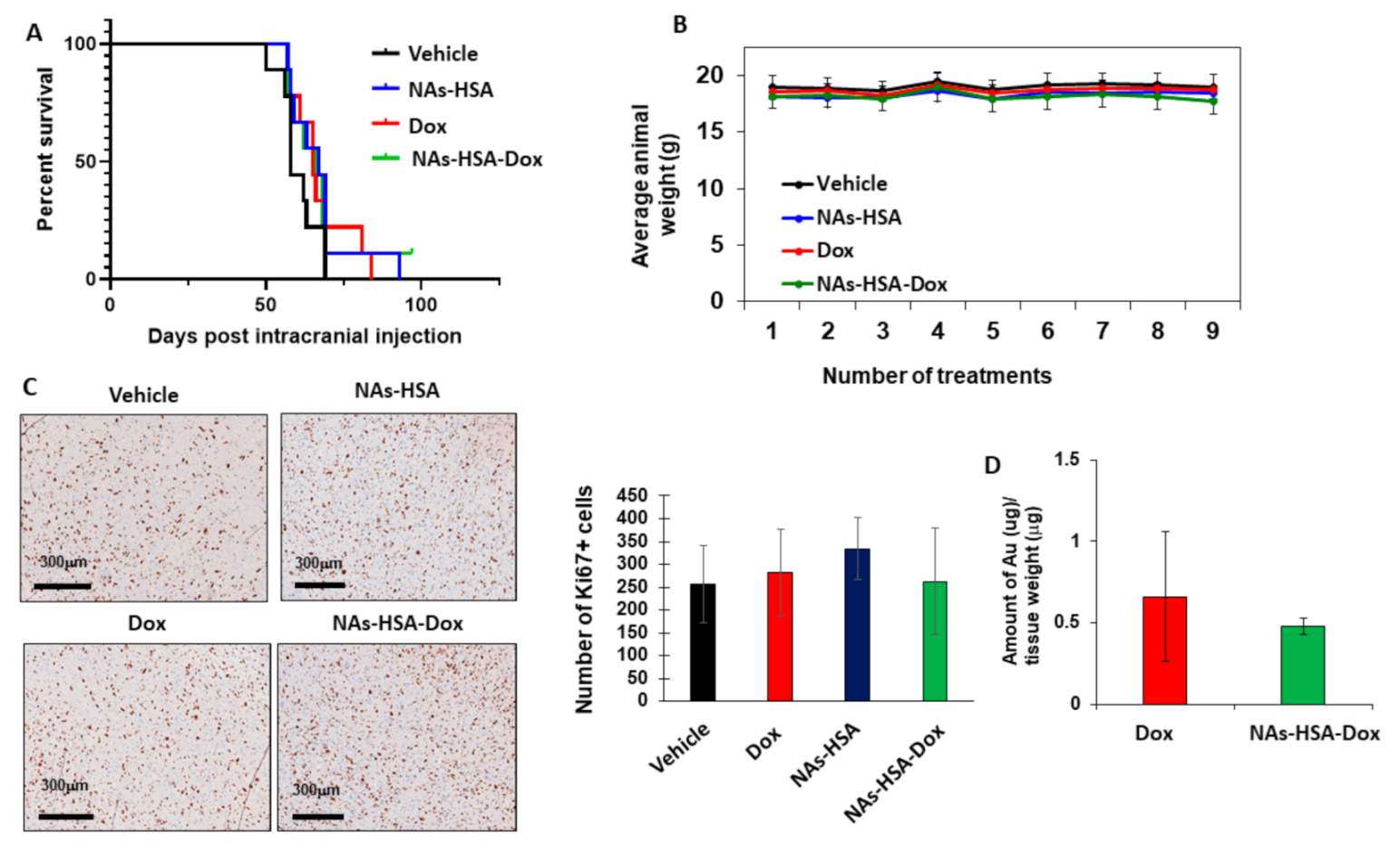
3.4. In Vivo Efficacy of Doxorubicin-Loaded Nanoparticles in an Orthotopic Patient-Derived DIPG Animal Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buczkowicz, P.; Hawkins, C. Pathology, molecular genetics, and epigenetics of diffuse intrinsic pontine glioma. Front. Oncol. 2015, 5, 147. [Google Scholar] [CrossRef]
- Gallitto, M.; Lazarev, S.; Wasserman, I.; Stafford, J.M.; Wolden, S.L.; Terezakis, S.A.; Bindra, R.S.; Bakst, R.L. Role of radiation therapy in the management of diffuse intrinsic pontine glioma: A systematic review. Adv. Radiat. Oncol. 2019, 4, 520–531. [Google Scholar] [CrossRef]
- Freeman, C.R.; Perilongo, G. Chemotherapy for brain stem gliomas. Childs Nerv. Syst. 1999, 15, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.K.; Rutka, J.T. Diffuse intrinsic pontine glioma: Clinical features, molecular genetics, and novel targeted therapeutics. J. Korean Neurosurg. Soc. 2018, 61, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Grill, J.; Puget, S.; Andreiuolo, F.; Philippe, C.; MacConaill, L.; Kieran, M.W. Critical oncogenic mutations in newly diagnosed pediatric diffuse intrinsic pontine glioma. Pediatr. Blood Cancer 2012, 58, 489–491. [Google Scholar] [CrossRef]
- Wu, G.; Diaz, A.K.; Paugh, B.S.; Rankin, S.L.; Ju, B.; Li, Y.; Zhu, X.; Qu, C.; Chen, X.; Zhang, J.; et al. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat. Genet. 2014, 46, 444–450. [Google Scholar]
- Paugh, B.S.; Broniscer, A.; Qu, C.; Miller, C.P.; Zhang, J.; Tatevossian, R.G.; Olson, J.M.; Geyer, J.R.; Chi, S.N.; da Silva, N.S.; et al. Genome-wide analyses identify recurrent amplifications of receptor tyrosine kinases and cell-cycle regulatory genes in diffuse intrinsic pontine glioma. J. Clin. Oncol. 2011, 29, 3999–4006. [Google Scholar] [CrossRef] [PubMed]
- Hankinson, T.C.; Campagna, E.J.; Foreman, N.K.; Handler, M.H. Interpretation of magnetic resonance images in diffuse intrinsic pontine glioma: A survey of pediatric neurosurgeons. J. Neurosurg. Pediatr. 2011, 8, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Chaves, C.; Decleves, X.; Taghi, M.; Menet, M.C.; Lacombe, J.; Varlet, P.; Olaciregui, N.G.; Carcaboso, A.M.; Cisternino, S. Characterization of the blood-brain barrier integrity and the brain transport of SN-38 in an orthotopic xenograft rat model of diffuse intrinsic pontine glioma. Pharmaceutics 2020, 12, 399. [Google Scholar] [CrossRef] [PubMed]
- Warren, K.E. Beyond the blood: Brain barrier: The importance of central nervous system (CNS) pharmacokinetics for the treatment of CNS tumors, including diffuse intrinsic pontine glioma. Front. Oncol 2018, 8, 239. [Google Scholar] [CrossRef]
- Khan, A.; Gamble, L.D.; Upton, D.H.; Ung, C.; Yu, D.M.T.; Ehteda, A.; Pandher, R.; Mayoh, C.; Hébert, S.; Jabado, N.; et al. Dual targeting of polyamine synthesis and uptake in diffuse intrinsic pontine gliomas. Nat. Commun. 2020, 12, 971. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Singh, R.; Souweidane, M.M. Convection-enhanced delivery for diffuse intrinsic pontine glioma treatment. Curr. Neuropharmacol. 2017, 15, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Souweidane, M.M.; Kramer, K.; Pandit-Taskar, N.; Zanzonico, P.; Zhou, Z.P.; Donzelli, M.; Lyashchenko, S.K.; Haque, S.; Thakur, S.B.; Cheung, N.K.V.; et al. A phase I study of convection enhanced delivery (CED) of I-124-8H9 radio-labeled monoclonal antibody in children with diffuse intrinsic pontine glioma (DIPG). J. Clin. Oncol. 2017, 35, 2010. [Google Scholar] [CrossRef]
- Singleton, W.G.B.; Bienemann, A.S.; Woolley, M.; Johnson, D.; Lewis, O.; Wyatt, M.J.; Damment, S.J.P.; Boulter, L.J.; Killick-Cole, C.L.; Asby, D.J.; et al. The distribution, clearance, and brainstem toxicity of panobinostat administered by convection-enhanced delivery. J. Neurosurg. Pediatr. 2018, 22, 288–296. [Google Scholar] [CrossRef]
- Etame, A.B.; Diaz, R.J.; O’Reilly, M.A.; Smith, C.A.; Mainprize, T.G.; Hynynen, K.; Rutka, J.T. Enhanced delivery of gold nanoparticles with therapeutic potential into the brain using MRI-guided focused ultrasound. Nanomedicine 2012, 8, 1133–1142. [Google Scholar] [CrossRef]
- Alli, S.; Figueiredo, C.A.; Golbourn, B.; Sabha, N.; Wu, M.Y.; Bondoc, A.; Luck, A.; Coluccia, D.; Maslink, C.; Smith, C.; et al. Brainstem blood brain barrier disruption using focused ultrasound: A demonstration of feasibility and enhanced doxorubicin delivery. J. Control. Release 2018, 281, 29–41. [Google Scholar] [CrossRef]
- Park, J.; Aryal, M.; Vykhodtseva, N.; Zhang, Y.Z.; McDannold, N. Evaluation of permeability, doxorubicin delivery, and drug retention in a rat brain tumor model after ultrasound-induced blood-tumor barrier disruption. J. Control. Release 2017, 250, 77–85. [Google Scholar] [CrossRef]
- D’Avella, D.; Cicciarello, R.; Albiero, F.; Mesiti, M.; Gagliardi, M.E.; Russi, E.; d’Aquino, A.; Tomasello, F.; d’Aquino, S. Quantitative study of blood-brain barrier permeability changes after experimental whole-brain radiation. Neurosurgery 1992, 30, 30–34. [Google Scholar] [CrossRef]
- Bart, J.; Nagengast, W.B.; Coppes, R.P.; Wegman, T.D.; van der Graaf, W.T.; Groen, H.J.; Vaalburg, W.; de Vries, E.G.; Hendrikse, N.H. Irradiation of rat brain reduces P-glycoprotein expression and function. Br. J. Cancer 2007, 97, 322–326. [Google Scholar] [CrossRef][Green Version]
- Khatri, A.; Gaber, M.W.; Brundage, R.C.; Naimark, M.D.; Hanna, S.K.; Stewart, C.F.; Kirstein, M.N. Effect of radiation on the penetration of irinotecan in rat cerebrospinal fluid. Cancer Chemother. Pharmacol. 2011, 68, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Ma, J.; Xiao, J.; Tang, Z. Effect of brain irradiation on blood-CSF barrier permeability of chemotherapeutic agents. Am. J. Clin. Oncol. 1997, 20, 263–265. [Google Scholar] [CrossRef]
- Ganipineni, L.P.; Danhier, F.; Preat, V. Drug delivery challenges and future of chemotherapeutic nanomedicine for glioblastoma treatment. J. Control. Release 2018, 281, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Akhter, S.; Ahmad, I.; Ahmad, M.Z.; Ramazani, F.; Singh, A.; Rahman, Z.; Ahmad, F.J.; Storm, G.; Kok, R.J. Nanomedicines as cancer therapeutics: Current status. Curr. Cancer Drug Targets 2013, 13, 362–378. [Google Scholar] [CrossRef]
- Kong, F.Y.; Zhang, J.W.; Li, R.F.; Wang, Z.X.; Wang, W.J.; Wang, W. Unique roles of gold nanoparticles in drug delivery, targeting and imaging applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef] [PubMed]
- Cabuzu, D.; Cirja, A.; Puiu, R.; Grumezescu, A.M. Biomedical applications of gold nanoparticles. Curr. Top. Med. Chem. 2015, 15, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandit, S.; Mokkapati, V.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef] [PubMed]
- Yah, C.S. The toxicity of gold nanoparticles in relation to their physiochemical properties. Biomed. Res. India 2013, 24, 400–413. [Google Scholar]
- Armanetti, P.; Pocoví-Martínez, S.; Flori, A.; Avigo, C.; Cassano, D.; Menichetti, L.; Voliani, V. Dual photoacoustic/ultrasound multi-parametric imaging from passion fruit-like nano-architectures. Nanomedicine 2018, 14, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Cassano, D.; Santi, M.; D’Autilia, F.; Mapanao, A.K.; Luin, S.; Voliani, V. Photothermal effect by NIR-responsive excretable ultrasmall-in-nano architectures. Mater. Horiz. 2019, 6, 531–537. [Google Scholar] [CrossRef]
- Cassano, D.; Santi, M.; Cappello, V.; Luin, S.; Signore, G.; Voliani, V. Biodegradable passion fruit-like nano-architectures as carriers for cisplatin prodrug. Part. Part. Syst. Char. 2016, 33, 818–824. [Google Scholar] [CrossRef]
- Santi, M.; Mapanao, A.K.; Cassano, D.; Vlamidis, Y.; Cappello, V.; Voliani, V. Endogenously-activated ultrasmall-in-nano therapeutics: Assessment on 3D head and neck squamous cell carcinomas. Cancers 2020, 12, 1063. [Google Scholar] [CrossRef] [PubMed]
- Cassano, D.; Mapanao, A.K.; Summa, M.; Vlamidis, Y.; Giannone, G.; Santi, M.; Guzzolino, E.; Pitto, L.; Poliseno, L.; Bertorelli, R.; et al. Biosafety and biokinetics of noble metals: The impact of their chemical nature. ACS Appl. Bio. Mater. 2019, 2, 4464–4470. [Google Scholar] [CrossRef]
- Mapanao, A.K.; Santi, M.; Voliani, V. Combined chemo-photothermal treatment of three-dimensional head and neck squamous cell carcinomas by gold nano-architectures. J. Colloid Interface Sci. 2021, 582, 1003–1011. [Google Scholar] [CrossRef]
- Lerra, L.; Farfalla, A.; Sanz, B.; Cirillo, G.; Vittorio, O.; Voli, F.; Grand, M.L.; Curcio, M.; Nicoletta, F.P.; Dubrovska, A.; et al. Graphene oxide functional nanohybrids with magnetic nanoparticles for improved vectorization of doxorubicin to neuroblastoma cells. Pharmaceutics 2019, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Cardoso Avila, P.E.; Rangel Mendoza, A.; Pichardo Molina, J.L.; Flores Villavicencio, L.L.; Castruita Dominguez, J.P.; Chilakapati, M.K.; Sabanero Lopez, M. Biological response of HeLa cells to gold nanoparticles coated with organic molecules. Toxicol. Vitr. 2017, 42, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Mapanao, A.K.; Santi, M.; Faraci, P.; Cappello, V.; Cassano, D.; Voliani, V. Endogenously triggerable ultrasmall-in-nano architectures: Targeting assessment on 3D pancreatic carcinoma spheroids. ACS Omega 2018, 3, 11796–11801. [Google Scholar] [CrossRef]
- Cassano, D.; Summa, M.; Pocoví-Martínez, S.; Mapanao, A.K.; Catelani, T.; Bertorelli, R.; Voliani, V. Biodegradable ultrasmall-in-nano gold architectures: Mid-period in vivo distribution and excretion assessment. Part. Part. Sys. Charact. 2019, 36. [Google Scholar] [CrossRef]
- Song, M.; Wang, J.; Lei, J.; Peng, G.; Zhang, W.; Zhang, Y.; Yin, M.; Li, J.; Liu, Y.; Wei, X.; et al. Preparation and evaluation of liposomes co-loaded with doxorubicin, phospholipase d inhibitor 5-fluoro-2-indolyl deschlorohalopemide (FIPI) and D-alpha tocopheryl acid succinate (α-TOS) for anti-metastasis. Nanoscale Res. Lett. 2019, 14, 138. [Google Scholar] [CrossRef]
- Ullah, K.; Sohail, M.; Murtaza, G.; Khan, S.A. Natural and synthetic materials based CMCh/PVA hydrogels for oxaliplatin delivery: Fabrication, characterization, In-vitro and in-vivo safety profiling. Int. J. Biol. Macromol. 2019, 122, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Unagolla, J.M.; Jayasuriya, A.C. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur. J. Pharm. Sci. 2018, 114, 199–209. [Google Scholar] [CrossRef]
- Faria, M.; Bjornmalm, M.; Thurecht, K.J.; Kent, S.J.; Parton, R.G.; Kavallaris, M.; Johnston, A.P.R.; Gooding, J.J.; Corrie, S.R.; Boyd, B.J.; et al. Minimum information reporting in bio-nano experimental literature. Nat. Nanotechnol. 2018, 13, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 1, 37–42. [Google Scholar] [CrossRef]
- Tsoli, M.; Shen, H.; Mayoh, C.; Franshaw, L.; Ehteda, A.; Upton, D.; Carvalho, D.; Vinci, M.; Meel, M.H.; van Vuurden, D.; et al. International experience in the development of patient-derived xenograft models of diffuse intrinsic pontine glioma. J. Neurooncol. 2019, 141, 253–263. [Google Scholar] [CrossRef]
- Tsoli, M.; Liu, J.; Franshaw, L.; Shen, H.; Cheng, C.; Jung, M.; Joshi, S.; Ehteda, A.; Khan, A.; Montero-Carcabosso, A.; et al. Dual targeting of mitochondrial function and mTOR pathway as a therapeutic strategy for diffuse intrinsic pontine glioma. Oncotarget 2018, 9, 7541–7556. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Mapanao, A.K.; Giannone, G.; Summa, M.; Ermini, M.L.; Zamborlin, A.; Santi, M.; Cassano, D.; Bertorelli, R.; Voliani, V. Biokinetics and clearance of inhaled gold ultrasmall-in-nano architectures. Nanoscale Adv. 2020, 2, 3815–3820. [Google Scholar] [CrossRef]
- Onafuye, H.; Pieper, S.; Mulac, D.; Cinatl, J., Jr.; Wass, M.N.; Langer, K.; Michaelis, M. Doxorubicin-loaded human serum albumin nanoparticles overcome transporter-mediated drug resistance in drug-adapted cancer cells. Beilstein J. Nanotechnol. 2019, 10, 1707–1715. [Google Scholar] [CrossRef]
- Dreis, S.; Rothweiler, F.; Michaelis, M.; Cinatl, J., Jr.; Kreuter, J.; Langer, K. Preparation, characterisation and maintenance of drug efficacy of doxorubicin-loaded human serum albumin (HSA) nanoparticles. Int. J. Pharm. 2007, 314, 207–214. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, B. Doxorubicin induces cardiotoxicity through upregulation of death receptors mediated apoptosis in cardiomyocytes. Sci. Rep. 2017, 7, 44735. [Google Scholar] [CrossRef]
- Mapanao, A.K.; Voliani, V. Three-dimensional tumor models: Promoting breakthroughs in nanotheranostics translational research. Appl. Mater. Today 2020, 19, 100552. [Google Scholar] [CrossRef]
- Sagnella, S.M.; Trieu, J.; Brahmbhatt, H.; MacDiarmid, J.A.; MacMillan, A.; Whan, R.M.; Fife, C.M.; McCarroll, J.A.; Gifford, A.J.; Ziegler, D.S.; et al. Targeted doxorubicin-loaded bacterially derived nano-cells for the treatment of neuroblastoma. Mol. Cancer Ther. 2018, 17, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Yu, M.; Tsoli, M.; Chang, C.; Joshi, S.; Liu, J.; Ryall, S.; Chornenkyy, Y.; Siddaway, R.; Hawkins, C.; et al. Targeting reduced mitochondrial DNA quantity as a therapeutic approach in pediatric high-grade gliomas. Neuro Oncol. 2020, 22, 139–151. [Google Scholar] [CrossRef] [PubMed]
- D’Amora, M.; Cassano, D.; Pocoví-Martínez, S.; Giordani, S.; Voliani, V. Biodistribution and biocompatibility of passion fruit-like nano-architectures in zebrafish. Nanotoxicology 2018, 12, 914–922. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems—A review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar]
- Katende, A.; Boyou, N.V.; Ismail, I.; Chung, D.Z.; Sagala, F.; Hussein, N.; Ismail, M.S. Improving the performance of oil based mud and water based mud in a high temperature hole using nanosilica nanoparticles. Colloids Surf. 2019, 577, 645–673. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, A.R.; Lee, S.S.; Bhattacharya, M.; Nam, J.S.; Chakraborty, C. Advances in nanocarriers enabled brain targeted drug delivery across blood brain barrier. Int. J. Pharm. 2019, 559, 360–372. [Google Scholar] [CrossRef]
- Maboudi, S.A.; Shojaosadati, S.A.; Arpanaei, A. Synthesis and characterization of multilayered nanobiohybrid magnetic particles for biomedical applications. Mater. Des. 2017, 115, 317–324. [Google Scholar] [CrossRef]
- Catanzaro, G.; Curcio, M.; Cirillo, G.; Spizzirri, U.G.; Besharat, Z.M.; Abballe, L.; Vacca, A.; Iemma, F.; Picci, N.; Ferretti, E. Albumin nanoparticles for glutathione-responsive release of cisplatin: New opportunities for medulloblastoma. Int. J. Pharm. 2017, 517, 168–174. [Google Scholar] [CrossRef]
- Baneshi, M.; Dadfarnia, S.; Shabani, A.M.H.; Sabbagh, S.K.; Haghgoo, S.; Bardania, H. A novel theranostic system of AS1411 aptamer-functionalized albumin nanoparticles loaded on iron oxide and gold nanoparticles for doxorubicin delivery. Int. J. Pharm. 2019, 564, 145–152. [Google Scholar] [CrossRef]
- Beňová, E.; Bergé-Lefranc, D.; Zeleňák, V.; Almáši, M.; Huntošová, V.; Hornebecq, V. Adsorption properties, the pH-sensitive release of 5-fluorouracil and cytotoxicity studies of mesoporous silica drug delivery matrix. Appl. Surf. Sci. 2020, 504, 144028. [Google Scholar] [CrossRef]
- Tian, Y.; Mi, G.; Chen, Q.; Chaurasiya, B.; Li, Y.; Shi, D.; Zhang, Y.; Webster, T.J.; Sun, C.; Shen, Y. Acid-induced activated cell-penetrating peptide-modified cholesterol-conjugated polyoxyethylene sorbitol oleate mixed micelles for pH-triggered drug release and efficient brain tumor targeting based on a charge reversal mechanism. ACS Appl. Mater. Interfaces 2018, 10, 43411–43428. [Google Scholar] [CrossRef]
- Curcio, M.; Diaz-Gomez, L.; Cirillo, G.; Concheiro, A.; Iemma, F.; Alvarez-Lorenzo, C. pH/redox dual-sensitive dextran nanogels for enhanced intracellular drug delivery. Eur. J. Pharm. Biopharm. 2017, 117, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Chávez, N.A.; Nosthas Aguiar, V.; Allegretto, J.A.; Albesa, A.G.; Giussi, J.M.; Longo, G.S. Triggering doxorubicin release from responsive hydrogel films by polyamine uptake. Soft Matter 2020, 16, 7492–7502. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, J.; Yang, K.; Cai, P.; Xiao, H. Novel multi-responsive and sugarcane bagasse cellulose-based nanogels for controllable release of doxorubicin hydrochloride. Mater. Sci. Eng. C Mater. Appl. Biol. 2021, 118, 111357. [Google Scholar] [CrossRef]
- Bredlau, A.L.; Dixit, S.; Chen, C.; Broome, A.M. Nanotechnology applications for diffuse intrinsic pontine glioma. Curr. Neuropharmacol. 2017, 15, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Shigdar, S.; Fang, D.Z.; Xiang, D.; Wei, M.Q.; Danks, A.; Kong, L.; Li, L.; Qiao, L.; Duan, W. Improved efficacy and reduced toxicity of doxorubicin encapsulated in sulfatide-containing nanoliposome in a glioma model. PLoS ONE 2014, 9, e103736. [Google Scholar]
- Venkatesh, H.S.; Tam, L.T.; Woo, P.J.; Lennon, J.; Nagaraja, S.; Gillespie, S.M.; Ni, J.; Duveau, D.Y.; Morris, P.J.; Zhao, J.J.; et al. Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma. Nature 2017, 549, 533–537. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and synaptic integration of glioma into neural circuits. Nature 2019, 573, 539–545. [Google Scholar] [CrossRef]
- Rocha, P.R.; Medeiros, M.C.; Kintzel, U.; Vogt, J.; Araujo, I.M.; Mestre, A.L.; Mailander, V.; Schlett, P.; Droge, M.; Schneider, L.; et al. Extracellular electrical recording of pH-triggered bursts in C6 glioma cell populations. Sci. Adv. 2016, 2, e1600516. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Pi, Z.; Yan, F.; Yeh, C.K.; Zeng, X.; Diao, X.; Hu, Y.; Chen, S.; Chen, X.; Zheng, H. Enhanced delivery of paclitaxel liposomes using focused ultrasound with microbubbles for treating nude mice bearing intracranial glioblastoma xenografts. Int. J. Nanomed. 2017, 12, 5613–5629. [Google Scholar] [CrossRef] [PubMed]
- Papachristodoulou, A.; Signorell, R.D.; Werner, B.; Brambilla, D.; Luciani, P.; Cavusoglu, M.; Grandjean, J.; Silginer, M.; Rudin, M.; Martin, E.; et al. Chemotherapy sensitization of glioblastoma by focused ultrasound-mediated delivery of therapeutic liposomes. J. Control. Release 2019, 295, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Guo, J.; Chen, G.; Chin, C.T.; Chen, X.; Chen, J.; Wang, F.; Chen, S.; Dan, G. Delivery of liposomes with different sizes to mice brain after sonication by focused ultrasound in the presence of microbubbles. Ultrasound Med. Biol. 2016, 42, 1499–1511. [Google Scholar] [CrossRef]
- Ye, D.; Sultan, D.; Zhang, X.; Yue, Y.; Heo, G.S.; Kothapalli, S.; Luehmann, H.; Tai, Y.C.; Rubin, J.B.; Liu, Y.; et al. Focused ultrasound-enabled delivery of radiolabeled nanoclusters to the pons. J. Control. Release 2018, 283, 143–150. [Google Scholar] [CrossRef]
- Hennika, T.; Hu, G.; Olaciregui, N.G.; Barton, K.L.; Ehteda, A.; Chitranjan, A.; Chang, C.; Gifford, A.J.; Tsoli, M.; Ziegler, D.S.; et al. Pre-clinical study of panobinostat in xenograft and genetically engineered murine diffuse intrinsic pontine glioma models. PLoS ONE 2017, 12, e0169485. [Google Scholar] [CrossRef]
- Meel, M.H.; de Gooijer, M.C.; Guillen Navarro, M.; Waranecki, P.; Breur, M.; Buil, L.C.M.; Wedekind, L.E.; Twisk, J.W.R.; Koster, J.; Hashizume, R.; et al. MELK inhibition in diffuse intrinsic pontine glioma. Clin. Cancer Res. 2018, 24, 5645–5657. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ung, C.; Tsoli, M.; Liu, J.; Cassano, D.; Pocoví-Martínez, S.; Upton, D.H.; Ehteda, A.; Mansfeld, F.M.; Failes, T.W.; Farfalla, A.; et al. Doxorubicin-Loaded Gold Nanoarchitectures as a Therapeutic Strategy against Diffuse Intrinsic Pontine Glioma. Cancers 2021, 13, 1278. https://doi.org/10.3390/cancers13061278
Ung C, Tsoli M, Liu J, Cassano D, Pocoví-Martínez S, Upton DH, Ehteda A, Mansfeld FM, Failes TW, Farfalla A, et al. Doxorubicin-Loaded Gold Nanoarchitectures as a Therapeutic Strategy against Diffuse Intrinsic Pontine Glioma. Cancers. 2021; 13(6):1278. https://doi.org/10.3390/cancers13061278
Chicago/Turabian StyleUng, Caitlin, Maria Tsoli, Jie Liu, Domenico Cassano, Salvador Pocoví-Martínez, Dannielle H. Upton, Anahid Ehteda, Friederike M. Mansfeld, Timothy W. Failes, Annafranca Farfalla, and et al. 2021. "Doxorubicin-Loaded Gold Nanoarchitectures as a Therapeutic Strategy against Diffuse Intrinsic Pontine Glioma" Cancers 13, no. 6: 1278. https://doi.org/10.3390/cancers13061278
APA StyleUng, C., Tsoli, M., Liu, J., Cassano, D., Pocoví-Martínez, S., Upton, D. H., Ehteda, A., Mansfeld, F. M., Failes, T. W., Farfalla, A., Katsinas, C., Kavallaris, M., Arndt, G. M., Vittorio, O., Cirillo, G., Voliani, V., & Ziegler, D. S. (2021). Doxorubicin-Loaded Gold Nanoarchitectures as a Therapeutic Strategy against Diffuse Intrinsic Pontine Glioma. Cancers, 13(6), 1278. https://doi.org/10.3390/cancers13061278









