Immune-Related Adverse Events (irAE) in Cancer Immune Checkpoint Inhibitors (ICI) and Survival Outcomes Correlation: To Rechallenge or Not?
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Procedures
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Initial ICI in Patients with an irAE
3.2. Initial and Sequential irAEs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Declarations
Abbreviations
| irAE | immune related adverse event |
| ICI | immune checkpoint inhibitor |
| mOS | median overall survival |
| PD-1 | programmed death 1 |
| PD-L1 | programmed death-ligand 1 |
| CTLA-4 | cytotoxic T-lymphocyte –associated protein 4 |
| CNS | central nervous system |
| RECIST | Response Evaluation Criteria In Solid Tumors |
| CTCAE | Common Terminology Criteria for Adverse Events |
| TPS | tumor proportion score |
| NCCN | National Comprehensive Cancer Network |
References
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Kartolo, A.; Sattar, J.; Sahai, V.; Baetz, T.; Lakoff, J.M. Predictors of immunotherapy-induced immune-related adverse events. Curr. Oncol. 2018, 25, e403–e410. [Google Scholar] [CrossRef] [PubMed]
- Defrance, T.; Taillardet, M.; Genestier, L. T cell-independent B cell memory. Curr. Opin. Immunol. 2011, 23, 330–336. [Google Scholar] [CrossRef]
- Lemke, A.; Kraft, M.; Roth, K.; Riedel, R.; Lammerding, D.; Hauser, A.E. Long-lived plasma cells are generated in mucosal immune responses and contribute to the bone marrow plasma cell pool in mice. Mucosal. Immunol. 2016, 9, 83–97. [Google Scholar] [CrossRef]
- Abu-Sbeih, H.; Tang, T.; Ali, F.S.; Owen, D.H.; Patel, S.; Otterson, G.A.; Kendra, K.; Ricciuti, B.; Chiari, R.; De Giglio, A.; et al. The impact of immune checkpoint inhibitor-related adverse events and their immunosuppressive treatment on patients’ outcomes. J. Immunother. Precis Oncol. 2018, 1, 7–18. [Google Scholar]
- Owen, D.H.; Wei, L.; Bertino, E.M.; Edd, T.; Villalona-Calero, M.A.; He, K.; Shields, P.G.; Carbone, D.P.; Otterson, G.A. Incidence, risk factors, and effect on survival of immune-related adverse events in patients with non-small-cell lung cancer. Clin. Lung Cancer 2018, 19, e893–e900. [Google Scholar] [CrossRef]
- Abu-Sbeih, H.; Ali, F.S.; Qiao, W.; Lu, Y.; Patel, S.; Diab, A.; Wang, Y. Immune checkpoint inhibitor-induced colitis as a predictor of survival in metastatic melanoma. Cancer Immunol. Immunother. 2019, 68, 553–561. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Horvat, T.Z.; Adel, N.G.; Dang, T.; Momtaz, P.; Postow, M.; Callahan, M.; Carvajal, R.D.; Dickson, M.; D’Angelo, S.P.; Woo, K.M.; et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J. Clin. Oncol. 2015, 33, 3193–3198. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Suo, A.; Chan, Y.; Beaulieu, C.; Kong, S.; Cheung, W.Y.; Monzon, J.G.; Smylie, M.; Walker, J.; Morris, D.; Cheng, T. Anti-PD1-Induced Immune-Related Adverse Events and Survival Outcomes in Advanced Melanoma. Oncologist 2020, 25, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Prior, L.; Harrold, E.; O’Leary, C.; Nugent, K.; Gleeson, J.P.; Watson, G.A.; Lim, M.C.; Kelly, D.; McCaffrey, J.; Kelly, C.M. Toxicities in immunotherapy: Can they predict response? J. Clin. Oncol. 2016, 34 (Suppl. 15), e14534. [Google Scholar] [CrossRef]
- Jamal-Hanjani, M.; Quezada, S.; Jarkin, J.; Swanton, C. Translational implications of tumor heterogeneity. Clin. Cancer Res. 2015, 21, 1258–1266. [Google Scholar] [CrossRef]
- Shankar, B.; Zhang, J.; Naqash, A.R.; Forde, P.M.; Feliciano, J.L.; Marrone, K.A.; Ettinger, D.S.; Hann, C.L.; Brahmer, J.R.; Ricciuti, B.; et al. Multisystem Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitors for Treatment of Non–Small Cell Lung Cancer. JAMA Oncol. 2020, 6, 1952–1956. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Cui, L.; Zhao, X.; Zhao, Z.; Chen, S.; Song, J.; Yang, L.; Qi, C.; Fang, Z.; Wang, Q.; et al. Use of Immunotherapy with Programmed Cell Death 1 vs Programmed Cell Death Ligand 1 Inhibitors in Patients with Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 6, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularua, E.; Roux, K.L.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef]
- Huang, X.Z.; Gao, P.; Song, Y.X.; Xu, Y.; Sun, J.X.; Chen, X.W.; Zhao, J.H.; Wang, Z.N. Antibiotic use and the efficacy of immune checkpoint inhibitors in cancer patients: A pooled analysis of 2740 cancer patients. Oncoimmunology 2019, 8, e1665973. [Google Scholar] [CrossRef]
- Arbour, K.C.; Mezquita, L.; Long, N.; Rizvi, H.; Auclin, E.; Ni, A.; Martinez-Bernal, G.; Ferrara, R.; Lai, W.V.; Hendriks, L.E.; et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients with Non-Small-Cell Lung Cancer. J Clin. Oncol. 2018, 36, 2872–2878. [Google Scholar] [CrossRef]
- Leighl, N.; Ghandi, L.; Hellmann, M.D. Pembrolizumab for NSCLC: Immune-mediated adverse events and corticosteroid use. J. Thorac. Oncol. 2015, 10, s233. [Google Scholar]
- Chatterjee, M.S.; Elassaiss-Schaap, J.; Lindauer, A.; Turner, D.C.; Sostelly, A.; Freshwater, T.; Mayawala, K.; Ahamadi, M.; Stone, J.A.; De Greef, R.; et al. Population pharmacokinetic/pharmacodynamic modeling of tumor size dynamics in pembrolizumab-treated advanced melanoma. CPT Pharmacomet. Syst. Pharmacol. 2016, 6, 29–39. [Google Scholar] [CrossRef]
- Jove, M.; Vilariño, N.; Nadal, E. Impact of baseline steroids on efficacy of programmed cell death-1 (PD-1) and programmed death-ligand 1 (PD-L1) blockade in patients with advanced non-small cell lung cancer. Transl. Lung Cancer Res. 2019, 8 (Suppl. 4), 364–368. [Google Scholar] [CrossRef] [PubMed]
- Giles, A.; Hutchinson, M.; Sonnemann, H.; Jung, J.; Fecci, P.; Ratnam, N.; Zhang, W.; Song, H.; Bailey, R.; Davis, D.; et al. Dexamethasone-induced immunosuppression: Mechanisms and implications for immunotherapy. J. Immunother. Cancer 2018, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, E.; Lipson, E.; Brahmer, J. Challenge of rechallenge: When to resume immunotherapy following an immune-related adverse event. J. Clin. Oncol. 2019, 37, 2714–2718. [Google Scholar] [CrossRef] [PubMed]
- Dolladille, C.; Ederhy, S.; Sassier, M.; Cautela, J.; Thuny, F.; Cohen, A.A.; Fedrizzi, S.; Chretien, B.; Da-Silva, A.; Plane, A.-F.; et al. Immune Checkpoint Inhibitor Rechallenge After Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2020, 6, 865–871. [Google Scholar] [CrossRef]
- Naqash, A.R.; Ricciuti, B.; Owen, D.H.; Florou, V.; Toi, Y.; Cherry, C.; Hafiz, M.; De Giglio, A.; Muzaffar, M.; Patel, S.H.; et al. Outcomes associated with immune-related adverse events in metastatic non-small cell lung cancer treated with nivolumab: A pooled exploratory analysis from a global cohort. Cancer Immunol. Immunother. 2020, 69, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Patients, No. (%) | ||
|---|---|---|---|
| Patients with irAE (N = 133) | Patients without irAE (N = 131) | p | |
| Median age, years (IQR) | 65 (59–73) | 63 (55–69) | 0.07 |
| Male Sex | 71 (53) | 80 (61) | 0.21 |
| Caucasian | 131 (99) | 124 (95) | 0.13 |
| Comorbid conditions present | 91 (68) | 106 (81) | 0.08 |
| ECOG Performance Status | |||
| 0–1 | 108 (81) | 101 (77) | 0.45 |
| 2–3 | 25 (19) | 30 (23) | 0.44 |
| Tumor Grade | |||
| Poorly differentiated | 66 (50) | 78 (60) | 0.10 |
| Cancer Stage | N = 114 | N = 118 | |
| III | 27 (20) | 28 (21) | 0.83 |
| IV | 87 (65) | 90 (69) | 0.57 |
| Cancer Type | |||
| Lung | 53 (40) | 59 (45) | 0.39 |
| Melanoma | 39 (29) | 25 (19) | 0.04 |
| Genitourinary | 14 (11) | 13 (10) | 0.87 |
| Other solid * | 9 (1) | 4 (3) | 0.57 |
| Hematologic ** | 3 (2) | 4 (3) | 0.61 |
| CNS disease present | 35 (26) | 42 (32) | 0.30 |
| Steroids required for CNS disease | 31 (89) | 34 (76) | 0.51 |
| Median observation period, months (IQR) | 14.5 (6–25) | 13.3 (4–23) | |
| Initial Immunotherapy | |||
| Anti-PD-1 | 87 (65) | 109 (83) | 0.45 |
| Anti-CTLA-4 | 10 (8) | 9 (7) | 0.84 |
| Combination anti-PD-1/CTLA-4 | 20 (15) | 1 (1) | <0.001 |
| Anti-PD-L1 | 16 (12) | 12 (9) | 0.05 |
| Steroid use within 30 days of ICI initiation | 33 (25) | 28 (21) | 0.51 |
| Antibiotic use within 30 days of ICI initiation | 31 (23) | 27 (21) | 0.60 |
| PD-L1 Status (% TPS) | N = 65 | N = 57 | |
| ≥50 | 21 (32) | 12 (21) | 0.04 |
| 1–49 | 19 (29) | 24 (42) | 0.03 |
| <1 | 25 (38) | 21 (37) | 0.9 |
| irAE (n = 133) | Patients, No. (%) |
|---|---|
| Hypothyroidism | 43 (32) |
| Rash | 30 (23) |
| Diarrhea/colitis | 22 (17) |
| Pneumonitis | 17 (13) |
| Adrenal Insufficiency | 12 (9) |
| Hepatitis | 9 (7) |
| Nephritis | 6 (5) |
| Hypophysitis | 3 (2) |
| Diabetes mellitus | 3 (2) |
| Myalgia | 3 (2) |
| Encephalopathy | 2 (2) |
| Other ** | 8 (6) |
| Grade of irAE (n = 115) | |
| 2 | 70 (61) |
| 3–4 | 45 (39) |
| Immunotherapy rechallenged after irAE | 98 (74) |
| Immunotherapy interrupted (with or without subsequent reinitiation) after irAE | 74 (56) |
| Identical Immunotherapy used after irAE (n = 98) | 83 (85) |
| Immunotherapy used after irAE (n = 98) | |
| Anti-PD-1 | 79 (81) |
| Anti-CTLA-4 | 2 (2) |
| Combination anti-PD-1/CTLA-4 | 5 (5) |
| Anti-PD-L1 | 12 (12) |
| Median duration of ICI therapy after irAE, cycles (IQR) | 7 (3–14.75) |
| Median duration from irAE to ICI resumption, days (IQR, n = 38) | 28 (16–44) |
| Median duration from ICI resumption to 2nd irAE, months (IQR, n = 38) | 3 (1–6) |
| Median duration from ICI resumption to 3rd irAE, months (IQR, n = 7) | 10 (6–18) |
| New IrAE after reinitiation of Immunotherapy (n = 98) | 41 (42) |
| Grade of second irAE (n = 41) | |
| 1–2 | 29 (71) |
| 3–4 | 12 (29) |
| IrAE after ICI interruption and reinitiated rechallenge the same as first irAE | 11 (27) |
| IrAE after second interruption and reinitiated rechallenge (n = 7) the same as first irAE | 4 (57) |
| Grade of irAE after second reinitiation (n = 7) | |
| 1–2 | 4 (57) |
| 3 | 3 (43) |
| Need for Immunosuppression | 80 (60) |
| PO/IV Steroids | 63 (79) |
| TNF-alpha inhibition | 3 (<1) |
| Topical steroids | 14 (18) |
| Characteristics | ICI Rechallenged Patients, No. (%) | ICI Discontinued and Not Reinitiated Patients, No. (%) | p |
|---|---|---|---|
| No. of patients | 98 | 35 | |
| Age, IQR | 64 (58.25–72) | 68 (60–74) | 0.10 |
| Alcohol status | |||
| Current | 27 (28) | 6 (17) | 0.56 |
| Former | 32 (33) | 15 (43) | |
| Smoking status | |||
| Current | 16 (16) | 7 (20) | 0.28 |
| Former | 53 (54) | 22 (63) | |
| Never | 29 (30) | 6 (17) | |
| Comorbid conditions present | 71 (72) | 20 (57) | 0.10 |
| ECOG Performance Status | |||
| 0–1 | 84 (86) | 29 (83) | 0.75 |
| 2–3 | 14 (14) | 6 (17) | |
| Median observed period, days | 434.5 (173.5–771.5) | 446 (222–707) | 0.81 |
| Median duration of ICI therapy, months (IQR) | 10 (4–17) | 3 (1–6) | <0.0001 |
| Median duration between initial ICI therapy and initial irAE, months (IQR) | 2 (1–4) | 3.5 (2–8) | 0.02 |
| Initial Immunotherapy | |||
| Anti-PD-1 | 65 (66) | 22 (63) | 0.97 |
| Anti-CTLA-4 | 7 (7) | 3 (9) | |
| Combination anti-PD-1/CTLA-4 | 15 (15) | 5 (14) | |
| Anti-PD-L1 | 11 (11) | 5 (14) | |
| Steroid use within 30 days of ICI initiation | 23 (23) | 10 (29) | 0.98 |
| Antibiotic use within 30 days of ICI initiation | 24 (24) | 7 (20) | 0.26 |
| CNS disease present | 26 (27) | 9 (26) | 0.82 |
| Steroids used for CNS disease | 23 (23) | 8 (23) | 0.98 |
| PD-L1 Status (% TPS) | 48 | 17 | |
| ≥50 | 15 (31) | 6 (35) | 0.32 |
| 1–49 | 15 (31) | 4 (11) | |
| <1 | 18 (38) | 7 (20) | |
| Grade of irAE | |||
| 2 | 63 (64) | 7 (20) | <0.0001 |
| 3–4 | 17 (17) | 28 (80) | |
| Median duration of ICI therapy prior to irAE, cycles (IQR) | 3 (2–6) | 4 (3–8.5) | 0.03 |
| Need for Immunosuppression | 50 (51) | 30 (86) | <0.0001 |
| PO/IV Steroids | 34 (67) | 29 (97) | |
| TNF-alpha inhibition | 2 (4) | 1 (3) | |
| Topical steroids | 14 (28) | 0 (0) | |
| Disease status after completion of ICI | 96 | 33 | |
| Complete response | 27 (28) | 10 (30) | 0.80 |
| Partial response | 10 (10) | 2 (6) | |
| Stable disease | 17 (18) | 8 (24) | |
| Progression of disease | 42 (44) | 13 (39) | |
| mOS, months (IQR) | 37.8 (19.7–51.7) | 24.9 (12.2-NR) | 0.7046 |
| Characteristics | ICI Interrupted, then Reinitiated Patients, No. (%) | ICI Interrupted and Not Reinitiated (i.e., Discontinued) Patients, No. (%) | p |
|---|---|---|---|
| No. of patients | 39 | 35 | |
| Age, IQR | 64 (59–71.5) | 68 (60–74) | 0.91 |
| Alcohol status | |||
| Current | 10 (26) | 6 (17) | 0.52 |
| Former | 11 (28) | 15 (43) | |
| Smoking status | |||
| Current | 7 (18) | 7 (20) | 0.60 |
| Former | 22 (56) | 22 (63) | |
| Never | 10 (26) | 6 (17) | |
| Comorbid conditions present | 27 (69) | 20 (57) | |
| ECOG Performance status | |||
| 0–1 | 35 (90) | 29 (83) | 0.44 |
| 2–3 | 4 (10) | 6 (17) | |
| Median observed period, months (IQR) | 16 (11–31) | 15 (7–24) | 0.38 |
| Median duration of total ICI therapy, months (IQR) | 12 (4–21.5) | 3 (1–8) | <0.001 |
| Median duration between initial ICI therapy and initial irAE, months (IQR) | 2 (1–4) | 3.5 (2–8) | 0.09 |
| Initial Immunotherapy | |||
| Anti-PD-1 | 20 (51) | 22 (63) | 0.75 |
| Anti-CTLA-4 | 5 (13) | 3 (9) | |
| Combination anti-PD-1/CTLA-4 | 8 (21) | 5 (14) | |
| Anti-PD-L1 | 6 (15) | 5 (14) | 0.58 |
| Steroid use within 30 days of ICI initiation | 8 (21) | 10 (29) | 0.90 |
| Antibiotic use within 30 days of ICI initiation | 11 (28) | 7 (20) | 0.13 |
| CNS disease present | 15 (38) | 9 (26) | 0.40 |
| Steroids used for CNS disease | 13 (33) | 8 (23) | 0.68 |
| PD-L1 Status | n = 48 | n = 17 | |
| ≥50 | 6 (15) | 6 (35) | 0.58 |
| 1–49 | 6 (15) | 4 (11) | |
| <1 | 8 (21) | 7 (20) | |
| Grade of irAE | |||
| 2 | 19 (49) | 7 (20) | <0.001 |
| 3–4 | 17 (44) | 28 (80) | |
| Median duration of ICI therapy prior to irAE, cycles (IQR) | 4 (2–6) | 4 (3–8.5) | |
| Need for Immunosuppression for irAE | n = 34 (87) | N = 30 (86) | 0.50 |
| PO/IV Steroids | 28 (82) | 29 (97) | |
| TNF-alpha inhibition | 2 (6) | 1 (3) | |
| Topical steroids | 4 (12) | 0 (0) | |
| Disease status after completion of ICI | 39 | 33 | |
| Complete response | 11 (31) | 10 (30) | |
| Partial response | 5 (14) | 2 (6) | |
| Stable disease | 8 (22) | 8 (24) | |
| Progression of disease | 15 (33) | 13 (39) | |
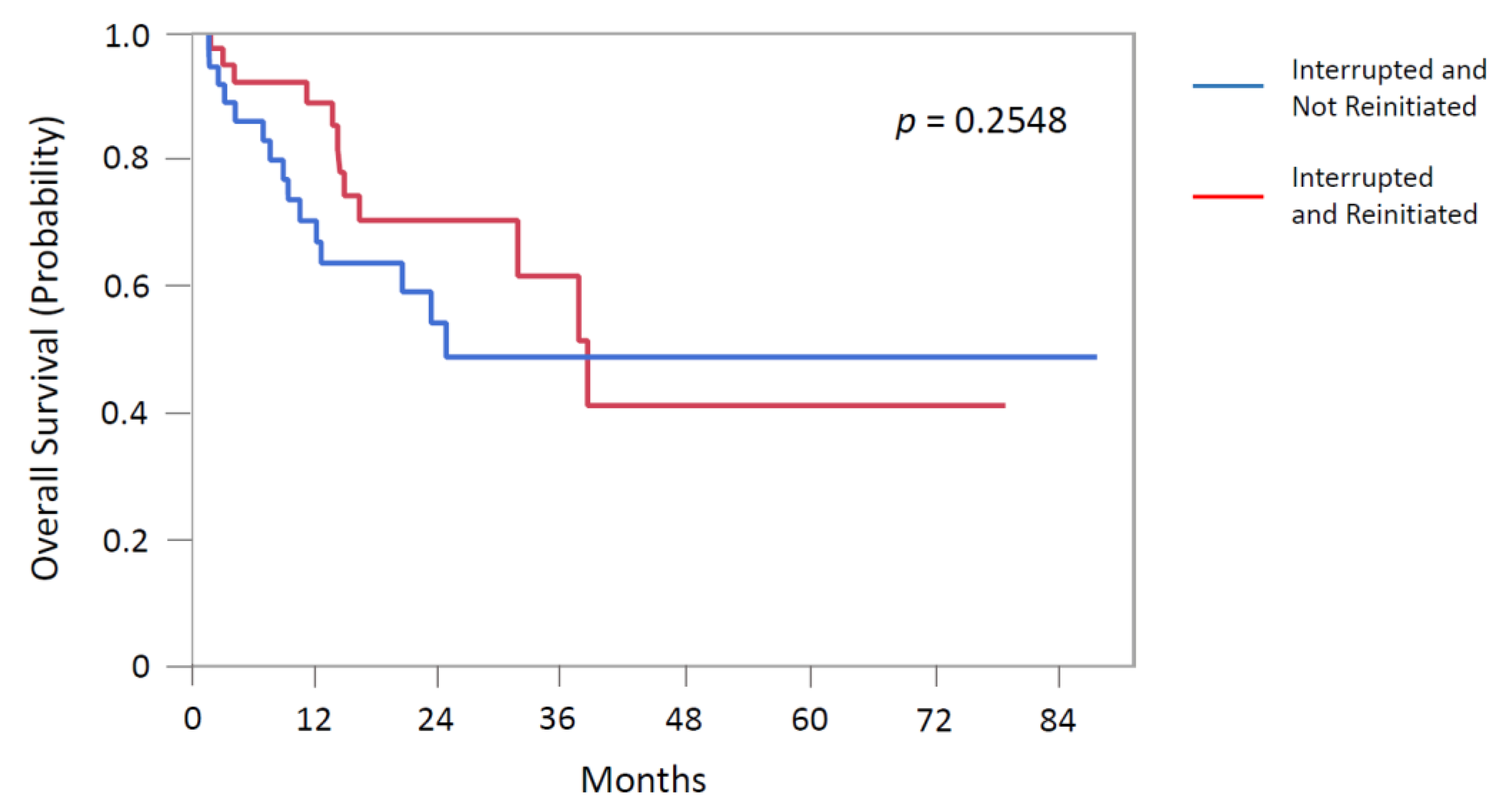
| mOS, months (IQR) | 38.6 (16.4-NR) | 24.9 (12.2-NR) | 0.2548 |
| Effect | Categories | Hazard Ratio * | Lower CI | Upper CI | p |
|---|---|---|---|---|---|
| Group | IrAE and not rechallenged (interrupted + non-reinitiated) | Ref | |||
| IrAE and rechallenged post-interruption (interrupted + reinitiated) | 1.19 | 0.70 | 2.03 | 0.52 | |
| Age | ≤60Y | Ref | |||
| >60Y | 1.59 | 0.90 | 2.79 | 0.11 | |
| Gender | Male | Ref | |||
| Female | 0.67 | 0.39 | 1.13 | 0.13 | |
| Immunotherapy type | Pembro/Nivo (PD-1) | Ref | |||
| Ipi & Ipi/Nivo (CTLA-4/PD-1) | 0.50 | 0.26 | 0.94 | 0.05 | |
| Atezo/Durva/Avelu (PD-L1) | 2.67 | 1.19 | 6.00 | 0.01 | |
| Antibiotic use | No | Ref | |||
| Yes | 1.40 | 0.78 | 2.49 | 0.13 | |
| IrAE Grade | 1–2 | Ref | |||
| 3–4 | 0.93 | 0.50 | 1.73 | 0.83 | |
| Median ICI duration, months | 0.96 | 0.94 | 0.98 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albandar, H.J.; Fuqua, J.; Albandar, J.M.; Safi, S.; Merrill, S.A.; Ma, P.C. Immune-Related Adverse Events (irAE) in Cancer Immune Checkpoint Inhibitors (ICI) and Survival Outcomes Correlation: To Rechallenge or Not? Cancers 2021, 13, 989. https://doi.org/10.3390/cancers13050989
Albandar HJ, Fuqua J, Albandar JM, Safi S, Merrill SA, Ma PC. Immune-Related Adverse Events (irAE) in Cancer Immune Checkpoint Inhibitors (ICI) and Survival Outcomes Correlation: To Rechallenge or Not? Cancers. 2021; 13(5):989. https://doi.org/10.3390/cancers13050989
Chicago/Turabian StyleAlbandar, Heidar J., Jacob Fuqua, Jasim M. Albandar, Salahuddin Safi, Samuel A. Merrill, and Patrick C. Ma. 2021. "Immune-Related Adverse Events (irAE) in Cancer Immune Checkpoint Inhibitors (ICI) and Survival Outcomes Correlation: To Rechallenge or Not?" Cancers 13, no. 5: 989. https://doi.org/10.3390/cancers13050989
APA StyleAlbandar, H. J., Fuqua, J., Albandar, J. M., Safi, S., Merrill, S. A., & Ma, P. C. (2021). Immune-Related Adverse Events (irAE) in Cancer Immune Checkpoint Inhibitors (ICI) and Survival Outcomes Correlation: To Rechallenge or Not? Cancers, 13(5), 989. https://doi.org/10.3390/cancers13050989







