Molecular Genetics of Follicular-Derived Thyroid Cancer
Abstract
Simple Summary
Abstract
1. Introduction
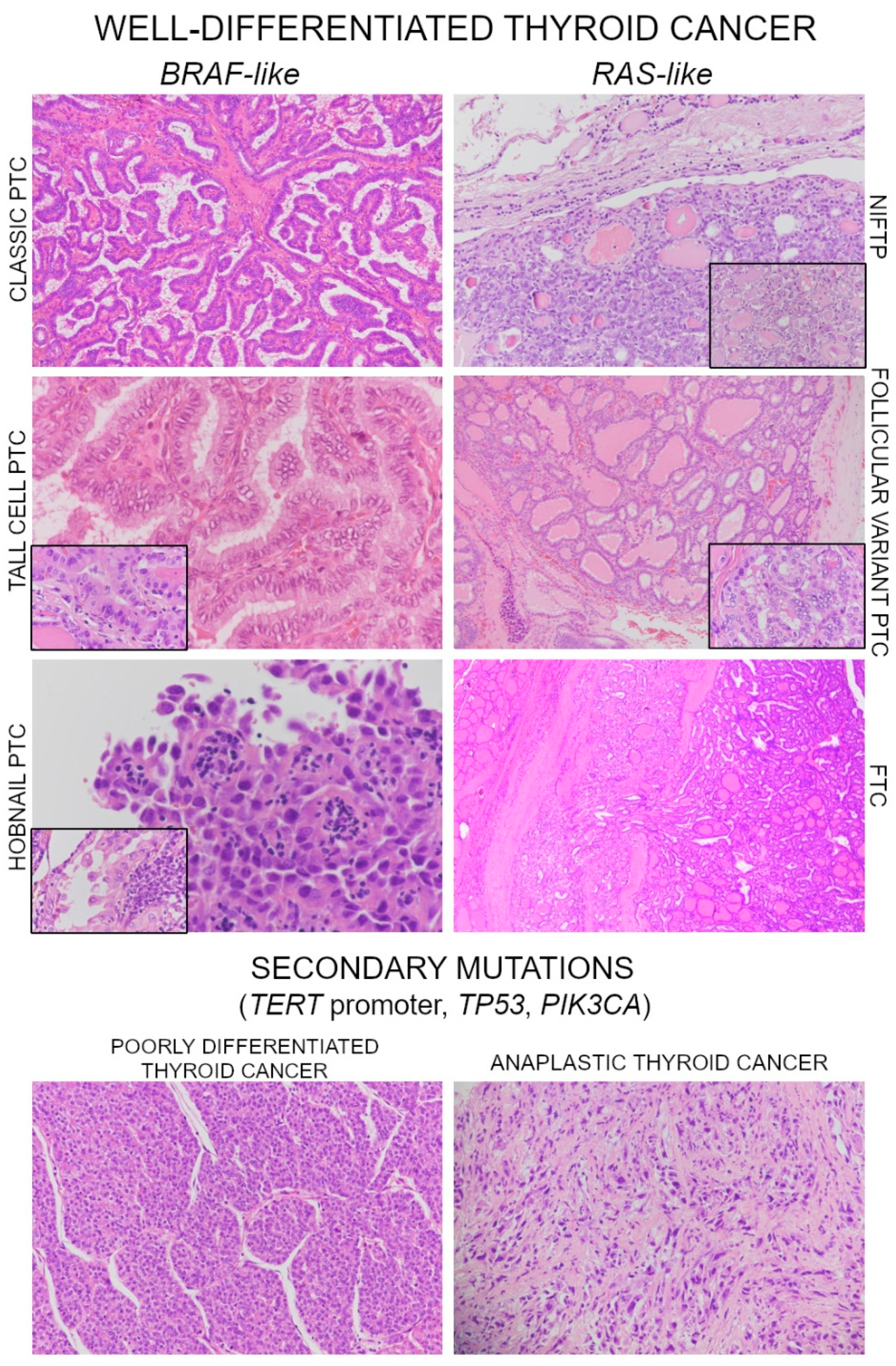
2. Molecular Landscape of Well-Differentiated Thyroid Carcinoma (WDTC)
Noninvasive Follicular Neoplasms with Papillary-Like Nuclear Features (NIFTP)
3. Poorly Differentiated and Anaplastic Thyroid Carcinoma
4. Molecular Alterations in Advanced Differentiated Cancers
5. Molecular Markers for Targeted Therapy in Thyroid Cancer
6. Copy Number Alterations, Gene Expression and microRNA in Thyroid Cancer
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dralle, H.; Machens, A.; Basa, J.; Fatourechi, V.; Franceschi, S.; Hay, I.D.; Nikiforov, Y.E.; Pacini, F.; Pasieka, J.L.; Sherman, S.I. Follicular Cell-Derived Thyroid Cancer. Nat. Rev. Dis. Primers 2015, 1, 15077. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.; Osamura, R.; Kloppel, G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs, 4th ed.; IARC Press: Lyon, France, 2017. [Google Scholar]
- Nikiforov, Y.E.; Seethala, R.R.; Tallini, G.; Baloch, Z.W.; Basolo, F.; Thompson, L.D.R.; Barletta, J.A.; Wenig, B.M.; Al Ghuzlan, A.; Kakudo, K.; et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA Oncol. 2016, 2, 1023. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, L.B.; Yan, F.; Morgan, P.F.; Kaczmar, J.M.; Fernandes, J.K.; Nguyen, S.A.; Jester, R.L.; Day, T.A. Hobnail Variant of Papillary Thyroid Carcinoma: A Systematic Review and Meta-Analysis. Endocrine 2020. [Google Scholar] [CrossRef] [PubMed]
- Pozdeyev, N.; Gay, L.M.; Sokol, E.S.; Hartmaier, R.; Deaver, K.E.; Davis, S.; French, J.D.; Borre, P.V.; LaBarbera, D.V.; Tan, A.-C.; et al. Genetic Analysis of 779 Advanced Differentiated and Anaplastic Thyroid Cancers. Clin. Cancer Res. 2018, 24, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Baloch, Z.W.; Shafique, K.; Flanagan, M.; LiVolsi, V.A. Encapsulated Classic and Follicular Variants of Papillary Thyroid Carcinoma: Comparative Clinicopathologic Study. Endocr. Pract. 2010, 16, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.-K.; Lee, S.; Kim, S.; Jee, H.-G.; Kim, B.-A.; Cho, H.; Song, Y.S.; Cho, S.W.; Won, J.-K.; Shin, J.-Y.; et al. Comprehensive Analysis of the Transcriptional and Mutational Landscape of Follicular and Papillary Thyroid Cancers. PLoS Genet. 2016, 12, e1006239. [Google Scholar] [CrossRef]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Shong, Y.K.; Kim, T.Y.; Viola, D.; Elisei, R.; Bendlová, B.; Yip, L.; Mian, C.; et al. Association Between BRAF V600E Mutation and Recurrence of Papillary Thyroid Cancer. JCO 2015, 33, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Bastos, A.U.; de Jesus, A.C.; Cerutti, J.M. ETV6-NTRK3 and STRN-ALK Kinase Fusions Are Recurrent Events in Papillary Thyroid Cancer of Adult Population. Eur. J. Endocrinol. 2018, 178, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, W.; Liu, C.; Li, J. Tall Cell Variant of Papillary Thyroid Carcinoma: Current Evidence on Clinicopathologic Features and Molecular Biology. Oncotarget 2016, 7, 40792–40799. [Google Scholar] [CrossRef]
- Vuong, H.G.; Odate, T.; Duong, U.N.P.; Mochizuki, K.; Nakazawa, T.; Katoh, R.; Kondo, T. Prognostic Importance of Solid Variant Papillary Thyroid Carcinoma: A Systematic Review and Meta-Analysis. Head Neck 2018, 40, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Liu, X.; Ren, X.; Zhang, H.; Wu, H.; Liang, Z. Mutation Profiles of Follicular Thyroid Tumors by Targeted Sequencing. Diagn. Pathol. 2019, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Bandoh, N.; Akahane, T.; Goto, T.; Kono, M.; Ichikawa, H.; Sawada, T.; Yamaguchi, T.; Nakano, H.; Kawase, Y.; Kato, Y.; et al. Targeted Next‑generation Sequencing of Cancer‑related Genes in Thyroid Carcinoma: A Single Institution’s Experience. Oncol. Lett. 2018. [Google Scholar] [CrossRef] [PubMed]
- Giordano, T.J. Genomic Hallmarks of Thyroid Neoplasia. Annu. Rev. Pathol. 2018, 13, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Gopal, R.K.; Kübler, K.; Calvo, S.E.; Polak, P.; Livitz, D.; Rosebrock, D.; Sadow, P.M.; Campbell, B.; Donovan, S.E.; Amin, S.; et al. Widespread Chromosomal Losses and Mitochondrial DNA Alterations as Genetic Drivers in Hürthle Cell Carcinoma. Cancer Cell 2018, 34, 242–255.e5. [Google Scholar] [CrossRef] [PubMed]
- Ganly, I.; Makarov, V.; Deraje, S.; Dong, Y.; Reznik, E.; Seshan, V.; Nanjangud, G.; Eng, S.; Bose, P.; Kuo, F.; et al. Integrated Genomic Analysis of Hürthle Cell Cancer Reveals Oncogenic Drivers, Recurrent Mitochondrial Mutations, and Unique Chromosomal Landscapes. Cancer Cell 2018, 34, 256–270.e5. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xing, M. TERT Promoter Mutations in Thyroid Cancer. Endocr. Relat. Cancer 2016, 23, R143–R155. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.; da Rocha, A.G.; Vinagre, J.; Batista, R.; Peixoto, J.; Tavares, C.; Celestino, R.; Almeida, A.; Salgado, C.; Eloy, C.; et al. TERT Promoter Mutations Are a Major Indicator of Poor Outcome in Differentiated Thyroid Carcinomas. J. Clin. Endocrinol. Metab. 2014, 99, E754–E765. [Google Scholar] [CrossRef]
- Liu, R.; Bishop, J.; Zhu, G.; Zhang, T.; Ladenson, P.W.; Xing, M. Mortality Risk Stratification by Combining BRAF V600E and TERT Promoter Mutations in Papillary Thyroid Cancer: Genetic Duet of BRAF and TERT Promoter Mutations in Thyroid Cancer Mortality. JAMA Oncol. 2017, 3, 202. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, T.B.; Sá, A.; Lopes, J.M.; Sobrinho-Simões, M.; Soares, P.; Vinagre, J. Telomere Maintenance Mechanisms in Cancer. Genes 2018, 9, 241. [Google Scholar] [CrossRef]
- Chu, Y.-H.; Sadow, P.M. Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features (NIFTP): Diagnostic Updates and Molecular Advances. Semin. Diagn. Pathol. 2020, 37, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Macerola, E.; Proietti, A.; Basolo, F. Noninvasive Follicular Neoplasm with Papillary-like Nuclear Features (NIFTP): A New Entity. Gland Surg. 2020, 9, S47–S53. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E.; Baloch, Z.W.; Hodak, S.P.; Giordano, T.J.; Lloyd, R.V.; Seethala, R.R.; Wenig, B.M. Change in Diagnostic Criteria for Noninvasive Follicular Thyroid Neoplasm With Papillarylike Nuclear Features. JAMA Oncol. 2018, 4, 1125. [Google Scholar] [CrossRef] [PubMed]
- Volante, M.; Collini, P.; Nikiforov, Y.E.; Sakamoto, A.; Kakudo, K.; Katoh, R.; Lloyd, R.V.; LiVolsi, V.A.; Papotti, M.; Sobrinho-Simoes, M.; et al. Poorly Differentiated Thyroid Carcinoma: The Turin Proposal for the Use of Uniform Diagnostic Criteria and an Algorithmic Diagnostic Approach. Am. J. Surg. Pathol. 2007, 31, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Hiltzik, D.; Carlson, D.L.; Tuttle, R.M.; Chuai, S.; Ishill, N.; Shaha, A.; Shah, J.P.; Singh, B.; Ghossein, R.A. Poorly Differentiated Thyroid Carcinomas Defined on the Basis of Mitosis and Necrosis: A Clinicopathologic Study of 58 Patients. Cancer 2006, 106, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Ghossein, R. Genomic Landscape of Poorly Differentiated and Anaplastic Thyroid Carcinoma. Endocr. Pathol. 2016, 27, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Ghossein, R. Poorly Differentiated Thyroid Carcinoma. Semin. Diagn. Pathol. 2020, 37, 243–247. [Google Scholar] [CrossRef]
- Chintakuntlawar, A.V.; Foote, R.L.; Kasperbauer, J.L.; Bible, K.C. Diagnosis and Management of Anaplastic Thyroid Cancer. Endocrinol. Metab. Clin. North Am. 2019, 48, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and Transcriptomic Hallmarks of Poorly Differentiated and Anaplastic Thyroid Cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Li, Y.; Hu, P.; Gao, J.; Ying, J.; Xu, W.; Zhao, D.; Wang, Z.; Ye, J.; Lizaso, A.; et al. Mutational Profiling of Poorly Differentiated and Anaplastic Thyroid Carcinoma by the Use of Targeted Next-generation Sequencing. Histopathology 2019, 75, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Fuchs, T.; Dogan, S.; Landa, I.; Katabi, N.; Fagin, J.A.; Tuttle, R.M.; Sherman, E.; Gill, A.J.; Ghossein, R. Dissecting Anaplastic Thyroid Carcinoma: A Comprehensive Clinical, Histologic, Immunophenotypic, and Molecular Study of 360 Cases. Thyroid 2020, 30, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Kunstman, J.W.; Juhlin, C.C.; Goh, G.; Brown, T.C.; Stenman, A.; Healy, J.M.; Rubinstein, J.C.; Choi, M.; Kiss, N.; Nelson-Williams, C.; et al. Characterization of the Mutational Landscape of Anaplastic Thyroid Cancer via Whole-Exome Sequencing. Hum. Mol. Genet. 2015, 24, 2318–2329. [Google Scholar] [CrossRef]
- Gerber, T.S.; Schad, A.; Hartmann, N.; Springer, E.; Zechner, U.; Musholt, T.J. Targeted Next-Generation Sequencing of Cancer Genes in Poorly Differentiated Thyroid Cancer. Endocr. Connect. 2018, 7, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Luthra, R.; Routbort, M.J.; Patel, K.P.; Cabanillas, M.E.; Broaddus, R.R.; Williams, M.D. Molecular Profile of Advanced Thyroid Carcinomas by Next-Generation Sequencing: Characterizing Tumors Beyond Diagnosis for Targeted Therapy. Mol. Cancer Ther. 2018, 17, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Sykorova, V.; Dvorakova, S.; Vcelak, J.; Vaclavikova, E.; Halkova, T.; Kodetova, D.; Lastuvka, P.; Betka, J.; Vlcek, P.; Reboun, M.; et al. Search for New Genetic Biomarkers in Poorly Differentiated and Anaplastic Thyroid Carcinomas Using next Generation Sequencing. Anticancer Res. 2015, 35, 2029–2036. [Google Scholar]
- Yoo, S.-K.; Song, Y.S.; Lee, E.K.; Hwang, J.; Kim, H.H.; Jung, G.; Kim, Y.A.; Kim, S.; Cho, S.W.; Won, J.-K.; et al. Integrative Analysis of Genomic and Transcriptomic Characteristics Associated with Progression of Aggressive Thyroid Cancer. Nat. Commun. 2019, 10, 2764. [Google Scholar] [CrossRef]
- Melo, M.; Gaspar da Rocha, A.; Batista, R.; Vinagre, J.; Martins, M.J.; Costa, G.; Ribeiro, C.; Carrilho, F.; Leite, V.; Lobo, C.; et al. TERT, BRAF, and NRAS in Primary Thyroid Cancer and Metastatic Disease. J. Clin. Endocrinol. Metab. 2017, 102, 1898–1907. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Song, D.E.; Ahn, J.; Kim, T.Y.; Kim, W.B.; Shong, Y.K.; Jeon, M.J.; Kim, W.G. Genetic Profile of Advanced Thyroid Cancers in Relation to Distant Metastasis. Endocr. Relat. Cancer 2020, 27, 285–293. [Google Scholar] [CrossRef]
- van der Tuin, K.; Ventayol Garcia, M.; Corver, W.E.; Khalifa, M.N.; Ruano Neto, D.; Corssmit, E.P.M.; Hes, F.J.; Links, T.P.; Smit, J.W.A.; Plantinga, T.S.; et al. Targetable Gene Fusions Identified in Radioactive Iodine Refractory Advanced Thyroid Carcinoma. Eur. J. Endocrinol. 2019, 180, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.; Cabanillas, M.E.; Urbanowitz, G.; et al. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600–Mutant Anaplastic Thyroid Cancer. JCO 2018, 36, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Babu, G.; Kainickal, C. Update on the Systemic Management of Radioactive Iodine Refractory Differentiated Thyroid Cancer (Review). Mol. Clin. Oncol. 2020, 14, 35. [Google Scholar] [CrossRef]
- Costante, G. Multikinase Inhibitors for the Treatment of Radioiodine Refractory Thyroid Cancer: What Have We Learned from the “real-World” Experience? Curr. Opin. Oncol. 2021, 33, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Oba, T.; Chino, T.; Soma, A.; Shimizu, T.; Ono, M.; Ito, T.; Kanai, T.; Maeno, K.; Ito, K. Comparative Efficacy and Safety of Tyrosine Kinase Inhibitors for Thyroid Cancer: A Systematic Review and Meta-Analysis. Endocr. J. 2020, 67, 1215–1226. [Google Scholar] [CrossRef]
- Basolo, A.; Matrone, A.; Elisei, R.; Santini, F. Effects of Tyrosine Kinase Inhibitors on Thyroid Function and Thyroid Hormone Metabolism. Semin. Cancer Biol. 2021, S1044579X20302686. [Google Scholar] [CrossRef]
- Pozdeyev, N.; Rose, M.M.; Bowles, D.W.; Schweppe, R.E. Molecular Therapeutics for Anaplastic Thyroid Cancer. Semin. Cancer Biol. 2020, 61, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion–Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in Patients with Advanced or Metastatic NTRK Fusion-Positive Solid Tumours: Integrated Analysis of Three Phase 1–2 Trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; Drilon, A.; Farago, A.F.; Brose, M.S.; McDermott, R.; Sohal, D.; Oh, D.-Y.; Almubarak, M.; Bauman, J.; Chu, E.; et al. 1916P Larotrectinib Treatment of Advanced TRK Fusion Thyroid Cancer. Ann. Oncol. 2020, 31, S1086. [Google Scholar] [CrossRef]
- Belli, C.; Anand, S.; Gainor, J.F.; Penault-Llorca, F.; Subbiah, V.; Drilon, A.; Andrè, F.; Curigliano, G. Progresses Toward Precision Medicine in RET-Altered Solid Tumors. Clin. Cancer Res. 2020, 26, 6102–6111. [Google Scholar] [CrossRef] [PubMed]
- Macerola, E.; Poma, A.M.; Basolo, F. NanoString in the Screening of Genetic Abnormalities Associated with Thyroid Cancer. Semin. Cancer Biol. 2020, S1044579X20302091. [Google Scholar] [CrossRef]
- Livhits, M.J.; Zhu, C.Y.; Kuo, E.J.; Nguyen, D.T.; Kim, J.; Tseng, C.-H.; Leung, A.M.; Rao, J.; Levin, M.; Douek, M.L.; et al. Effectiveness of Molecular Testing Techniques for Diagnosis of Indeterminate Thyroid Nodules: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, 70. [Google Scholar] [CrossRef]
- Celakovsky, P.; Kovarikova, H.; Chrobok, V.; Mejzlik, J.; Laco, J.; Vosmikova, H.; Chmelarova, M.; Ryska, A. MicroRNA Deregulation in Papillary Thyroid Cancer and Its Relationship With BRAF V600E Mutation. Vivo 2021, 35, 319–323. [Google Scholar] [CrossRef]
- Ramírez-Moya, J.; Santisteban, P. MiRNA-Directed Regulation of the Main Signaling Pathways in Thyroid Cancer. Front. Endocrinol. 2019, 10, 430. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Shirvani-Farsani, Z.; Taheri, M. The Role of MicroRNAs in the Pathogenesis of Thyroid Cancer. Non-Coding RNA Res. 2020, 5, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Santiago, K.; Chen Wongworawat, Y.; Khan, S. Differential MicroRNA-Signatures in Thyroid Cancer Subtypes. J. Oncol. 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Celano, M.; Rosignolo, F.; Maggisano, V.; Pecce, V.; Iannone, M.; Russo, D.; Bulotta, S. MicroRNAs as Biomarkers in Thyroid Carcinoma. Int. J. Genom. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Ohori, N.P.; Singhal, R.; Nikiforova, M.N.; Yip, L.; Schoedel, K.E.; Coyne, C.; McCoy, K.L.; LeBeau, S.O.; Hodak, S.P.; Carty, S.E.; et al. BRAF Mutation Detection in Indeterminate Thyroid Cytology Specimens: Underlying Cytologic, Molecular, and Pathologic Characteristics of Papillary Thyroid Carcinoma. Cancer Cytopathol. 2013, 121, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Ravella, L.; Lopez, J.; Descotes, F.; Giai, J.; Lapras, V.; Denier, M.-L.; Borson-Chazot, F.; Lifante, J.-C.; Decaussin-Petrucci, M. Preoperative Role of RAS or BRAF K601E in the Guidance of Surgery for Indeterminate Thyroid Nodules. World J. Surg. 2020, 44, 2264–2271. [Google Scholar] [CrossRef]
- Censi, S.; Barollo, S.; Grespan, E.; Watutantrige-Fernando, S.; Manso, J.; Iacobone, M.; Casal Ide, E.; Galuppini, F.; Fassina, A.; Bertazza, L.; et al. Prognostic Significance of TERT Promoter and BRAF Mutations in TIR-4 and TIR-5 Thyroid Cytology. Eur. J. Endocrinol. 2019, 181, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Tavares, C.; Melo, M.; Cameselle-Teijeiro, J.M.; Soares, P.; Sobrinho-Simões, M. ENDOCRINE TUMOURS: Genetic Predictors of Thyroid Cancer Outcome. Eur. J. Endocrinol. 2016, 174, R117–R126. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. Molecular Pathogenesis and Mechanisms of Thyroid Cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef] [PubMed]
| Gene | PDTC | ATC | ||||
|---|---|---|---|---|---|---|
| n° Mutant/n° Total | Frequency Range | Pooled Frequency | n° Mutant/n° Total | Frequency Range | Pooled Frequency | |
| BRAF | 57/220 | 15–33% | 26% | 166/395 | 20–56% | 42% |
| RAS | 48/220 | 9–39% | 22% | 100/395 | 20–33% | 25% |
| EIF1AX | 11/125 | 5–11% | 9% | 22/181 | 8–14% | 12% |
| PIK3CA | 15/220 | 2–20% | 7% | 65/395 | 9–44% | 16% |
| PTEN | 6/220 | 4–33% | 3% | 45/395 | 11–20% | 11% |
| TERT | 43/125 | 22–40% | 34% | 242/355 | 56–75% | 68% |
| TP53 | 45/220 | 8–67% | 20% | 244/395 | 25–80% | 62% |
| RET fusion | 11/125 | 6–15% | 9% | 5/355 | 0–2% | 1% |
| PPARG fusion | 4/125 | 2–4% | 3% | 0/159 | 0% | 0% |
| ALK fusion | 4/125 | 2–4% | 3% | 0/355 | 0% | 0% |
| NTRK fusion | 1/41 | 0–2% | 2% | 5/322 | 1–4% | 2% |
| Gene | Advanced PTC | Advanced FTC | ||||
|---|---|---|---|---|---|---|
| n° Mutant/n° Total | Frequency Range | Pooled Frequency | n° Mutant/n° Total | Frequency Range | Pooled Frequency | |
| BRAF | 583/894 | 45–71% | 65% | 6/136 1 | 0–8% | 4% |
| RAS2 | 68/890 | 1–23% | 8% | 83/136 | 8–90% | 61% |
| EIF1AX | 3/62 | 0–10% | 5% | 5/88 | 0–40% | 6% |
| PIK3CA | 36/669 | 3–6% | 5% | 2/100 | 0–3% | 2% |
| PTEN | 10/669 | 0–2% | 1% | 9/100 | 0–14% | 9% |
| TERT | 314/651 | 13–62% | 48% | 68/103 | 50–82% | 66% |
| TP53 | 64/669 | 3–13% | 10% | 9/100 | 0–12% | 9% |
| RET fusion | 37/558 | 3–7% | 7% | 0/89 | 0% | 0% |
| PPARG fusion | 0/59 | 0% | 0% | 0/89 | 0% | 0% |
| ALK fusion | 3/527 | <1–2% | 1% | 0/89 | 0% | 0% |
| NTRK fusion | 8/527 | 1–5% | 2% | 0/89 | 0% | 0% |
| BRAF fusion | 14/527 | 0–3% | 3% | 0/89 | 0% | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macerola, E.; Poma, A.M.; Vignali, P.; Basolo, A.; Ugolini, C.; Torregrossa, L.; Santini, F.; Basolo, F. Molecular Genetics of Follicular-Derived Thyroid Cancer. Cancers 2021, 13, 1139. https://doi.org/10.3390/cancers13051139
Macerola E, Poma AM, Vignali P, Basolo A, Ugolini C, Torregrossa L, Santini F, Basolo F. Molecular Genetics of Follicular-Derived Thyroid Cancer. Cancers. 2021; 13(5):1139. https://doi.org/10.3390/cancers13051139
Chicago/Turabian StyleMacerola, Elisabetta, Anello Marcello Poma, Paola Vignali, Alessio Basolo, Clara Ugolini, Liborio Torregrossa, Ferruccio Santini, and Fulvio Basolo. 2021. "Molecular Genetics of Follicular-Derived Thyroid Cancer" Cancers 13, no. 5: 1139. https://doi.org/10.3390/cancers13051139
APA StyleMacerola, E., Poma, A. M., Vignali, P., Basolo, A., Ugolini, C., Torregrossa, L., Santini, F., & Basolo, F. (2021). Molecular Genetics of Follicular-Derived Thyroid Cancer. Cancers, 13(5), 1139. https://doi.org/10.3390/cancers13051139








