Histopathological and Immune Prognostic Factors in Colo-Rectal Liver Metastases
Abstract
Simple Summary
Abstract
1. Introduction
2. Histopathological Parameters
2.1. Metastatic Tumor Invasion
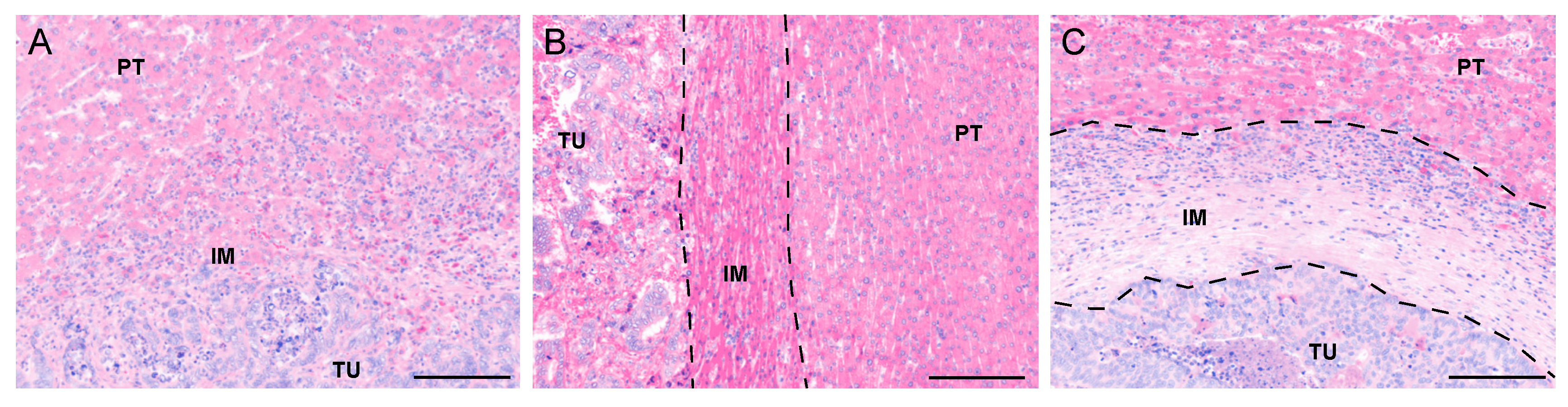
2.2. Histopathological Growth Patterns (HGP)
2.3. Tumor Regression Grade (TRG)
2.4. Chemotherapy-Associated Liver Injury (CALI)
3. Tissue Immune Parameters
3.1. The Prognostic Relevance of T Cells in CLM
3.2. The Potential of Macrophages as Prognostic Factors: Not an Easy Task
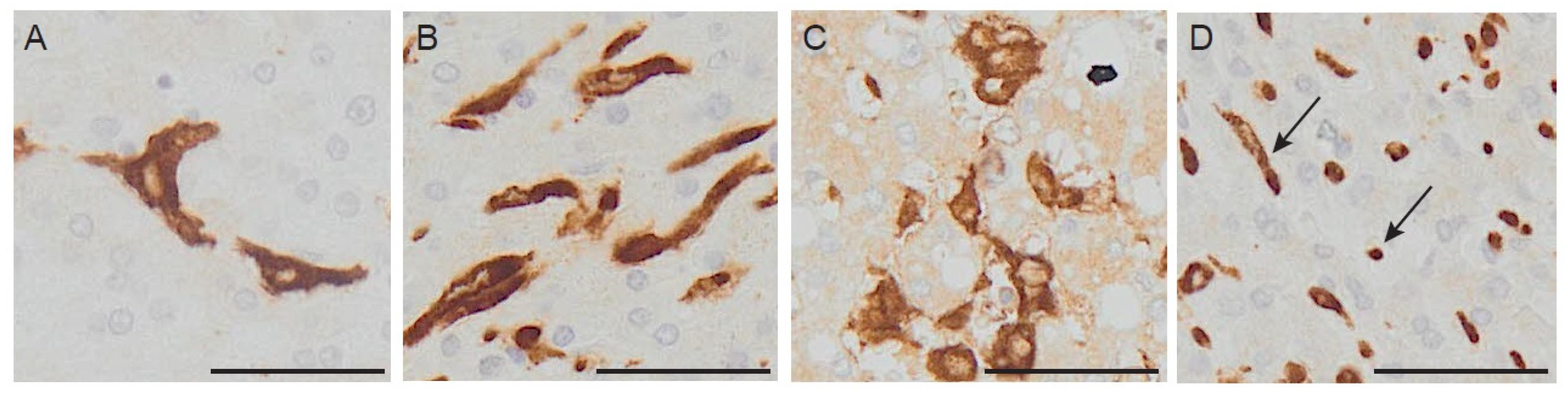
3.3. Macrophage Morphology: A New Feature to Be Considered?
4. Conclusions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Baldin, P.; Van den Eynde, M.; Mlecnik, B.; Bindea, G.; Beniuga, G.; Carrasco, J.; Haicheur, N.; Marliot, F.; Lafontaine, L.; Fredriksen, T.; et al. Prognostic assessment of resected colorectal liver metastases integrating pathological features, RAS mutation and Immunoscore. J. Pathol. Clin. Res. 2021, 7, 27–41. [Google Scholar] [CrossRef]
- Galon, J.; Pages, F.; Marincola, F.M.; Angell, H.K.; Thurin, M.; Lugli, A.; Zlobec, I.; Berger, A.; Bifulco, C.; Botti, G.; et al. Cancer classification using the Immunoscore: A worldwide task force. J. Transl. Med. 2012, 10, 205. [Google Scholar] [CrossRef]
- Galon, J.; Mlecnik, B.; Bindea, G.; Angell, H.K.; Berger, A.; Lagorce, C.; Lugli, A.; Zlobec, I.; Hartmann, A.; Bifulco, C.; et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J. Pathol. 2014, 232, 199–209. [Google Scholar] [CrossRef]
- Mlecnik, B.; Van den Eynde, M.; Bindea, G.; Church, S.E.; Vasaturo, A.; Fredriksen, T.; Lafontaine, L.; Haicheur, N.; Marliot, F.; Debetancourt, D.; et al. Comprehensive Intrametastatic Immune Quantification and Major Impact of Immunoscore on Survival. J. Natl. Cancer Inst. 2018, 110, 97–108. [Google Scholar] [CrossRef]
- Fridman, W.H.; Zitvogel, L.; Sautès-Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Fonseca, G.M.; Herman, P.; Faraj, S.F.; Kruger, J.A.P.; Coelho, F.F.; Jeismann, V.B.; Cecconello, I.; Alves, V.A.F.; Pawlik, T.M.; de Mello, E.S. Pathological factors and prognosis of resected liver metastases of colorectal carcinoma: Implications and proposal for a pathological reporting protocol. Histopathology 2018, 72, 377–390. [Google Scholar] [CrossRef]
- De Oliveira, C.V.C.; Fonseca, G.M.; Kruger, J.A.P.; de Mello, E.S.; Coelho, F.F.; Herman, P. Histopathological prognostic factors for colorectal liver metastases: A systematic review and meta-analysis of observational studies. Histol. Histopathol. 2020, 18274. [Google Scholar] [CrossRef]
- Yamamoto, J.; Sugihara, K.; Kosuge, T.; Takayama, T.; Shimada, K.; Yamasaki, S.; Sakamoto, M.; Hirohashi, S. Pathologic support for limited hepatectomy in the treatment of liver metastases from colorectal cancer. Ann. Surg. 1995, 221, 74–78. [Google Scholar] [CrossRef]
- Yamamoto, J.; Shimada, K.; Kosuge, T.; Yamasaki, S.; Sakamoto, M.; Fukuda, H. Factors influencing survival of patients undergoing hepatectomy for colorectal metastases. Br. J. Surg. 1999, 86, 332–337. [Google Scholar] [CrossRef]
- Knijn, N.; de Ridder, J.A.; Punt, C.J.; de Wilt, J.H.; Nagtegaal, I.D. Histopathological evaluation of resected colorectal cancer liver metastases: What should be done. Histopathology 2013, 63, 149–156. [Google Scholar] [CrossRef]
- de Ridder, J.A.; Knijn, N.; Wiering, B.; de Wilt, J.H.; Nagtegaal, I.D. Lymphatic Invasion is an Independent Adverse Prognostic Factor in Patients with Colorectal Liver Metastasis. Ann. Surg. Oncol. 2015, 22 (Suppl. 3), S638–S645. [Google Scholar] [CrossRef][Green Version]
- Fonseca, G.M.; de Mello, E.S.; Faraj, S.F.; Kruger, J.A.P.; Coelho, F.F.; Jeismann, V.B.; Lupinacci, R.M.; Cecconello, I.; Alves, V.A.F.; Pawlik, T.M.; et al. Prognostic significance of poorly differentiated clusters and tumor budding in colorectal liver metastases. J. Surg. Oncol. 2018, 117, 1364–1375. [Google Scholar] [CrossRef]
- Lupinacci, R.M.; Paye, F.; Coelho, F.F.; Kruger, J.A.; Herman, P. Lymphatic drainage of the liver and its implications in the management of colorectal cancer liver metastases. Updates Surg. 2014, 66, 239–245. [Google Scholar] [CrossRef]
- Sasaki, A.; Aramaki, M.; Kawano, K.; Yasuda, K.; Inomata, M.; Kitano, S. Prognostic significance of intrahepatic lymphatic invasion in patients with hepatic resection due to metastases from colorectal carcinoma. Cancer 2002, 95, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Bockhorn, M.; Sotiropoulos, G.; Neuhaus, J.; Sgourakis, G.; Sheu, S.Y.; Molmenti, E.; Fingas, C.; Trarbach, T.; Frilling, A.; Broelsch, C.E. Prognostic impact of intrahepatic lymphatic and microvascular involvement in cases of colorectal liver metastases. Int. J. Colorectal. Dis. 2009, 24, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Latorre Fragua, R.A.; Manuel Vazquez, A.; Rodrigues Figueira, Y.; Ramiro Pérez, C.; López Marcano, A.J.; de la Plaza Llamas, R.; Ramia Ángel, J.M. Intrabiliary metastases in colorectal cancer: A systematic review. J. Hepato Biliary Pancreat. Sci. 2019, 26, 270–280. [Google Scholar] [CrossRef]
- Barresi, V.; Fioravanzo, A.; Pecori, S.; Tomezzoli, A.; Reggiani Bonetti, L. The histopathologic report of surgically resected colorectal liver metastases: What is clinically relevant. Pathol. Res. Pract. 2019, 215, 152547. [Google Scholar] [CrossRef]
- Kawakatsu, S.; Kaneoka, Y.; Maeda, A.; Takayama, Y.; Fukami, Y.; Onoe, S. Intrapancreatic bile duct metastasis from colon cancer after resection of liver metastasis with intrabiliary growth: A case report. World J. Surg. Oncol. 2015, 13, 254. [Google Scholar] [CrossRef][Green Version]
- Cortese, N.; Rigamonti, A.; Mantovani, A.; Marchesi, F. The neuro-immune axis in cancer: Relevance of the peripheral nervous system to the disease. Immunol. Lett. 2020, 227, 60–65. [Google Scholar] [CrossRef]
- Marchesi, F.; Piemonti, L.; Mantovani, A.; Allavena, P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev. 2010, 21, 77–82. [Google Scholar] [CrossRef]
- Demir, I.E.; Friess, H.; Ceyhan, G.O. Neural plasticity in pancreatitis and pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Stift, J.; Graf, A.; Schwarz, C.; Tamandl, D.; Starlinger, P.; Herac, M.; Beer, A.; Wrba, F.; Bodingbauer, M.; Kaczirek, K.; et al. Microscopic biliary and perineural invasion and clinical outcome after neoadjuvant bevacizumab-based chemotherapy and liver resection in patients with colorectal liver metastases. Eur. J. Surg. Oncol. 2018, 44, 139–147. [Google Scholar] [CrossRef] [PubMed]
- van Dam, P.J.; van der Stok, E.P.; Teuwen, L.A.; Van den Eynden, G.G.; Illemann, M.; Frentzas, S.; Majeed, A.W.; Eefsen, R.L.; Coebergh van den Braak, R.R.J.; Lazaris, A.; et al. International consensus guidelines for scoring the histopathological growth patterns of liver metastasis. Br. J. Cancer 2017, 117, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Jass, J.R.; Love, S.B.; Northover, J.M. A new prognostic classification of rectal cancer. Lancet 1987, 1, 1303–1306. [Google Scholar] [CrossRef]
- Van den Eynden, G.G.; Bird, N.C.; Majeed, A.W.; Van Laere, S.; Dirix, L.Y.; Vermeulen, P.B. The histological growth pattern of colorectal cancer liver metastases has prognostic value. Clin. Exp. Metastasis 2012, 29, 541–549. [Google Scholar] [CrossRef]
- Frentzas, S.; Simoneau, E.; Bridgeman, V.L.; Vermeulen, P.B.; Foo, S.; Kostaras, E.; Nathan, M.; Wotherspoon, A.; Gao, Z.H.; Shi, Y.; et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat. Med. 2016, 22, 1294–1302. [Google Scholar] [CrossRef]
- Brunner, S.M.; Kesselring, R.; Rubner, C.; Martin, M.; Jeiter, T.; Boerner, T.; Ruemmele, P.; Schlitt, H.J.; Fichtner-Feigl, S. Prognosis according to histochemical analysis of liver metastases removed at liver resection. Br. J. Surg. 2014, 101, 1681–1691. [Google Scholar] [CrossRef]
- van Dam, P.J.; Daelemans, S.; Ross, E.; Waumans, Y.; Van Laere, S.; Latacz, E.; Van Steen, R.; De Pooter, C.; Kockx, M.; Dirix, L.; et al. Histopathological growth patterns as a candidate biomarker for immunomodulatory therapy. Semin. Cancer Biol. 2018, 52, 86–93. [Google Scholar] [CrossRef]
- Cucchetti, A.; Ferrero, A.; Cescon, M.; Donadon, M.; Russolillo, N.; Ercolani, G.; Stacchini, G.; Mazzotti, F.; Torzilli, G.; Pinna, A.D. Cure model survival analysis after hepatic resection for colorectal liver metastases. Ann. Surg. Oncol. 2015, 22, 1908–1914. [Google Scholar] [CrossRef]
- Vera, R.; González-Flores, E.; Rubio, C.; Urbano, J.; Valero Camps, M.; Ciampi-Dopazo, J.J.; Orcajo Rincón, J.; Morillo Macías, V.; Gomez Braco, M.A.; Suarez-Artacho, G. Multidisciplinary management of liver metastases in patients with colorectal cancer: A consensus of SEOM, AEC, SEOR, SERVEI, and SEMNIM. Clin. Transl. Oncol. 2020, 22, 647–662. [Google Scholar] [CrossRef]
- Tomasello, G.; Petrelli, F.; Ghidini, M.; Russo, A.; Passalacqua, R.; Barni, S. FOLFOXIRI Plus Bevacizumab as Conversion Therapy for Patients With Initially Unresectable Metastatic Colorectal Cancer: A Systematic Review and Pooled Analysis. JAMA Oncol. 2017, 3, e170278. [Google Scholar] [CrossRef] [PubMed]
- Viganò, L.; Capussotti, L.; De Rosa, G.; De Saussure, W.O.; Mentha, G.; Rubbia-Brandt, L. Liver resection for colorectal metastases after chemotherapy: Impact of chemotherapy-related liver injuries, pathological tumor response, and micrometastases on long-term survival. Ann. Surg. 2013, 258, 731–740, discussion 741. [Google Scholar] [CrossRef] [PubMed]
- Blazer, D.G.; Kishi, Y.; Maru, D.M.; Kopetz, S.; Chun, Y.S.; Overman, M.J.; Fogelman, D.; Eng, C.; Chang, D.Z.; Wang, H.; et al. Pathologic response to preoperative chemotherapy: A new outcome end point after resection of hepatic colorectal metastases. J. Clin. Oncol. 2008, 26, 5344–5351. [Google Scholar] [CrossRef]
- Adam, R.; Wicherts, D.A.; de Haas, R.J.; Aloia, T.; Lévi, F.; Paule, B.; Guettier, C.; Kunstlinger, F.; Delvart, V.; Azoulay, D.; et al. Complete pathologic response after preoperative chemotherapy for colorectal liver metastases: Myth or reality. J. Clin. Oncol. 2008, 26, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Ribero, D.; Wang, H.; Donadon, M.; Zorzi, D.; Thomas, M.B.; Eng, C.; Chang, D.Z.; Curley, S.A.; Abdalla, E.K.; Ellis, L.M.; et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer 2007, 110, 2761–2767. [Google Scholar] [CrossRef]
- Maru, D.M.; Kopetz, S.; Boonsirikamchai, P.; Agarwal, A.; Chun, Y.S.; Wang, H.; Abdalla, E.K.; Kaur, H.; Charnsangavej, C.; Vauthey, J.N.; et al. Tumor thickness at the tumor-normal interface: A novel pathologic indicator of chemotherapy response in hepatic colorectal metastases. Am. J. Surg. Pathol. 2010, 34, 1287–1294. [Google Scholar] [CrossRef]
- Rubbia-Brandt, L.; Giostra, E.; Brezault, C.; Roth, A.D.; Andres, A.; Audard, V.; Sartoretti, P.; Dousset, B.; Majno, P.E.; Soubrane, O.; et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann. Oncol. 2007, 18, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Tanis, E.; Julié, C.; Emile, J.F.; Mauer, M.; Nordlinger, B.; Aust, D.; Roth, A.; Lutz, M.P.; Gruenberger, T.; Wrba, F.; et al. Prognostic impact of immune response in resectable colorectal liver metastases treated by surgery alone or surgery with perioperative FOLFOX in the randomised EORTC study 40983. Eur. J. Cancer 2015, 51, 2708–2717. [Google Scholar] [CrossRef]
- Inoue, Y.; Hazama, S.; Suzuki, N.; Tokumitsu, Y.; Kanekiyo, S.; Tomochika, S.; Tsunedomi, R.; Tokuhisa, Y.; Iida, M.; Sakamoto, K.; et al. Cetuximab strongly enhances immune cell infiltration into liver metastatic sites in colorectal cancer. Cancer Sci. 2017, 108, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Dagenborg, V.J.; Marshall, S.E.; Yaqub, S.; Grzyb, K.; Boye, K.; Lund-Iversen, M.; Høye, E.; Berstad, A.E.; Fretland, Å.A.; Edwin, B.; et al. Neoadjuvant chemotherapy is associated with a transient increase of intratumoral T-cell density in microsatellite stable colorectal liver metastases. Cancer Biol. Ther. 2020, 21, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Lu, X.; Zhu, X.; Ju, H.; Sun, W.; Wu, W. Histological tumor response assessment in colorectal liver metastases after neoadjuvant chemotherapy: Impact of the variation in tumor regression grading and peritumoral lymphocytic infiltration. J. Cancer 2019, 10, 5852–5861. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; van Mierlo, K.M.C.; Gómez-Ramírez, J.; Kim, H.; Pilgrim, C.H.C.; Pessaux, P.; Rensen, S.S.; van der Stok, E.P.; Schaap, F.G.; Soubrane, O.; et al. Systematic review of the influence of chemotherapy-associated liver injury on outcome after partial hepatectomy for colorectal liver metastases. Br. J. Surg. 2017, 104, 990–1002. [Google Scholar] [CrossRef] [PubMed]
- Bruni, D.; Angell, H.K.; Galon, J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat. Rev. Cancer 2020, 20, 662–680. [Google Scholar] [CrossRef]
- Massalha, H.; Halpern, K.B.; Abu-Gazala, S.; Jana, T.; Massasa, E.; Moor, A.; Buchauer, L.; Rozenberg, M.; Pikarsky, E.; Amid, I.; et al. A single cell atlas of the human liver tumor microenvironment. Mol. Syst. Biol. 2020, 16, e9682. [Google Scholar]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Cortese, N.; Soldani, C.; Franceschini, B.; Barbagallo, M.; Marchesi, F.; Torzilli, G.; Donadon, M. Macrophages in Colorectal Cancer Liver Metastases. Cancers 2019, 11, 633. [Google Scholar] [CrossRef]
- Halama, N.; Spille, A.; Lerchl, T.; Brand, K.; Herpel, E.; Welte, S.; Keim, S.; Lahrmann, B.; Klupp, F.; Kahlert, C.; et al. Hepatic metastases of colorectal cancer are rather homogeneous but differ from primary lesions in terms of immune cell infiltration. Oncoimmunology 2013, 2, e24116. [Google Scholar] [CrossRef]
- Van den Eynde, M.; Mlecnik, B.; Bindea, G.; Fredriksen, T.; Church, S.E.; Lafontaine, L.; Haicheur, N.; Marliot, F.; Angelova, M.; Vasaturo, A.; et al. The Link between the Multiverse of Immune Microenvironments in Metastases and the Survival of Colorectal Cancer Patients. Cancer Cell 2018, 34, 1012–1026.e3. [Google Scholar] [CrossRef]
- Donadon, M.; Hudspeth, K.; Cimino, M.; Di Tommaso, L.; Preti, M.; Tentorio, P.; Roncalli, M.; Mavilio, D.; Torzilli, G. Increased Infiltration of Natural Killer and T Cells in Colorectal Liver Metastases Improves Patient Overall Survival. J. Gastrointest. Surg. 2017, 21, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.C.; Pillarisetty, V.; Bamboat, Z.M.; Shia, J.; Hedvat, C.; Gonen, M.; Jarnagin, W.; Fong, Y.; Blumgart, L.; D’Angelica, M.; et al. T cell infiltrate predicts long-term survival following resection of colorectal cancer liver metastases. Ann. Surg. Oncol. 2009, 16, 2524–2530. [Google Scholar] [CrossRef] [PubMed]
- Halama, N.; Michel, S.; Kloor, M.; Zoernig, I.; Benner, A.; Spille, A.; Pommerencke, T.; von Knebel, D.M.; Folprecht, G.; Luber, B.; et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011, 71, 5670–5677. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.C.; Bamboat, Z.M.; Maker, A.V.; Shia, J.; Pillarisetty, V.G.; Yopp, A.C.; Hedvat, C.V.; Gonen, M.; Jarnagin, W.R.; Fong, Y.; et al. Regulatory T cell infiltration predicts outcome following resection of colorectal cancer liver metastases. Ann. Surg. Oncol. 2013, 20, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Tanaka, K.; Homma, Y.; Nojiri, K.; Kumamoto, T.; Takeda, K.; Endo, I. Low infiltration of peritumoral regulatory T cells predicts worse outcome following resection of colorectal liver metastases. Ann. Surg. Oncol. 2015, 22, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Donadon, M.; Lleo, A.; Di Tommaso, L.; Soldani, C.; Franceschini, B.; Roncalli, M.; Torzilli, G. The Shifting Paradigm of Prognostic Factors of Colorectal Liver Metastases: From Tumor-Centered to Host Immune-Centered Factors. Front. Oncol. 2018, 8, 181. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Noy, R.; Pollard, J.W. Tumor-associated macrophages: From mechanisms to therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Krenkel, O.; Tacke, F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 2017, 17, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Z.; Skrzypczynska, K.M.; Fang, Q.; Zhang, W.; O’Brien, S.A.; He, Y.; Wang, L.; Zhang, Q.; Kim, A.; et al. Single-Cell Analyses Inform Mechanisms of Myeloid-Targeted Therapies in Colon Cancer. Cell 2020, 181, 442–459.e29. [Google Scholar] [CrossRef] [PubMed]
- Cortese, N.; Carriero, R.; Laghi, L.; Mantovani, A.; Marchesi, F. Prognostic significance of tumor-associated macrophages: Past, present and future. Semin. Immunol. 2020, 48, 101408. [Google Scholar] [CrossRef] [PubMed]
- Bonnardel, J.; T’Jonck, W.; Gaublomme, D.; Browaeys, R.; Scott, C.L.; Martens, L.; Vanneste, B.; De Prijck, S.; Nedospasov, S.A.; Kremer, A.; et al. Stellate Cells, Hepatocytes, and Endothelial Cells Imprint the Kupffer Cell Identity on Monocytes Colonizing the Liver Macrophage Niche. Immunity 2019, 51, 638–654.e9. [Google Scholar] [CrossRef] [PubMed]
- Freire Valls, A.; Knipper, K.; Giannakouri, E.; Sarachaga, V.; Hinterkopf, S.; Wuehrl, M.; Shen, Y.; Radhakrishnan, P.; Klose, J.; Ulrich, A.; et al. VEGFR1+ Metastasis-associated Macrophages Contribute to Metastatic Angiogenesis and influence Colorectal Cancer PAtient Outcome. Clin. Cancer Res. 2019, 25, 5674–5685. [Google Scholar] [CrossRef]
- Chandrasekaran, S.N.; Ceulemans, H.; Boyd, J.D.; Carpenter, A.E. Image-based profiling for drug discovery: Due for a machine-learning upgrade. Nat. Rev. Drug Discov. 2020, 20, 145–159. [Google Scholar] [CrossRef]
- Donadon, M.; Torzilli, G.; Cortese, N.; Soldani, C.; Di Tommaso, L.; Franceschini, B.; Carriero, R.; Barbagallo, M.; Rigamonti, A.; Anselmo, A.; et al. Macrophage morphology correlates with single-cell diversity and prognosis in colorectal liver metastasis. J. Exp. Med. 2020, 217, e20191847. [Google Scholar] [CrossRef]
- Waldo, S.W.; Li, Y.; Buono, C.; Zhao, B.; Billings, E.M.; Chang, J.; Kruth, H.S. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am. J. Pathol. 2008, 172, 1112–1126. [Google Scholar] [CrossRef]
- Russell, D.G.; Cardona, P.J.; Kim, M.J.; Allain, S.; Altare, F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat. Immunol. 2009, 10, 943–948. [Google Scholar] [CrossRef]
- McWhorter, F.Y.; Wang, T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. USA 2013, 110, 17253–17258. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigamonti, A.; Feuerhake, F.; Donadon, M.; Locati, M.; Marchesi, F. Histopathological and Immune Prognostic Factors in Colo-Rectal Liver Metastases. Cancers 2021, 13, 1075. https://doi.org/10.3390/cancers13051075
Rigamonti A, Feuerhake F, Donadon M, Locati M, Marchesi F. Histopathological and Immune Prognostic Factors in Colo-Rectal Liver Metastases. Cancers. 2021; 13(5):1075. https://doi.org/10.3390/cancers13051075
Chicago/Turabian StyleRigamonti, Alessandra, Friedrich Feuerhake, Matteo Donadon, Massimo Locati, and Federica Marchesi. 2021. "Histopathological and Immune Prognostic Factors in Colo-Rectal Liver Metastases" Cancers 13, no. 5: 1075. https://doi.org/10.3390/cancers13051075
APA StyleRigamonti, A., Feuerhake, F., Donadon, M., Locati, M., & Marchesi, F. (2021). Histopathological and Immune Prognostic Factors in Colo-Rectal Liver Metastases. Cancers, 13(5), 1075. https://doi.org/10.3390/cancers13051075






