Angiogenesis in the Normal Adrenal Fetal Cortex and Adrenocortical Tumors
Abstract
Simple Summary
Abstract
1. Introduction
2. Angiogenesis Regulation
- (1)
- Sprouting angiogenesis, one the most well characterized mechanism leading to angiogenesis, relies on endothelial cells function specification into either tip or stalk cells. Tip cells are derived from the parent vessel, degrade the basement membrane, extend large filopodia which can sense angiogenic factor gradients, such as vascular endothelial growth factor (VEGF), and migrate along the chemotactic paths. In contrast, stalk cells proliferate behind tip cells to form the sprout body, start the process of lumen formation, and connect with neighboring vessels [5,6,7].
- (2)
- Intussusceptive angiogenesis is a process that consists in the splitting of pre-existing vessels into two new vessels. It starts with the formation of transluminal tissue pillars through the invagination of opposing capillary endothelial cells into the vascular lumen, creating a zone of contact. Commonly, intussusceptive and sprouting angiogenesis are complementary mechanisms [5,8].
- (3)
- Recruitment of endothelial progenitor cells and vasculogenesis, a process through which endothelial progenitor cells are recruited in response to several growth factors, cytokines and/or hypoxia-inducible factors. Endothelial progenitor cells differentiate into mature endothelial cells and are incorporated into the angiogenic sprout, thus contributing to new blood vessel formation [4,9].
- (4)
2.1. VEGF Pathway in Angiogenesis Regulation
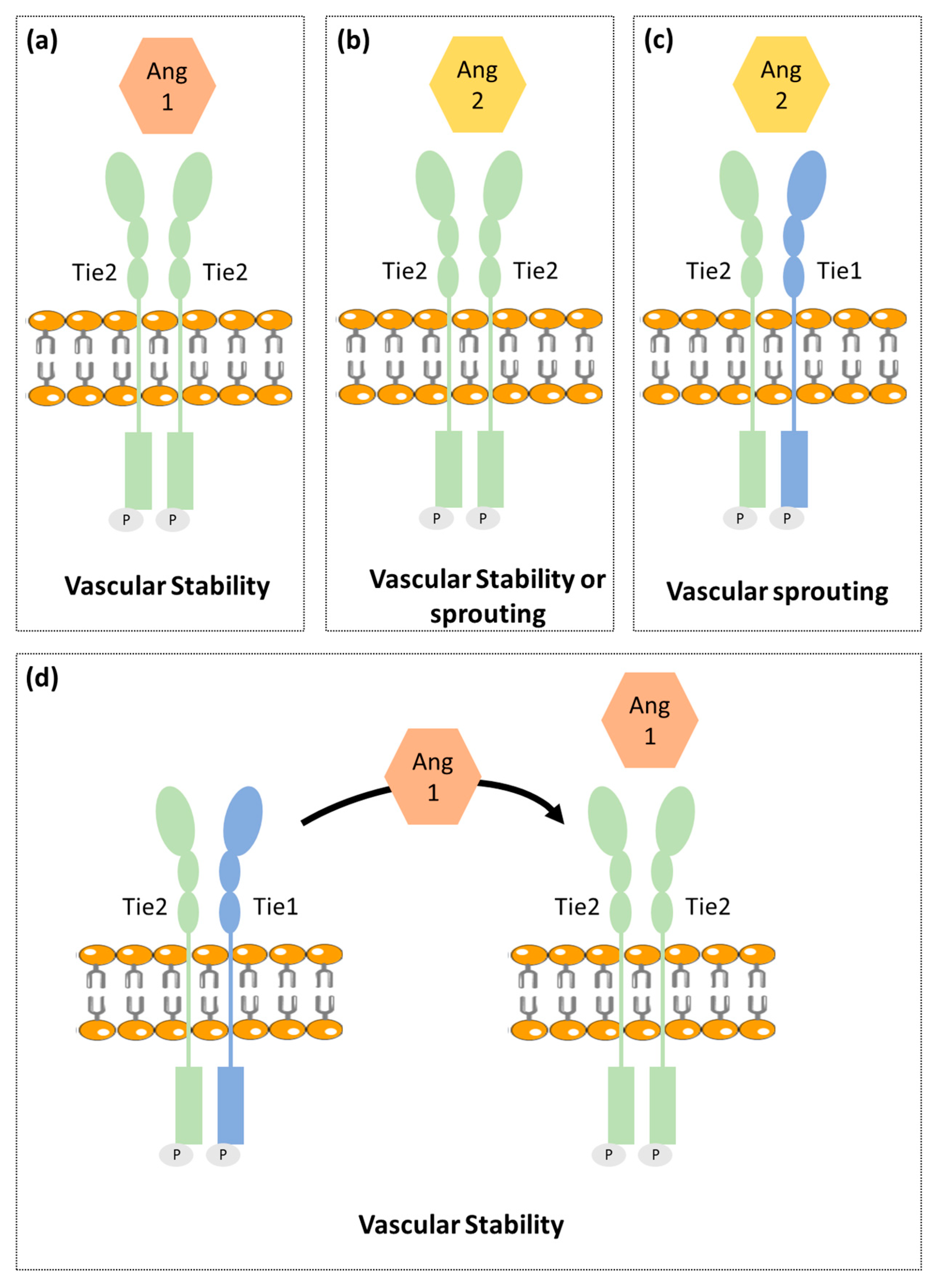
2.2. Ang-Tie Pathway in Angiogenesis Regulation
3. Angiogenesis in Normal Adrenal Cortex
3.1. Fetal Adrenal Cortex
3.2. Adult Adrenal Cortex
4. Angiogenesis in Adrenocortical Tumors
5. Anti-Angiogenic Agents’ Efficacy in Adrenocortical Carcinomas Treatment
| Anti-Angiogenic Drug | Mechanism of Action | Study Type | Patient Population | Results | Ref. |
|---|---|---|---|---|---|
| Bevacizumab (+capecitabine) | Monoclonal anti-VEGF antibody | Observational retrospective cohort study | Patients with refractory ACC (n = 10) | PFS: 59 days OS: 124 days | [71] |
| Thalidomide | Immunomodulatory agent that targets TNF-α, ILs, VEGF, bFGF | Observational retrospective cohort study | Patients with refractory ACC (n = 27) | PFS: 11.2 weeks (4.4–22.8 weeks) OS: 36.4 weeks (5.1–111.1 weeks) | [75] |
| Lenvatinib (+pembrolizumab) | Multi-TKI that inhibits VEGFR-1, VEGFR-2 and VEGFR-3, FGFRs, PDGFR-α, KIT, RET | Observational retrospective cohort study | Patients with recurrent and/or metastatic ACC (n = 8) | PFS: 5.5 months OS: NA | [76] |
| Cabozatinib | TKI that targets VEGFR-2 and c-Met | Observational retrospective cohort study | Patients with refractory metastatic ACC (n = 16) | PFS: 16.2 weeks (2.8–61 weeks) OS: 56 weeks (5.6–83.1 weeks) | [77] |
| Sorafenib (+paclitaxel) | Multi-TKI inhibitor that VEGFR-2 VEGFR-3, PDGFR and RAF-1 | Phase II, single-arm, open label clinical trial | Patients with refractory metastatic ACC (n = 10) | Trial interrupted due disease progression in all enrolled patients | [72] |
| Sunitinib | Multi-TKI that inhibits VEGFR-1 and VEGFR-2, c-KIT, FLT3 and PDGFR | Phase II, single-arm, open label clinical trial | Patients with advanced ACC after mitotane or others cytotoxic drugs (n = 35) | PFS: 2.8 months (5.6–11.2 months) OS: 5.4 months (14.0–35.5 months) | [73] |
| Axitinib | Selective inhibitor of VEGFR-1, VEGFR-2 and VEGFR-3 | Phase II, single-arm, open label clinical trial | Patients with metastatic ACC previously treated with at least one chemotherapy regimen (n = 13) | PFS: 5.48 months (1.8–10.92 months) OS: 13.7 months | [74] |
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef]
- Chung, A.S.; Ferrara, N. Developmental and Pathological Angiogenesis. Annu. Rev. Cell Dev. Biol. 2011, 27, 563–584. [Google Scholar] [CrossRef]
- Zuazo-Gaztelu, I.; Casanovas, O. Unraveling the Role of Angiogenesis in Cancer Ecosystems. Front. Oncol. 2018, 8, 248. [Google Scholar] [CrossRef]
- Mentzer, S.J.; Konerding, M.A. Intussusceptive angiogenesis: Expansion and remodeling of microvascular networks. Angiogenesis 2014, 17, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Duran, C.L.; Howell, D.W.; Dave, J.M.; Smith, R.L.; Torrie, M.E.; Essner, J.J.; Bayless, K.J. Molecular Regulation of Sprouting Angiogenesis. Compr. Physiol. 2017, 8, 153–235. [Google Scholar] [CrossRef]
- Makanya, A.N.; Hlushchuk, R.; Djonov, V.G. Intussusceptive angiogenesis and its role in vascular morphogenesis, patterning, and remodeling. Angiogenesis 2009, 12, 113. [Google Scholar] [CrossRef]
- Kolte, D.; McClung, J.A.; Aronow, W.S. Vasculogenesis and angiogenesis. In Translational Research in Coronary Artery Disease; Elsevier: Amsterdam, The Netherlands, 2016; pp. 49–65. [Google Scholar]
- Ge, H.; Luo, H. Overview of advances in vasculogenic mimicry—A potential target for tumor therapy. Cancer Manag. Res. 2018, 10, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cortés, M.; Delgado-Bellido, D.; Oliver, F.J. Vasculogenic Mimicry: Become an Endothelial Cell “But Not So Much”. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Saharinen, P.; Eklund, L.; Pulkki, K.; Bono, P.; Alitalo, K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol. Med. 2011, 17, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Shibuya, M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin. Sci. 2005, 109, 227–241. [Google Scholar] [CrossRef]
- Guo, H.-F.; Vander Kooi, C.W. Neuropilin Functions as an Essential Cell Surface Receptor. J. Biol. Chem. 2015, 290, 29120–29126. [Google Scholar] [CrossRef]
- Chiodelli, P.; Mitola, S.; Ravelli, C.; Oreste, P.; Rusnati, M.; Presta, M. Heparan sulfate proteoglycans mediate the angiogenic activity of the vascular endothelial growth factor receptor-2 agonist gremlin. Arterioscler. Thromb. Vasc. Biol. 2011, 31, e116–e127. [Google Scholar] [CrossRef]
- Cross, M.J.; Dixelius, J.; Matsumoto, T.; Claesson-Welsh, L. VEGF-receptor signal transduction. Trends Biochem. Sci. 2003, 28, 488–494. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- LeCouter, J.; Kowalski, J.; Foster, J.; Hass, P.; Zhang, Z.; Dillard-Telm, L.; Frantz, G.; Rangell, L.; DeGuzman, L.; Keller, G.A.; et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature 2001, 412, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Augustin, H.G.; Koh, G.Y.; Thurston, G.; Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 2009, 10, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Cho, C.H.; Hwang, S.J.; Choi, H.H.; Kim, K.T.; Ahn, S.Y.; Kim, J.H.; Oh, J.L.; Lee, G.M.; Koh, G.Y. Biological characterization of angiopoietin-3 and angiopoietin. FASEB J 2004, 18, 1200–1208. [Google Scholar] [CrossRef]
- Eklund, L.; Saharinen, P. Angiopoietin signaling in the vasculature. Exp. Cell Res. 2013, 319, 1271–1280. [Google Scholar] [CrossRef]
- Fagiani, E.; Christofori, G. Angiopoietins in angiogenesis. Cancer Lett. 2013, 328, 18–26. [Google Scholar] [CrossRef]
- Davis, S.; Aldrich, T.H.; Jones, P.F.; Acheson, A.; Compton, D.L.; Jain, V.; Ryan, T.E.; Bruno, J.; Radziejewski, C.; Maisonpierre, P.C.; et al. Isolation of Angiopoietin-1, a Ligand for the TIE2 Receptor, by Secretion-Trap Expression Cloning. Cell 1996, 87, 1161–1169. [Google Scholar] [CrossRef]
- Dumont, D.J.; Gradwohl, G.; Fong, G.H.; Puri, M.C.; Gertsenstein, M.; Auerbach, A.; Breitman, M.L. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994, 8, 1897–1909. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.T.; Khankin, E.V.; Karumanchi, S.A.; Parikh, S.M. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol. Cell Biol. 2009, 29, 2011–2022. [Google Scholar] [CrossRef] [PubMed]
- Barton, W.A.; Tzvetkova-Robev, D.; Miranda, E.P.; Kolev, M.V.; Rajashankar, K.R.; Himanen, J.P.; Nikolov, D.B. Crystal structures of the Tie2 receptor ectodomain and the angiopoietin-2-Tie2 complex. Nat. Struct. Mol. Biol. 2006, 13, 524–532. [Google Scholar] [CrossRef]
- Seegar, T.C.M.; Eller, B.; Tzvetkova-Robev, D.; Kolev, M.V.; Henderson, S.C.; Nikolov, D.B.; Barton, W.A. Tie1-Tie2 interactions mediate functional differences between angiopoietin ligands. Mol. Cell 2010, 37, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Porat, R.M.; Grunewald, M.; Globerman, A.; Itin, A.; Barshtein, G.; Alhonen, L.; Alitalo, K.; Keshet, E. Specific Induction of tie1 Promoter by Disturbed Flow in Atherosclerosis-Prone Vascular Niches and Flow-Obstructing Pathologies. Circ. Res. 2004, 94, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Puri, M.C.; Rossant, J.; Alitalo, K.; Bernstein, A.; Partanen, J. The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J. 1995, 14, 5884–5891. [Google Scholar] [CrossRef] [PubMed]
- La Porta, S.; Roth, L.; Singhal, M.; Mogler, C.; Spegg, C.; Schieb, B.; Qu, X.; Adams, R.H.; Baldwin, H.S.; Savant, S.; et al. Endothelial Tie1-mediated angiogenesis and vascular abnormalization promote tumor progression and metastasis. J. Clin. Investig. 2018, 128, 834–845. [Google Scholar] [CrossRef]
- Zhang, Y.; Kontos, C.D.; Annex, B.H.; Popel, A.S. Angiopoietin-Tie Signaling Pathway in Endothelial Cells: A Computational Model. iScience 2019, 20, 497–511. [Google Scholar] [CrossRef]
- Liggins, G.C. Adrenocortical-related maturational events in the fetus. Am. J. Obstet. Gynecol. 1976, 126, 931–941. [Google Scholar] [CrossRef]
- Mesiano, S.; Jaffe, R.B. Developmental and functional biology of the primate fetal adrenal cortex. Endocr. Rev. 1997, 18, 378–403. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, H.; Jaffe, R.B. Development and function of the human fetal adrenal cortex: A key component in the feto-placental unit. Endocr. Rev. 2011, 32, 317–355. [Google Scholar] [CrossRef]
- Lanman, J.T. The fetal zone of the adrenal gland: Its developmental course, comparative anatomy, and possible physiologic functions. Medicine 1953, 32, 389–430. [Google Scholar] [CrossRef] [PubMed]
- Johannisson, E. The foetal adrenal cortex in the human. Its ultrastructure at different stages of development and in different functional states. Acta Endocrinol. 1968, 58, S7–S107. [Google Scholar] [CrossRef]
- Spencer, S.J.; Mesiano, S.; Lee, J.Y.; Jaffe, R.B. Proliferation and apoptosis in the human adrenal cortex during the fetal and perinatal periods: Implications for growth and remodeling. J. Clin. Endocrinol. Metab. 1999, 84, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- McNutt, N.S.; Jones, A.L. Observations on the ultrastructure of cytodifferentiation in the human fetal adrenal cortex. Lab. Investig. J. Tech. Methods Pathol. 1970, 22, 513–527. [Google Scholar]
- Sucheston, M.E.; Cannon, M.S. Development of zonular patterns in the human adrenal gland. J. Morphol. 1968, 126, 477–491. [Google Scholar] [CrossRef]
- Mesiano, S.; Coulter, C.L.; Jaffe, R.B. Localization of cytochrome P450 cholesterol side-chain cleavage, cytochrome P450 17 alpha-hydroxylase/17, 20-lyase, and 3 beta-hydroxysteroid dehydrogenase isomerase steroidogenic enzymes in human and rhesus monkey fetal adrenal glands: Reappraisal of functional zonation. J. Clin. Endocrinol. Metab. 1993, 77, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- McClellan, M.C.; Brenner, R.M. Development of the fetal adrenals in nonhuman primates: Electron microscopy. In Fetal Endocrinology; Novy, M.J., Resko, J.A., Eds.; Academic Press: Cambridge, MA, USA, 1981; Volume 1, pp. 383–403. [Google Scholar] [CrossRef]
- Ishimoto, H.; Ginzinger, D.G.; Jaffe, R.B. Adrenocorticotropin preferentially up-regulates angiopoietin 2 in the human fetal adrenal gland: Implications for coordinated adrenal organ growth and angiogenesis. J. Clin. Endocrinol. Metab. 2006, 91, 1909–1915. [Google Scholar] [CrossRef]
- Ishimoto, H.; Minegishi, K.; Higuchi, T.; Furuya, M.; Asai, S.; Kim, S.H.; Tanaka, M.; Yoshimura, Y.; Jaffe, R.B. The periphery of the human fetal adrenal gland is a site of angiogenesis: Zonal differential expression and regulation of angiogenic factors. J. Clin. Endocrinol. Metab. 2008, 93, 2402–2408. [Google Scholar] [CrossRef][Green Version]
- Pitynski, K.; Litwin, J.A.; Nowogrodzka-Zagorska, M.; Miodonski, A.J. Vascular architecture of the human fetal adrenal gland: A SEM study of corrosion casts. Ann. Anat. Anat. Anz Off. Organ Anat. Ges. 1996, 178, 215–222. [Google Scholar] [CrossRef]
- Mesiano, S.; Mellon, S.H.; Gospodarowicz, D.; Di Blasio, A.M.; Jaffe, R.B. Basic fibroblast growth factor expression is regulated by corticotropin in the human fetal adrenal: A model for adrenal growth regulation. Proc. Natl. Acad. Sci. USA 1991, 88, 5428–5432. [Google Scholar] [CrossRef] [PubMed]
- Shifren, J.L.; Mesiano, S.; Taylor, R.N.; Ferrara, N.; Jaffe, R.B. Corticotropin regulates vascular endothelial growth factor expression in human fetal adrenal cortical cells. J. Clin. Endocrinol. Metab. 1998, 83, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Ozisik, G.; Achermann, J.C.; Meeks, J.J.; Jameson, J.L. SF1 in the development of the adrenal gland and gonads. Horm. Res. 2003, 59 (Suppl. 1), 94–98. [Google Scholar] [CrossRef]
- Ferraz-de-Souza, B.; Lin, L.; Shah, S.; Jina, N.; Hubank, M.; Dattani, M.T.; Achermann, J.C. ChIP-on-chip analysis reveals angiopoietin 2 (Ang2, ANGPT2) as a novel target of steroidogenic factor-1 (SF-1, NR5A1) in the human adrenal gland. FASEB J. 2010, 25, 1166–1175. [Google Scholar] [CrossRef]
- Sapirstein, L.A.; Goldman, H. Adrenal blood flow in the albino rat. Am. J. Physiol. 1959, 196, 159–162. [Google Scholar] [CrossRef]
- Bassett, J.R.; West, S.H. Vascularization of the adrenal cortex: Its possible involvement in the regulation of steroid hormone release. Microsc. Res. Tech. 1997, 36, 546–557. [Google Scholar] [CrossRef]
- Thomas, M.; Keramidas, M.; Monchaux, E.; Feige, J.J. Role of adrenocorticotropic hormone in the development and maintenance of the adrenal cortical vasculature. Microsc. Res. Tech. 2003, 61, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Vittet, D.; Ciais, D.; Keramidas, M.; De Fraipont, F.; Feige, J.J. Paracrine control of the adult adrenal cortex vasculature by vascular endothelial growth factor. Endocr. Res. 2000, 26, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Heck, D.; Wortmann, S.; Kraus, L.; Ronchi, C.L.; Sinnott, R.O.; Fassnacht, M.; Sbiera, S. Role of Endocrine Gland-Derived Vascular Endothelial Growth Factor (EG-VEGF) and Its Receptors in Adrenocortical Tumors. Horm. Cancer 2015, 6, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Senger, D.R. Vascular endothelial growth factor: Much more than an angiogenesis factor. Mol. Biol. Cell 2010, 21, 377–379. [Google Scholar] [CrossRef]
- Gomez-Sanchez, C.E. Regulation of Adrenal Arterial Tone by Adrenocorticotropin: The Plot Thickens. Endocrinology 2007, 148, 3566–3568. [Google Scholar] [CrossRef] [PubMed]
- Else, T.; Kim, A.C.; Sabolch, A.; Raymond, V.M.; Kandathil, A.; Caoili, E.M.; Jolly, S.; Miller, B.S.; Giordano, T.J.; Hammer, G.D. Adrenocortical Carcinoma. Endocr. Rev. 2014, 35, 282–326. [Google Scholar] [CrossRef]
- Libé, R. Adrenocortical carcinoma (ACC): Diagnosis, prognosis, and treatment. Front. Cell Dev. Biol. 2015, 3, 45. [Google Scholar] [CrossRef]
- Fassnacht, M.; Arlt, W.; Bancos, I.; Dralle, H.; Newell-Price, J.; Sahdev, A.; Tabarin, A.; Terzolo, M.; Tsagarakis, S.; Dekkers, O.M. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2016, 175, G1–G34. [Google Scholar] [CrossRef]
- Kolomecki, K.; Stepien, H.; Bartos, M.; Kuzdak, K. Usefulness of VEGF, MMP-2, MMP-3 and TIMP-2 serum level evaluation in patients with adrenal tumours. Endocr. Regul. 2001, 35, 9–16. [Google Scholar]
- Zacharieva, S.; Atanassova, I.; Orbetzova, M.; Nachev, E.; Kalinov, K.; Kirilov, G.; Shigarminova, R.; Ivanova, R.; Dashev, G. Circulating vascular endothelial growth factor and active renin concentrations and prostaglandin E2 urinary excretion in patients with adrenal tumours. Eur. J. Endocrinol. 2004, 150, 345–349. [Google Scholar] [CrossRef]
- Bernini, G.P.; Moretti, A.; Bonadio, A.G.; Menicagli, M.; Viacava, P.; Naccarato, A.G.; Iacconi, P.; Miccoli, P.; Salvetti, A. Angiogenesis in Human Normal and Pathologic Adrenal Cortex. J. Clin. Endocrinol. Metab. 2002, 87, 4961–4965. [Google Scholar] [CrossRef] [PubMed]
- Kroiss, M.; Reuss, M.; Kühner, D.; Johanssen, S.; Beyer, M.; Zink, M.; Hartmann, M.F.; Dhir, V.; Wudy, S.A.; Arlt, W.; et al. Sunitinib Inhibits Cell Proliferation and Alters Steroidogenesis by Down-Regulation of HSD3B2 in Adrenocortical Carcinoma Cells. Front. Endocrinol. 2011, 2, 27. [Google Scholar] [CrossRef]
- de Fraipont, F.; El Atifi, M.; Gicquel, C.; Bertagna, X.; Chambaz, E.M.; Feige, J.J. Expression of the Angiogenesis Markers Vascular Endothelial Growth Factor-A, Thrombospondin-1, and Platelet-Derived Endothelial Cell Growth Factor in Human Sporadic Adrenocortical Tumors: Correlation with Genotypic Alter. J. Clin. Endocrinol. Metab. 2000, 85, 4734–4741. [Google Scholar] [CrossRef]
- Xu, Y.Z.; Zhu, Y.; Shen, Z.J.; Sheng, J.Y.; He, H.C.; Ma, G.; Qi, Y.C.; Zhao, J.P.; Wu, Y.X.; Rui, W.B.; et al. Significance of heparanase-1 and vascular endothelial growth factor in adrenocortical carcinoma angiogenesis: Potential for therapy. Endocrine 2011, 40, 445–451. [Google Scholar] [CrossRef]
- Diaz-Cano, S.J.; De Miguel, M.; Blanes, A.; Galera, H.; Wolfe, H.J. Contribution of the microvessel network to the clonal and kinetic profiles of adrenal cortical proliferative lesions. Hum. Pathol. 2001, 32, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Sasano, H.; Ohashi, Y.; Suzuki, T.; Nagura, H. Vascularity in human adrenal cortex. Mod. Pathol. 1998, 11, 329–333. [Google Scholar] [PubMed]
- Zhu, Y.; Xu, Y.; Chen, D.; Zhang, C.; Rui, W.; Zhao, J.; Zhu, Q.; Wu, Y.; Shen, Z.; Wang, W.; et al. Expression of STAT3 and IGF2 in adrenocortical carcinoma and its relationship with angiogenesis. Clin. Transl. Oncol. 2014, 16, 644–649. [Google Scholar] [CrossRef]
- Pereira, S.S.; Costa, M.M.; Guerreiro, S.G.; Monteiro, M.P.; Pignatelli, D. Angiogenesis and Lymphangiogenesis in the Adrenocortical Tumors. Pathol. Oncol. Res. 2018, 24, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Logie, J.J.; Ali, S.; Marshall, K.M.; Heck, M.M.S.; Walker, B.R.; Hadoke, P.W.F. Glucocorticoid-mediated inhibition of angiogenic changes in human endothelial cells is not caused by reductions in cell proliferation or migration. PLoS ONE 2010, 5, e14476. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Wortmann, S.; Quinkler, M.; Ritter, C.; Kroiss, M.; Johanssen, S.; Hahner, S.; Allolio, B.; Fassnacht, M. Bevacizumab plus capecitabine as a salvage therapy in advanced adrenocortical carcinoma. Eur. J. Endocrinol. 2010, 162, 349. [Google Scholar] [CrossRef][Green Version]
- Berruti, A.; Sperone, P.; Ferrero, A.; Germano, A.; Ardito, A.; Priola, A.M.; Francia, S.D.; Volante, M.; Daffara, F.; Generali, D.; et al. Phase II study of weekly paclitaxel and sorafenib as second/third-line therapy in patients with adrenocortical carcinoma. Eur. J. Endocrinol. 2012, 166, 451. [Google Scholar] [CrossRef]
- Kroiss, M.; Quinkler, M.; Johanssen, S.; van Erp, N.P.; Lankheet, N.; Pöllinger, A.; Laubner, K.; Strasburger, C.J.; Hahner, S.; Müller, H.-H.; et al. Sunitinib in Refractory Adrenocortical Carcinoma: A Phase II, Single-Arm, Open-Label Trial. J. Clin. Endocrinol. Metab. 2012, 97, 3495–3503. [Google Scholar] [CrossRef]
- O’Sullivan, C.; Edgerly, M.; Velarde, M.; Wilkerson, J.; Venkatesan, A.M.; Pittaluga, S.; Yang, S.X.; Nguyen, D.; Balasubramaniam, S.; Fojo, T. The VEGF inhibitor axitinib has limited effectiveness as a therapy for adrenocortical cancer. J. Clin. Endocrinol. Metab. 2014, 99, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Kroiss, M.; Deutschbein, T.; Schlötelburg, W.; Ronchi, C.L.; Hescot, S.; Körbl, D.; Megerle, F.; Beuschlein, F.; Neu, B.; Quinkler, M.; et al. Treatment of Refractory Adrenocortical Carcinoma with Thalidomide: Analysis of 27 Patients from the European Network for the Study of Adrenal Tumours Registry. Exp. Clin. Endocrinol. Diabetes 2019, 127, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Bedrose, S.; Miller, K.C.; Altameemi, L.; Ali, M.S.; Nassar, S.; Garg, N.; Daher, M.; Eaton, K.D.; Yorio, J.T.; Daniel, D.B.; et al. Combined lenvatinib and pembrolizumab as salvage therapy in advanced adrenal cortical carcinoma. J. Immunother. Cancer 2020, 8, e001009. [Google Scholar] [CrossRef] [PubMed]
- Kroiss, M.; Megerle, F.; Kurlbaum, M.; Zimmermann, S.; Wendler, J.; Jimenez, C.; Lapa, C.; Quinkler, M.; Scherf-Clavel, O.; Habra, M.A.; et al. Objective Response and Prolonged Disease Control of Advanced Adrenocortical Carcinoma with Cabozantinib. J. Clin. Endocrinol. Metab. 2020, 105. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Kurzrock, R.; Mulay, M.; Rasmussen, E.; Wu, B.M.; Bass, M.B.; Zhong, Z.D.; Friberg, G.; Rosen, L.S. A phase 1b, open-label study of trebananib plus bevacizumab or motesanib in patients with solid tumours. Oncotarget 2014, 5, 11154–11167. [Google Scholar] [CrossRef][Green Version]
- Kroiss, M.; Quinkler, M.; Lutz, W.K.; Allolio, B.; Fassnacht, M. Drug interactions with mitotane by induction of CYP3A4 metabolism in the clinical management of adrenocortical carcinoma. Clin. Endocrinol. 2011, 75, 585–591. [Google Scholar] [CrossRef]
- Raj, N.; Zheng, Y.; Kelly, V.; Katz, S.S.; Chou, J.; Do, R.K.G.; Capanu, M.; Zamarin, D.; Saltz, L.B.; Ariyan, C.E.; et al. PD-1 Blockade in Advanced Adrenocortical Carcinoma. J. Clin. Oncol. 2020, 38, 71–80. [Google Scholar] [CrossRef]
- Qin, S.; Ren, Z.; Meng, Z.; Chen, Z.; Chai, X.; Xiong, J.; Bai, Y.; Yang, L.; Zhu, H.; Fang, W.; et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: A multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 571–580. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, G.; Huang, Y.; Zhou, J.; Lin, L.; Feng, J.; Wang, Z.; Shu, Y.; Shi, J.; Hu, Y.; et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): A randomised, open-label, multicentre, phase 3 trial. Lancet Respir. Med. 2020. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Y.; Chen, X.; Li, J.; Pan, J.; He, X.; Lin, L.; Shi, Y.; Feng, W.; Xiong, J.; et al. 912MO A single-arm, open-label, multicenter phase II study of camrelizumab in patients with recurrent or metastatic (R/M) nasopharyngeal carcinoma (NPC) who had progressed on ≥2 lines of chemotherapy: CAPTAIN study. Ann. Oncol. 2020, 31, S659. [Google Scholar] [CrossRef]
| Patient Group Comparisons | Results | |
|---|---|---|
| VEGF | Patients with ACT vs. Healthy individuals | ↑ VEGF serum levels in patients with ACT [59,60] |
| Aldosterone secreting ACA vs. Non-functioning ACA | ↑ VEGF tumor expression in aldosterone producing ACA [61] | |
| Cortisol secreting ACA vs. Aldosterone secreting ACA | ↑ VEGF serum levels patients with cortisol secreting ACA [60] | |
| ACC vs. Normal adrenal glands | ↑ VEGF expression in ACC [61,62] | |
| ACC vs. ACA | ↑ VEGF serum levels in ACC ↑ VEGF tumor expression in ACC [59,61,63,64] | |
| Patients with recurrent ACC vs. Patients with non-recurrent ACC | ↑ VEGF serum levels in recurrent ACC ↑ VEGF tumor expression in recurrent ACC [60,63] | |
| Localized ACC vs. Invasive ACC | No difference in VEGF tumor expression [63] | |
| VEGF-R2 | ACC vs. Normal adrenal glands | ↑ VEGF-R2 tumor expression in ACC [62] |
| ACC vs. ACA | ↑ VEGF-R2 tumor expression in ACC [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, S.S.; Oliveira, S.; Monteiro, M.P.; Pignatelli, D. Angiogenesis in the Normal Adrenal Fetal Cortex and Adrenocortical Tumors. Cancers 2021, 13, 1030. https://doi.org/10.3390/cancers13051030
Pereira SS, Oliveira S, Monteiro MP, Pignatelli D. Angiogenesis in the Normal Adrenal Fetal Cortex and Adrenocortical Tumors. Cancers. 2021; 13(5):1030. https://doi.org/10.3390/cancers13051030
Chicago/Turabian StylePereira, Sofia S., Sofia Oliveira, Mariana P. Monteiro, and Duarte Pignatelli. 2021. "Angiogenesis in the Normal Adrenal Fetal Cortex and Adrenocortical Tumors" Cancers 13, no. 5: 1030. https://doi.org/10.3390/cancers13051030
APA StylePereira, S. S., Oliveira, S., Monteiro, M. P., & Pignatelli, D. (2021). Angiogenesis in the Normal Adrenal Fetal Cortex and Adrenocortical Tumors. Cancers, 13(5), 1030. https://doi.org/10.3390/cancers13051030






