Current Trends in Endoscopic Diagnosis and Treatment of Early Esophageal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Barrett’s Esophagus, High-Grade Dysplasia, and Early Adenocarcinoma
2.1. Screening for the Presence of Barrett’s Esophagus
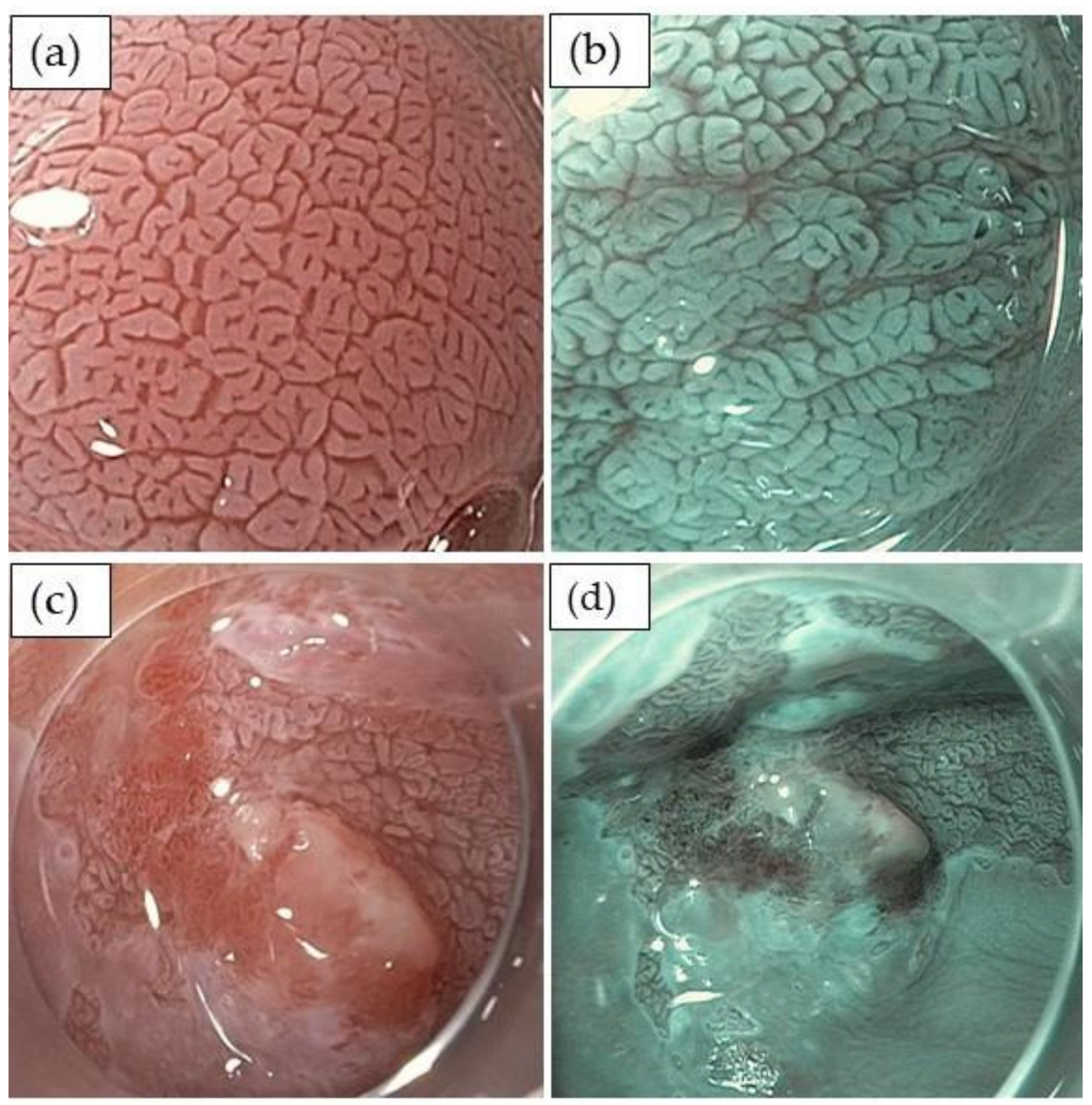
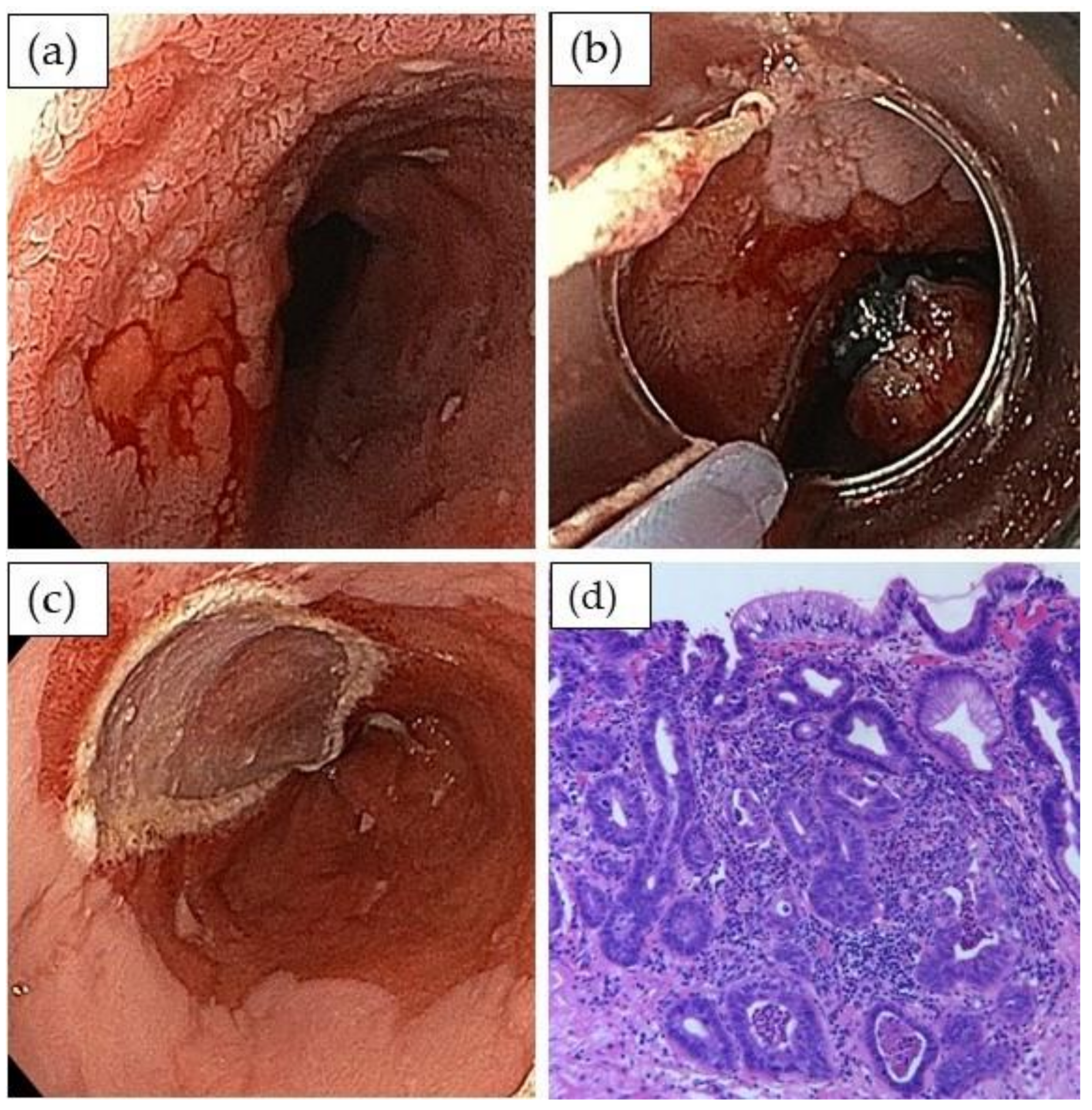
2.2. Detection and Evaluation of High-Grade Dysplasia and Early Adenocarcinoma
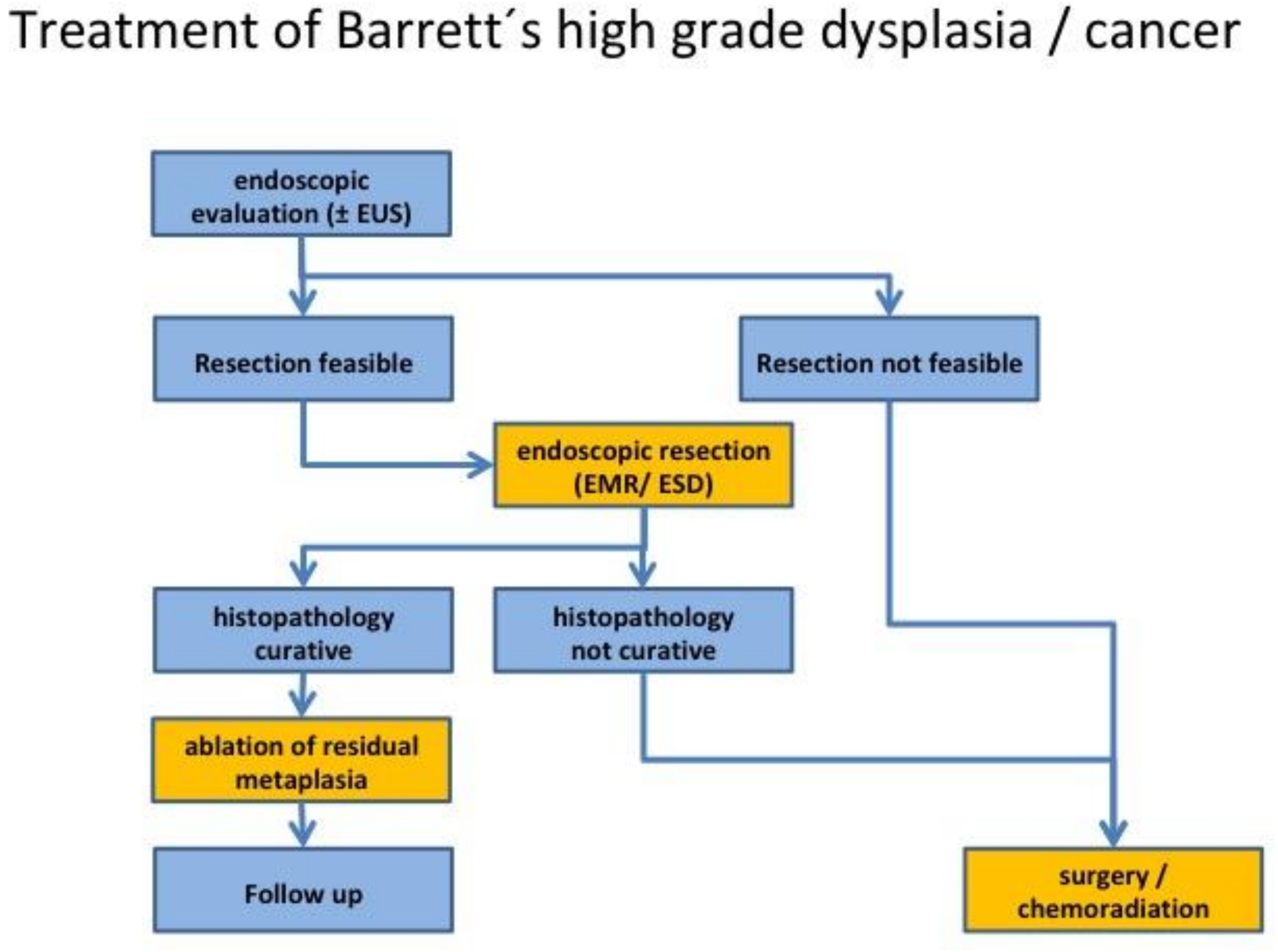
2.3. Endoscopic Treatment of Dysplasia and Early Adenocarcinoma
2.4. Additional Treatment and Follow-Up
3. Squamous Cell High-Grade Dysplasia and Early Cancer
3.1. Screening for Squamous Cell Dysplasia or Early Cancer
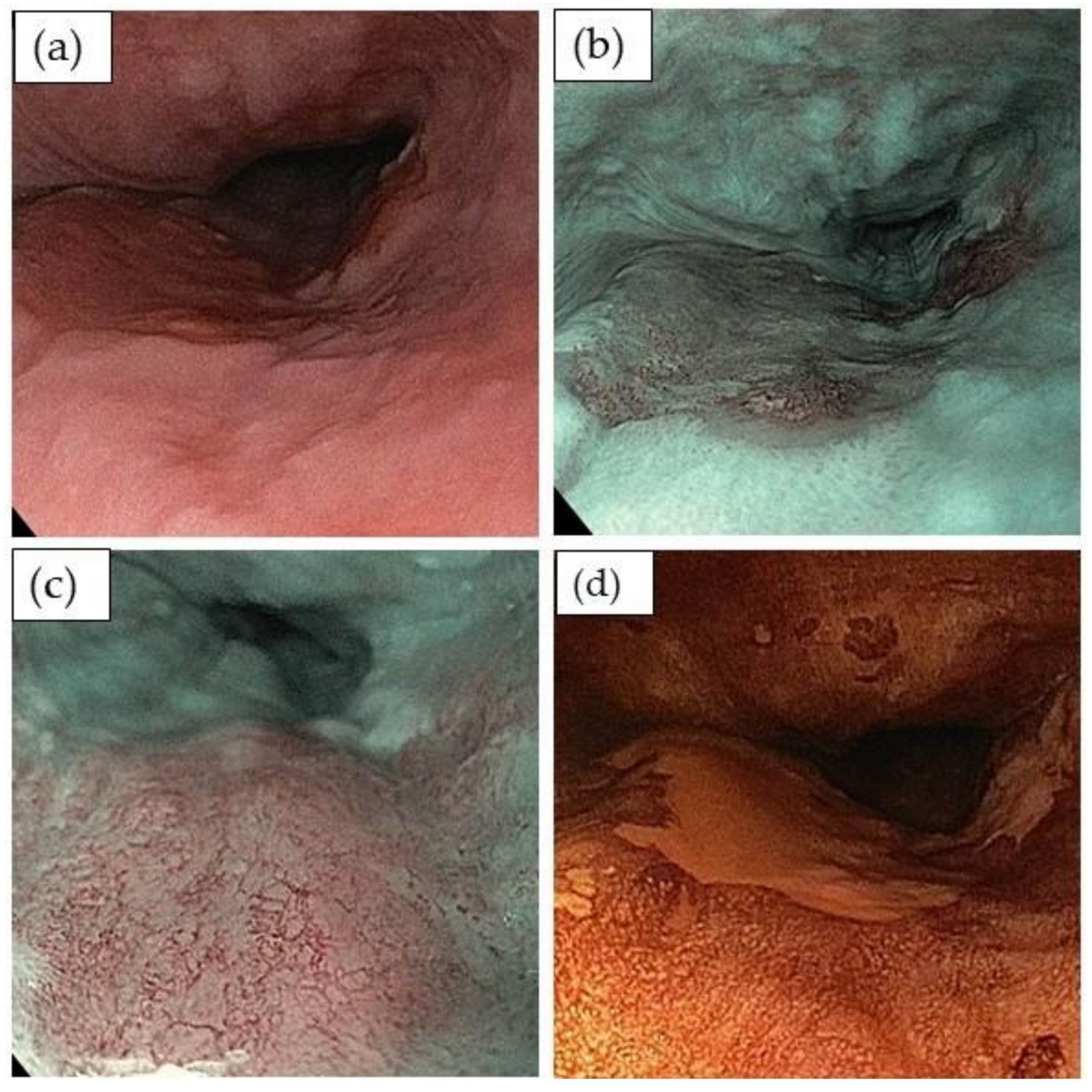
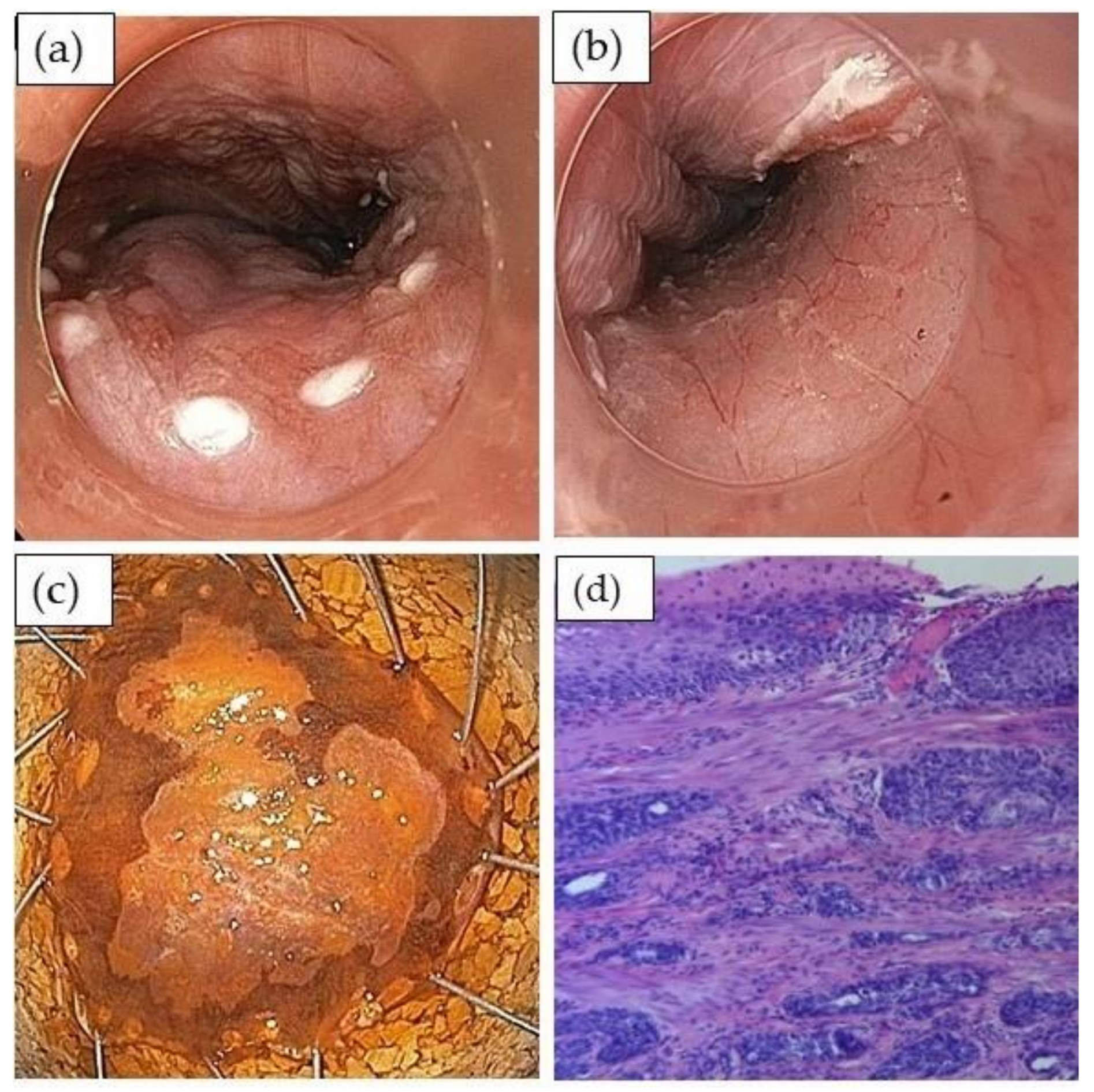
3.2. Detection and Evaluation of Squamous Cell Dysplasia or Early Cancer
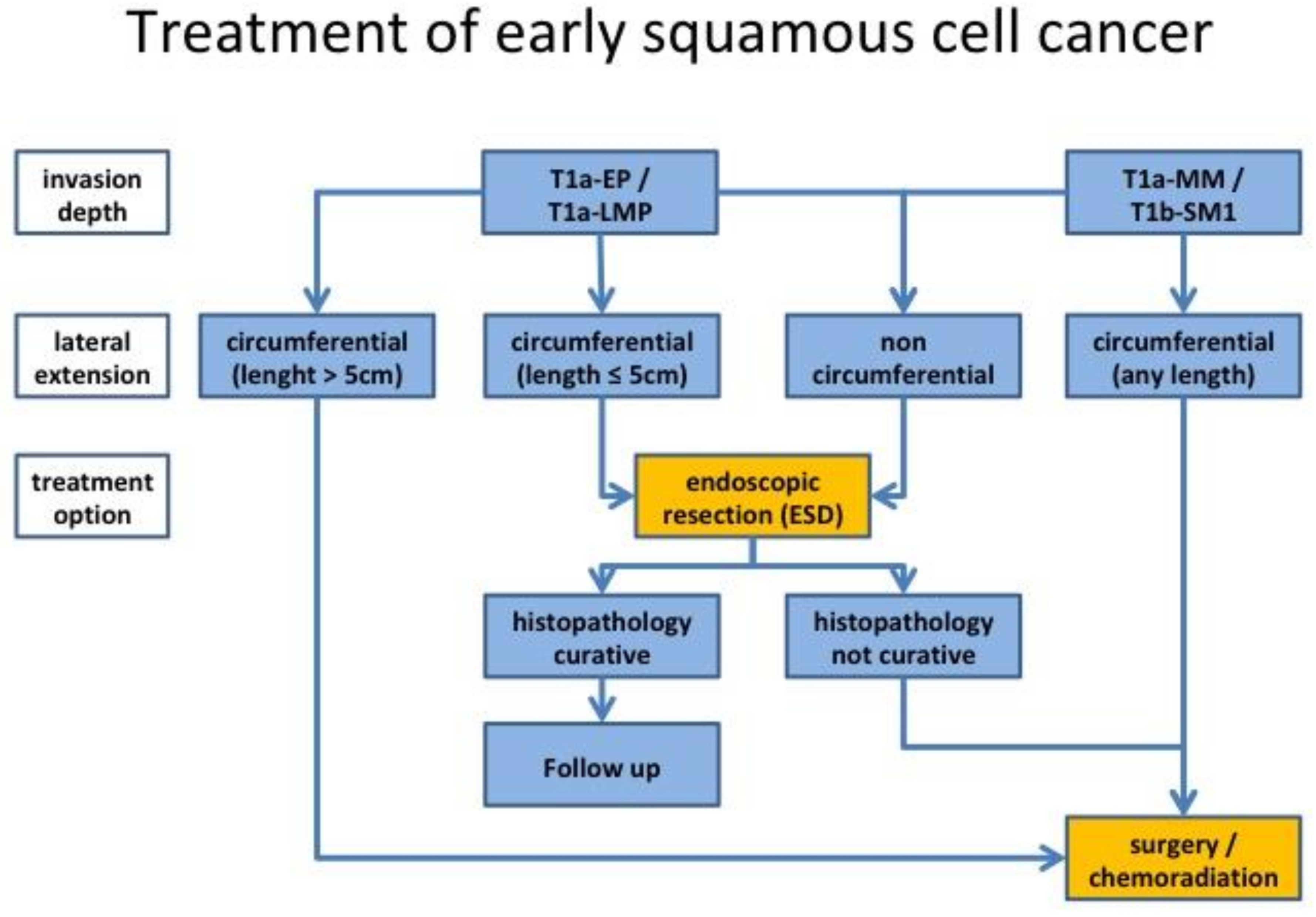
3.3. Endoscopic Treatment
3.4. Additional Treatment and Follow-Up
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349. [Google Scholar] [CrossRef]
- Malhotra, G.K.; Yanala, U.; Ravipati, A.; Follet, M.; Vijayakumar, M.; Are, C. Global trends in esophageal cancer. J. Surg. Oncol. 2017, 115, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Que, J.; Garman, K.S.; Souza, R.F.; Spechler, S.J. Pathogenesis and cells of origin of Barrett’s esophagus. Gastroenterology 2019, 157, 349–364. [Google Scholar] [CrossRef]
- Desai, M.; Lieberman, D.A.; Kennedy, K.F.; Hamade, N.; Thota, P.; Parasa, S.; Gorrepati, V.S.; Bansal, A.; Gupta, N.; Gaddam, S.; et al. Increasing prevalence of high-grade dysplasia and adenocarcinoma on index endoscopy in Barrett’s esophagus over the past 2 decades: Data from a multicenter U.S. consortium. Gastrointest. Endosc. 2019, 89, 257–263. [Google Scholar] [CrossRef]
- Peters, Y.; Al-Kaabi, A.; Shaheen, N.J.; Chak, A.; Blum, A.; Souza, R.F.; Di Pietro, M.; Iyer, P.G.; Pech, O.; Fitzgerald, R.C.; et al. Barrett oesophagus. Nat. Rev. Dis. Prim. 2019, 5, 35. [Google Scholar] [CrossRef]
- Spechler, S.J.; Sharma, P.; Souza, R.F.; Inadomi, J.M.; Shaheen, N.J. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011, 140, 1084–1091. [Google Scholar] [CrossRef]
- Fitzgerald, R.C.; Di Pietro, M.; Ragunath, K.; Ang, Y.; Kang, J.Y.; Watson, P.; De Caestecker, J. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut 2014, 63, 7–42. [Google Scholar] [CrossRef] [PubMed]
- Weusten, B.; Bisschops, R.; Coron, E.; Dinis-Ribeiro, M.; Dumonceau, J.-M.; Esteban, J.-M.; Hassan, C.; Pech, O.; Repici, A.; Bergman, J.; et al. Endoscopic management of Barrett’s esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Eur. Soc. Gastrointest. Endosc. Position Statement 2017, 49, 191–198. [Google Scholar] [CrossRef]
- Qumseya, B.; Sultan, S.; Bain, P.; Jamil, L.; Jacobson, B.; Anandasabapathy, S.; Agrawal, D.; Buxbaum, J.L.; Fishman, D.S.; Gurudu, S.R.; et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest. Endosc. 2019, 90, 335–359. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Thrift, A.P.; Rugge, M.; El-Serag, H.B. Prevalence of Barrett’s esophagus and performance of societal screening guidelines in an unreferred primary care population of U.S. veterans. Gastrointest. Endosc. e1. [CrossRef] [PubMed]
- Fitzgerald, R.C.; di Pietro, M.; O’Donovan, M.; Maroni, R.; Muldrew, B.; Debiram-Beecham, I.; Gaw, M. Cytosponge-trefoil factor 3 versus usual care to identify Barrett’s oesophagus in a primary care setting: A multicentre, pragmatic, randomised controlled trial. Lancet 2020, 396, 333–344. [Google Scholar] [CrossRef]
- Peters, Y.; Schrauwen, R.W.M.; Tan, A.C.; Bogers, S.K.; De Jong, B.; Siersema, P.D. Detection of Barrett’s oesophagus through exhaled breath using an electronic nose device. Gut 2020, 69, 1169–1172. [Google Scholar] [CrossRef]
- Rodríguez de Santiago, E.; Hernanz, N.; Marcos-Prieto, H.M.; De-Jorge-Turrión M, Á.; Barreiro-Alonso, E.; Rodríguez-Escaja, C.; Albillos, A. Rate of missed oesophageal cancer at routine endoscopy and survival outcomes: A multicentric cohort study. United Eur. Gastroenterol. J. 2019, 7, 189–198. [Google Scholar]
- Parasa, S.; Desai, M.; Vittal, A.; Chandrasekar, V.T.; Pervez, A.; Kennedy, K.F.; Gupta, N.; Shaheen, N.J.; Sharma, P. Estimating neoplasia detection rate (NDR) in patients with Barrett’s oesophagus based on index endoscopy: A systematic review and meta-analysis. Gut 2019, 68, 2122–2128. [Google Scholar] [CrossRef] [PubMed]
- Bergman, J.; De Groof, A.J.; Pech, O.; Ragunath, K.; Armstrong, D.; Mostafavi, N.; Lundell, L.; Dent, J.; Vieth, M.; Tytgat, G.; et al. An Interactive Web-Based Educational Tool Improves Detection and Delineation of Barrett’s Esophagus–Related Neoplasia. Gastroenterology 2019, 156, 1299–1308. [Google Scholar] [CrossRef]
- Kandiah, K.; Chedgy, F.J.Q.; Subramaniam, S.; Longcroft-Wheaton, G.; Bassett, P.; Repici, A.; Sharma, P.; Pech, O.; Bhandari, P. International development and validation of a classification system for the identification of Barrett’s neoplasia using acetic acid chromoendoscopy: The Portsmouth acetic acid classification (PREDICT). Gut 2017, 67, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Chedgy, F.J.; Subramaniam, S.; Kandiah, K.; Thayalasekaran, S.; Bhandari, P. Acetic acid chromoendoscopy: Improving neoplasia detection in Barrett’s esophagus. World J. Gastroenterol. 2016, 22, 5753. [Google Scholar] [CrossRef][Green Version]
- Sharma, P.; Bergman, J.J.G.H.M.; Goda, K.; Kato, M.; Messmann, H.; Alsop, B.R.; Gupta, N.; Vennalaganti, P.; Hall, M.; A Konda, V.J.; et al. Development and Validation of a Classification System to Identify High-Grade Dysplasia and Esophageal Adenocarcinoma in Barrett’s Esophagus Using Narrow-Band Imaging. Gastroenterol. 2016, 150, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, R.; Goda, K.; Oyama, T. Endoscopic diagnosis and treatment of esophageal adenocarcinoma: Introduction of Japan Esophageal Society classification of Barrett’s esophagus. J. Gastroenterol. 2018, 54, 1–9. [Google Scholar] [CrossRef]
- Iwaya, Y.; Rowsell, C.; Gupta, V.; Marcon, N. Buried Barrett’s Adenocarcinoma Clearly Demonstrated with Acetic Acid Chromoendoscopy. Am. J. Gastroenterol. 2018, 113, 1580. [Google Scholar] [CrossRef]
- Ishihara, R.; Arima, M.; Iizuka, T.; Oyama, T.; Katada, C.; Kato, M.; Goda, K.; Goto, O.; Tanaka, K.; Yano, T.; et al. Endoscopic submucosal dissection/endoscopic mucosal resection guidelines for esophageal cancer. Dig. Endosc. 2020, 32, 452–493. [Google Scholar] [CrossRef]
- Ahmed, O.; A Ajani, J.; Lee, J.H. Endoscopic management of esophageal cancer. World J. Gastrointest. Oncol. 2019, 11, 830–841. [Google Scholar] [CrossRef]
- Raphael, K.L.; Stewart, M.; Sejpal, D.V.; Cheung, M.; Whitson, M.J.; Han, D.; Trindade, A.J. Adjunctive yield of wide-area transepithelial sampling for dysplasia detection after advanced imaging and random biopsies in Barrett’s esophagus. Clin. Transl. Gastroenterol. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- de Groof, A.J.; Struyvenberg, M.R.; Van Der Putten, J.; Van Der Sommen, F.; Fockens, K.N.; Curvers, W.L.; Zinger, S.; Pouw, R.E.; Coron, E.; Baldaque-Silva, F.; et al. Deep-Learning System Detects Neoplasia in Patients With Barrett’s Esophagus With Higher Accuracy Than Endoscopists in a Multistep Training and Validation Study With Benchmarking. Gastroenterologgy 2020, 158, 915–929. [Google Scholar] [CrossRef] [PubMed]
- Ebigbo, A.; Mendel, R.; Probst, A.; Manzeneder, J.A.D.S.; Papa, J.P.; Palm, C.; Messmann, H. Computer-aided diagnosis using deep learning in the evaluation of early oesophageal adenocarcinoma. Gut 2013, 68, 1143. [Google Scholar] [CrossRef]
- Hashimoto, R.; Requa, J.; Dao, T.; Ninh, A.; Tran, E.; Mai, D.; Lugo, M.; Chehade, N.E.-H.; Chang, K.J.; Karnes, W.E.; et al. Artificial intelligence using convolutional neural networks for real-time detection of early esophageal neoplasia in Barrett’s esophagus (with video). Gastrointest. Endosc. 2020, 91, 1264–1271. [Google Scholar] [CrossRef]
- Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy 2005, 37, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Pech, O.; May, A.; Manner, H.; Behrens, A.; Pohl, J.; Weferling, M.; Hartmann, U.; Manner, N.; Huijsmans, J.; Gossner, L.; et al. Long-term Efficacy and Safety of Endoscopic Resection for Patients With Mucosal Adenocarcinoma of the Esophagus. Gastroenterol. 2014, 146, 652–660. [Google Scholar] [CrossRef]
- Manner, H.; Pech, O.; Heldmann, Y.; May, A.; Pohl, J.; Behrens, A.; Gossner, L.; Stolte, M.; Vieth, M.; Ell, C. Efficacy, Safety, and Long-term Results of Endoscopic Treatment for Early Stage Adenocarcinoma of the Esophagus with Low-risk sm1 Invasion. Clin. Gastroenterol. Hepatol. 2013, 11, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Shaheen, N.J.; Katzka, D.; Bergman, J.J. AGA clinical practice update on endoscopic treatment of Barrett’s esophagus with dysplasia and/or early cancer: Expert review. Gastroenterology 2020, 158, 760–769. [Google Scholar]
- Noordzij, I.C.; Curvers, W.L.; Schoon, E.J. Endoscopic resection for early esophageal carcinoma. J. Thorac. Dis. 2019, 11, S713–S722. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Nunes, P.; Dinis-Ribeiro, M.; Ponchon, T.; Repici, A.; Vieth, M.; De Ceglie, A.; Amato, A.; Berr, F.; Bhandari, P.; Bialek, A.; et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015, 47, 829–854. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Shaheen, N.J.; Katzka, D.; Bergman, J. Clinical Practice Update: Endoscopic Treatment of Barrett’s Esophagus With Dysplasia and/or Early Cancer. Gastroenterology 2019. [Google Scholar] [PubMed]
- Manner, H.; Rabenstein, T.; Pech, O.; Braun, K.; May, M.A.; Pohl, J.; Behrens, A.; Vieth, M.; Ell, C. Ablation of residual Barrett’s epithelium after endoscopic resection: A randomized long-term follow-up study of argon plasma coagulation vs. surveillance (APE study). Endoscopy 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Lal, P.; Thota, P.N. Cryotherapy in the management of premalignant and malignant conditions of the esophagus. World J. Gastroenterol. 2018, 24, 4862–4869. [Google Scholar] [CrossRef] [PubMed]
- Hamade, N.; Desai, M.; Thoguluva Chandrasekar, V.; Chalhoub, J.; Patel, M.; Duvvuri, A.; Sharma, P. Efficacy of cryotherapy as first line therapy in patients with Barrett’s neoplasia: A systematic review and pooled analysis. Dis. Esophagus 2019, 32, doz040. [Google Scholar] [CrossRef]
- Wani, S.; Han, S.; Kushnir, V.; Early, D.; Mullady, D.; Hammad, H.; Brauer, B.; Thaker, A.; Simon, V.; Ezekwe, E.; et al. Recurrence Is Rare Following Complete Eradication of Intestinal Metaplasia in Patients With Barrett’s Esophagus and Peaks at 18 Months. Clin. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- di Pietro, M.; Fitzgerald, R.C. Revised British Society of Gastroenterology recommendation on the diagnosis and management of Barrett’s oesophagus with low-grade dysplasia. Gut 2018, 67, 392–393. [Google Scholar] [CrossRef]
- de Matos, M.V.; da Ponte-Neto, A.M.; de Moura DT, H.; Maahs, E.D.; Chaves, D.M.; Baba, E.R.; de Moura EG, H. Treatment of high-grade dysplasia and intramucosal carcinoma using radiofrequency ablation or endoscopic mucosal resection+ radiofrequency ablation: Meta-analysis and systematic review. World J. Gastrointest. Endosc. 2019, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- van de Ven SE, M.; Bugter, O.; Hardillo, J.A.; Bruno, M.J.; Baatenburg de Jong, R.J.; Koch, A.D. Screening for head and neck second primary tumors in patients with esophageal squamous cell cancer: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2019, 7, 1304–1311. [Google Scholar]
- Maekawa, A.; Ishihara, R.; Iwatsubo, T.; Nakagawa, K.; Ohmori, M.; Iwagami, H.; Matsuno, K.; Inoue, S.; Arao, M.; Nakahira, H.; et al. High incidence of head and neck cancers after endoscopic resection for esophageal cancer in younger patients. J. Gastroenterol. 2019, 55, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Bugter, O.; van de Ven, S.E.; Hardillo, J.A.; Bruno, M.J.; Koch, A.D.; Baatenburg de Jong, R.J. Early detection of esophageal second primary tumors using Lugol chromoendoscopy in patients with head and neck cancer: A systematic review and meta-analysis. Head Neck 2019, 41, 1122–1130. [Google Scholar]
- Su, H.-A.; Hsiao, S.-W.; Hsu, Y.-C.; Wang, L.-Y.; Yen, H.-H. Superiority of NBI endoscopy to PET/CT scan in detecting esophageal cancer among head and neck cancer patients: A retrospective cohort analysis. BMC Cancer 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Oyama, T.; Inoue, H.; Arima, M.; Momma, K.; Tomori, A.; Ishihara, R.; Hirasawa, D.; Takeuchi, M.; Goda, K. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: Magnifying endoscopic classification of the Japan Esophageal Society. Esophagus 2017, 14, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Oyama, T. Counter Traction Makes Endoscopic Submucosal Dissection Easier. Clin. Endosc. 2012, 45, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Raman, V.; Jawitz, O.K.; Voigt, S.L.; Yang, C.-F.J.; Harpole, D.H.; D’Amico, T.A.; Hartwig, M.G. The effect of age on survival after endoscopic resection versus surgery for T1a esophageal cancer. J. Thorac. Cardiovasc. Surg. 2020, 160, 295–302. [Google Scholar] [CrossRef]
- Minashi, K.; Nihei, K.; Mizusawa, J.; Takizawa, K.; Yano, T.; Ezoe, Y.; Muto, M. Efficacy of endoscopic resection and selective chemoradiotherapy for stage I esophageal squamous cell carcinoma. Gastroenterology 2019, 157, 382–390. [Google Scholar] [CrossRef] [PubMed]
| Classification System (Barrett’s Esophagus) |
|---|
| Paris Classification (‘generic” classification of superficial GI neoplasia) |
| 0-Ip (pedunculated), 0-Is (sessile) |
| 0-IIa (slightly elevated), 0-IIb (completely flat) 0-IIc (slightly depressed) |
| 0-III (excavated/ulcerous) |
| Barrett’s International NBI Group (BING) Classification |
| mucosal pattern regular |
| • circular, ridged/villous, or tubular patterns |
| mucosal pattern irregular: |
| • absent or irregular patterns |
| vascular pattern regular |
| • blood vessels situated regularly along or between mucosal ridges and/or showing normal, long, branching patterns |
| vascular pattern irregular |
| • focally or diffusely distributed vessels not following the normal architecture of the mucosa |
| Japan Esophageal Society classification of Barrett’s esophagus |
| mucosal pattern regular: |
| • form/size: similar; arrangement: regular; density: low or same as surrounding area; white zone: clearly visible and/or of homogeneous width. |
| mucosal pattern irregular: |
| • form/size: variable; arrangement: irregular; density: high; white zone: obscure/invisible or of heterogeneous width. |
| vascular pattern regular: |
| • form: similar or bending and branching gently or regularly; caliber change gradual; location between or in mucosal pattern |
| vascular pattern irregular: |
| • form: various or bending and branching steeply or irregularly; caliber change: abrupt; location: beyond or regardless of mucosal patterns |
| flat pattern (classified as regular) |
| • completely flat surface without a clear demarcation line; greenish thick and/or long branching vessels |
| Portsmouth acetic acid (PREDICT) Classification |
| acetowhitening non-neoplastic: |
| • no focal loss of acetowhitening |
| acetowhitening neoplastic: |
| • focal loss of acetowhitening |
| surface pattern non-neoplastic: |
| • uniform evenly spaced pits with normal pit density |
| surface pattern neoplastic: |
| • compactly packed small pits with increased pit density; focal irregularity or disorganized pits; absent pit pattern |
| Criteria for Curative Endoscopic Resection |
|---|
| • Resection en bloc/R0 (vertical margin) |
| • Grading G1/G21 |
| • No infiltration of lymphatic/blood vessels |
| • Submucosal infiltration depth ≤500 µm |
| Japan Esophageal Society (JES) Classification of Early Squamous Cell Cancer to Assess Tumor Infiltration Depth |
|---|
| Vascular pattern regular |
| Type A vessels |
| Type B vessels |
| Abnormal microvessels (severe irregularity/highly dilated abnormal vessels) • Type B1 with a loop-like formation • Type B2 without a loop-like formation • Type B3 highly dilated vessels, the calibers of which appear to be more than three times that of usual B2 vessels |
| Criteria for Curative Endoscopic Resection |
|---|
| • Resection en bloc/R0 |
| • Grading G1/G21 |
| • No infiltration of lymphatic/blood vessels |
| • Infiltration depth pT1a-EP 1 or pT1a-LPM 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumoulin, F.L.; Hildenbrand, R.; Oyama, T.; Steinbrück, I. Current Trends in Endoscopic Diagnosis and Treatment of Early Esophageal Cancer. Cancers 2021, 13, 752. https://doi.org/10.3390/cancers13040752
Dumoulin FL, Hildenbrand R, Oyama T, Steinbrück I. Current Trends in Endoscopic Diagnosis and Treatment of Early Esophageal Cancer. Cancers. 2021; 13(4):752. https://doi.org/10.3390/cancers13040752
Chicago/Turabian StyleDumoulin, Franz Ludwig, Ralf Hildenbrand, Tsuneo Oyama, and Ingo Steinbrück. 2021. "Current Trends in Endoscopic Diagnosis and Treatment of Early Esophageal Cancer" Cancers 13, no. 4: 752. https://doi.org/10.3390/cancers13040752
APA StyleDumoulin, F. L., Hildenbrand, R., Oyama, T., & Steinbrück, I. (2021). Current Trends in Endoscopic Diagnosis and Treatment of Early Esophageal Cancer. Cancers, 13(4), 752. https://doi.org/10.3390/cancers13040752






