Toll-Like Receptor 7 Mediates Inflammation Resolution and Inhibition of Angiogenesis in Non-Small Cell Lung Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Increased mRNA Expression of TLR7 Associated Directly with Pro-Resolving and Inversely with Pro-Angiogenic Markers in NSCLC Patients
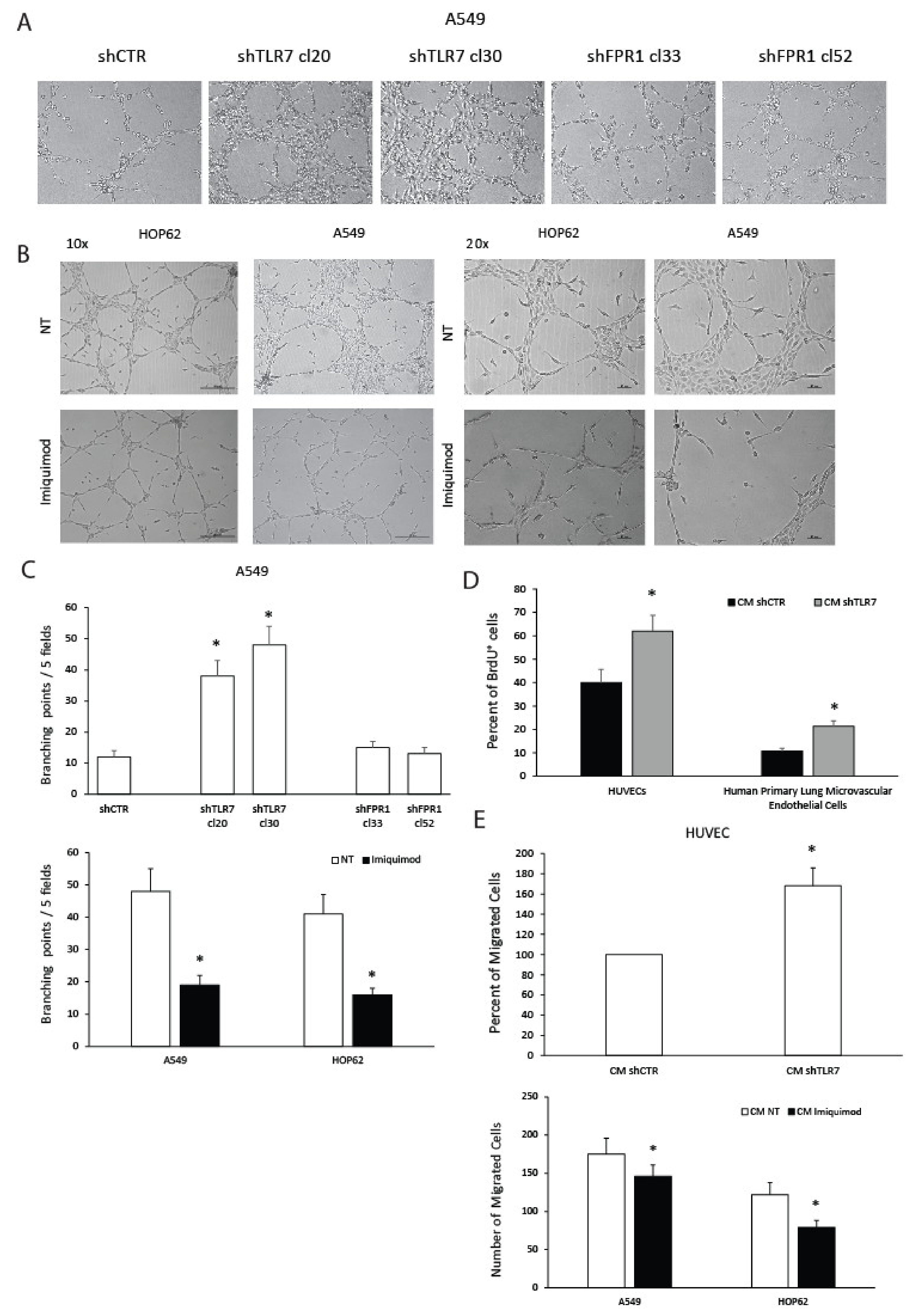
2.2. TLR7 Regulates the Angiogenic Potential of NSCLC Cells
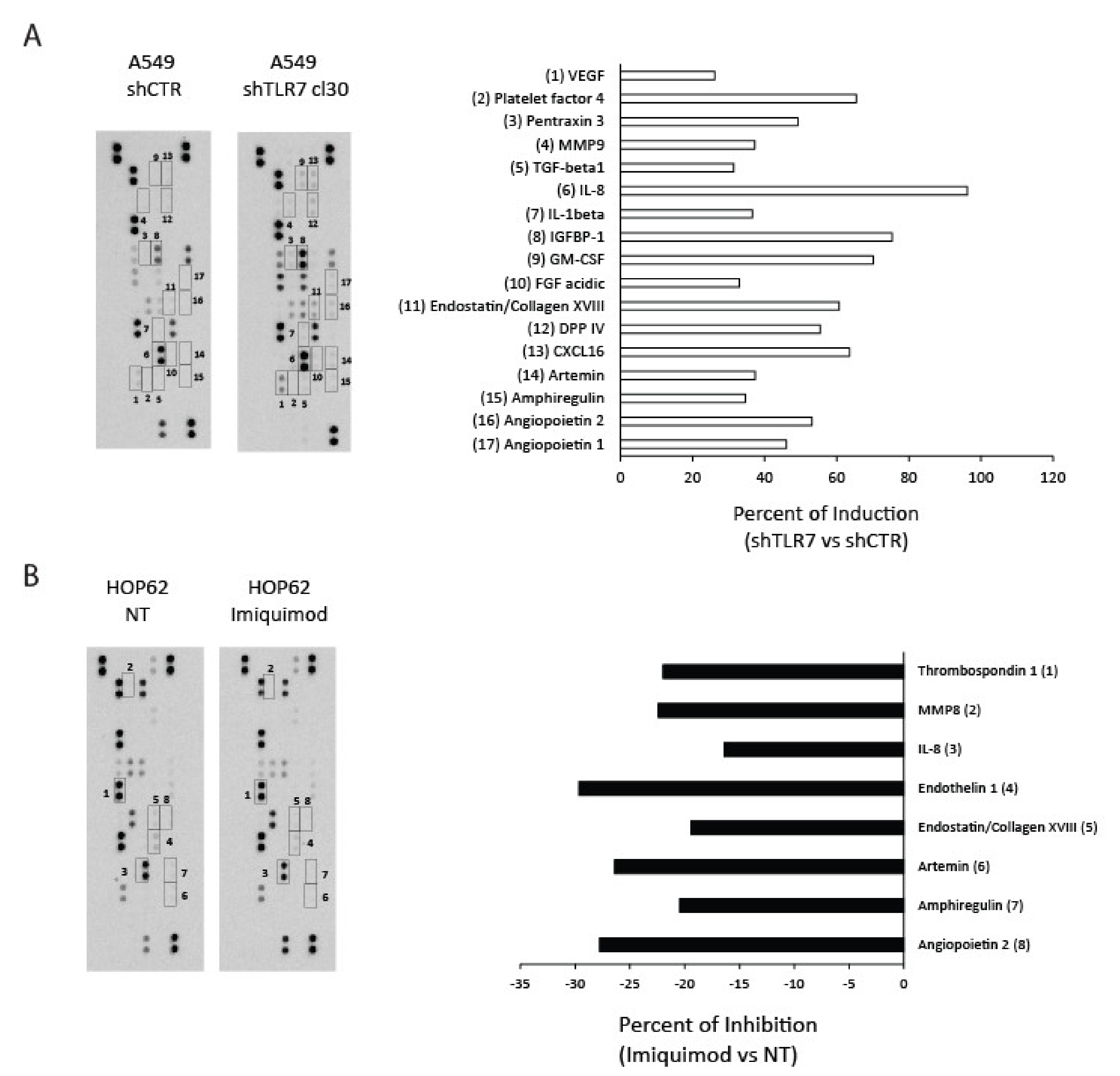
2.3. TLR7 Expression/Activation Status Correlates with the Expression of Angiogenic Mediators in NSCLC Cells
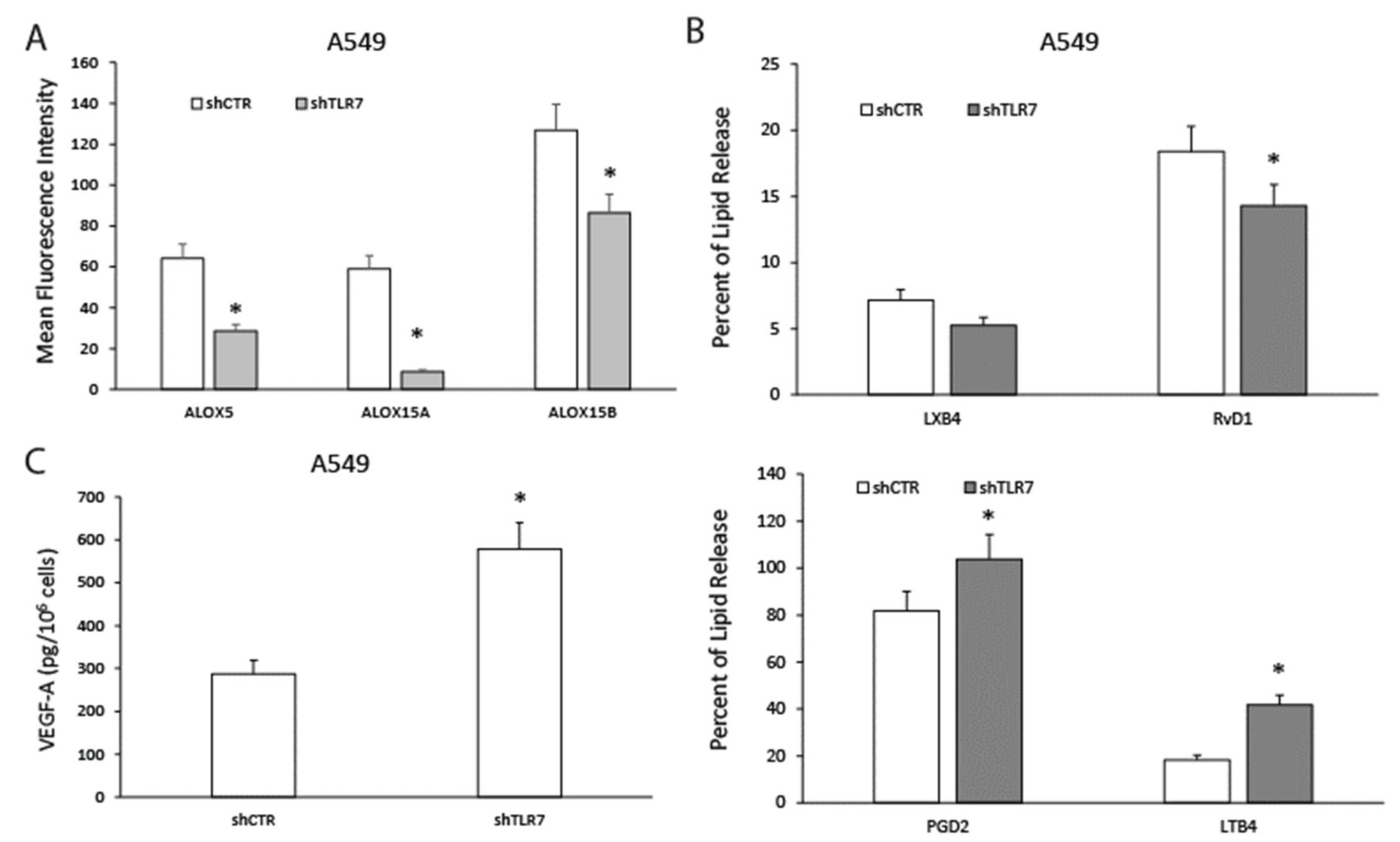
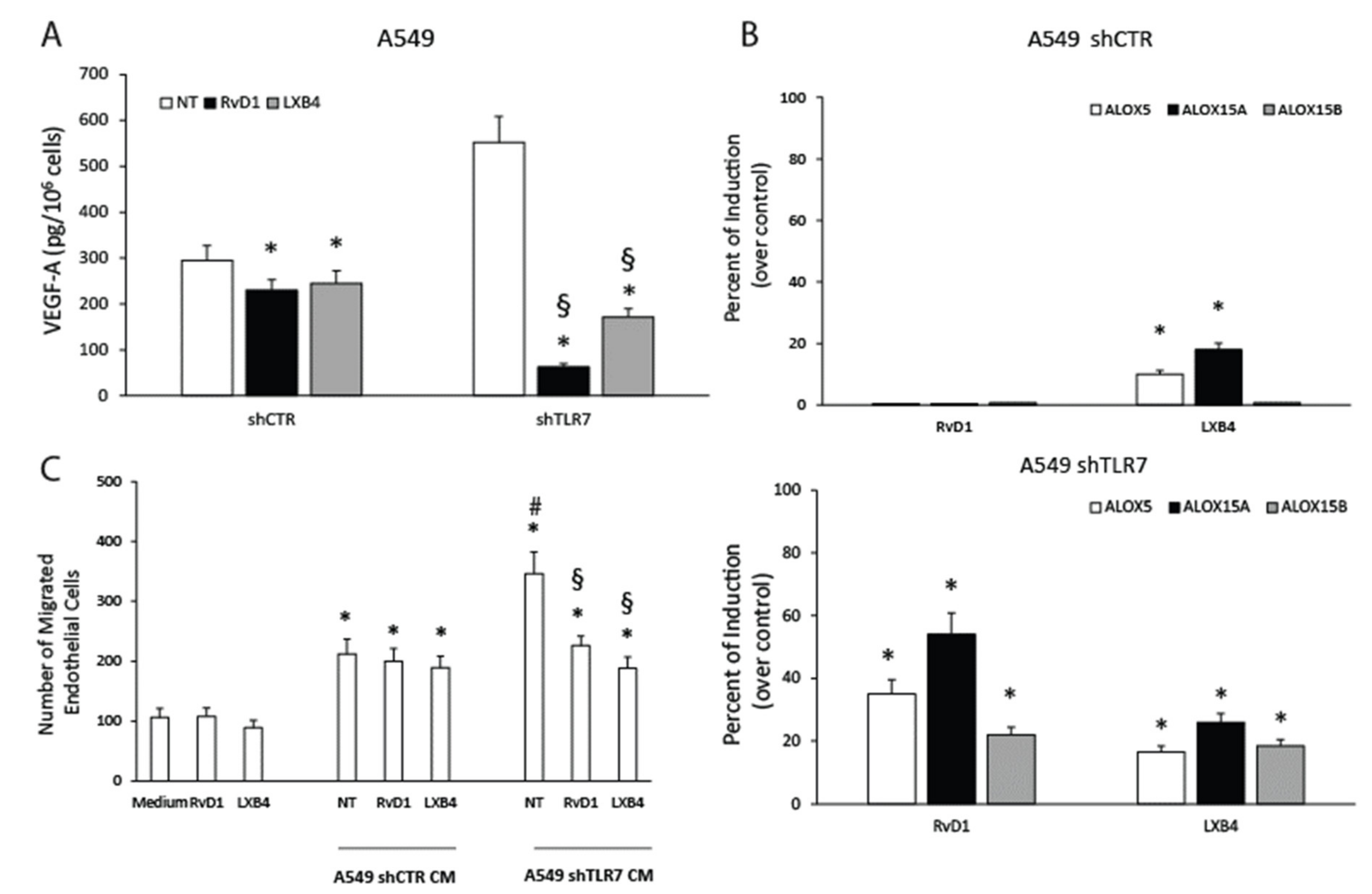
2.4. TLR7-Silencing Decreases Pro-Resolving Potential and Increases Vascular Endothelial Growth Factor A (VEGF-A) Release of NSCLC Cells
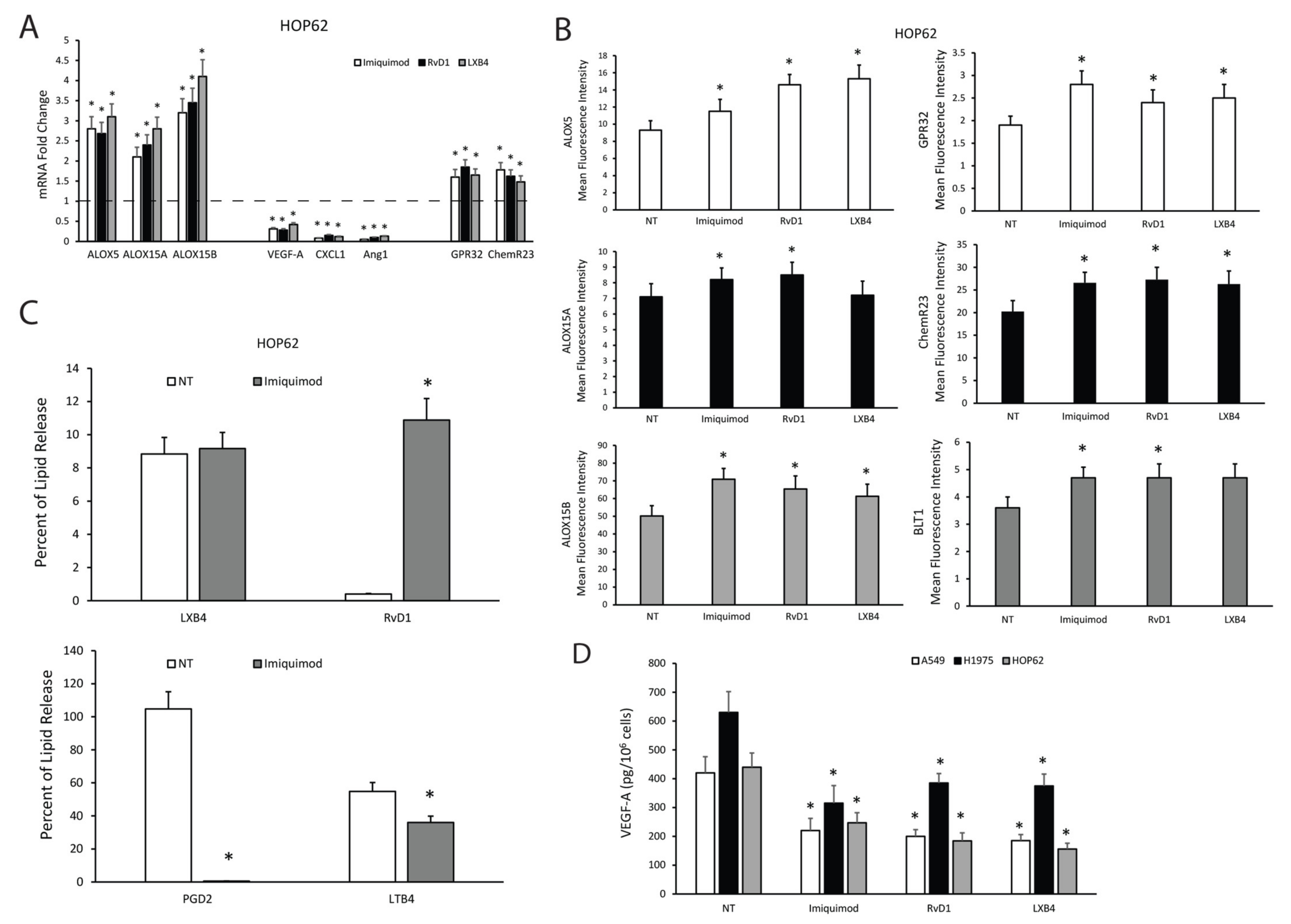
2.5. TLR7 Activation Sustains Pro-Resolving Pathway Components’ Expression and Inhibits Angiogenic Mediators’ Production in NSCLC Cells
2.6. SPMs Inhibit the Production of Angiogenic Mediators and Restore the Expression of Pro-Resolving Pathway Components in NSCLC Cells
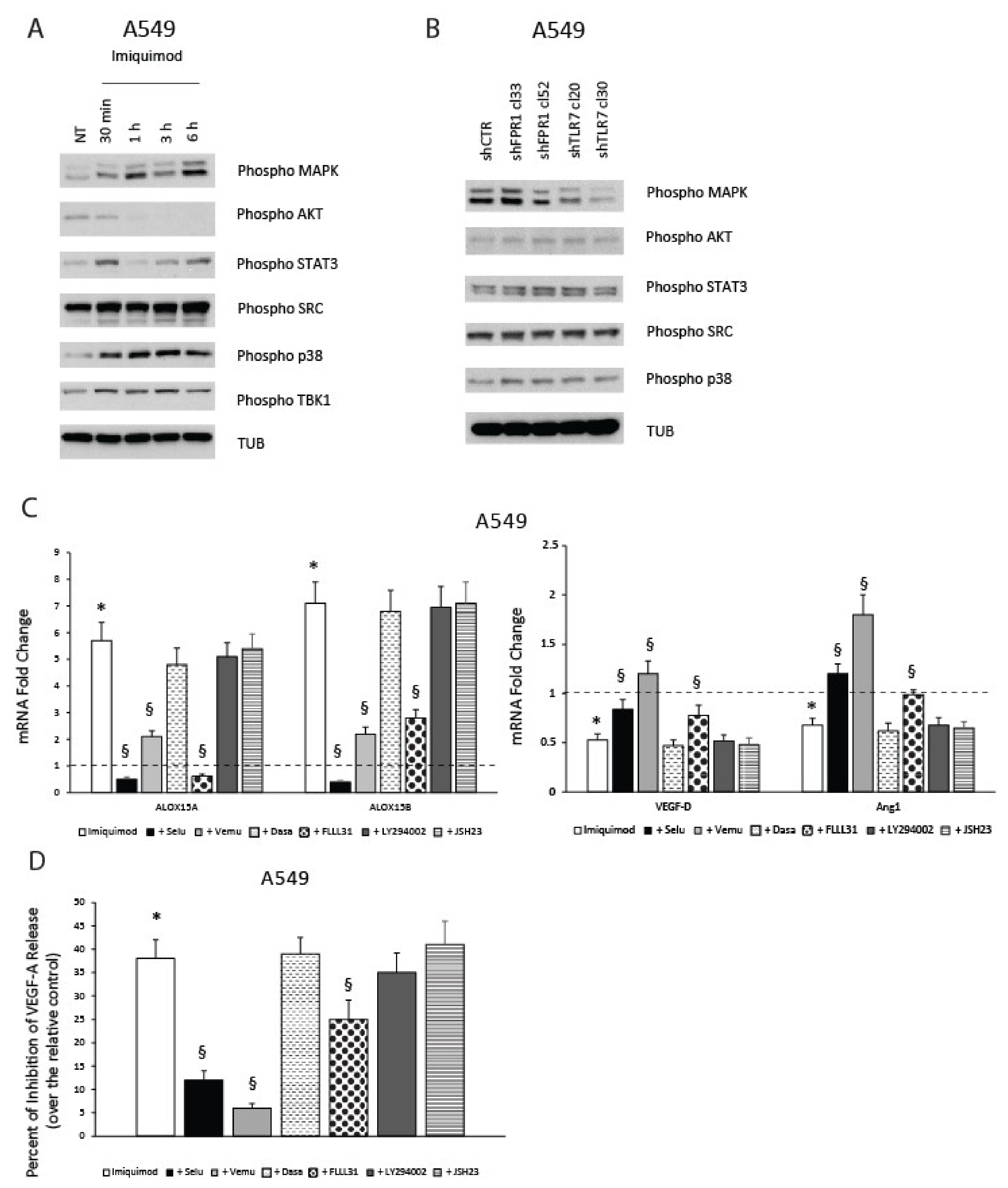
2.7. TLR7-Mediated Pro-Resolving Response in NSCLC Cells Requires MAPK and STAT3 Activation
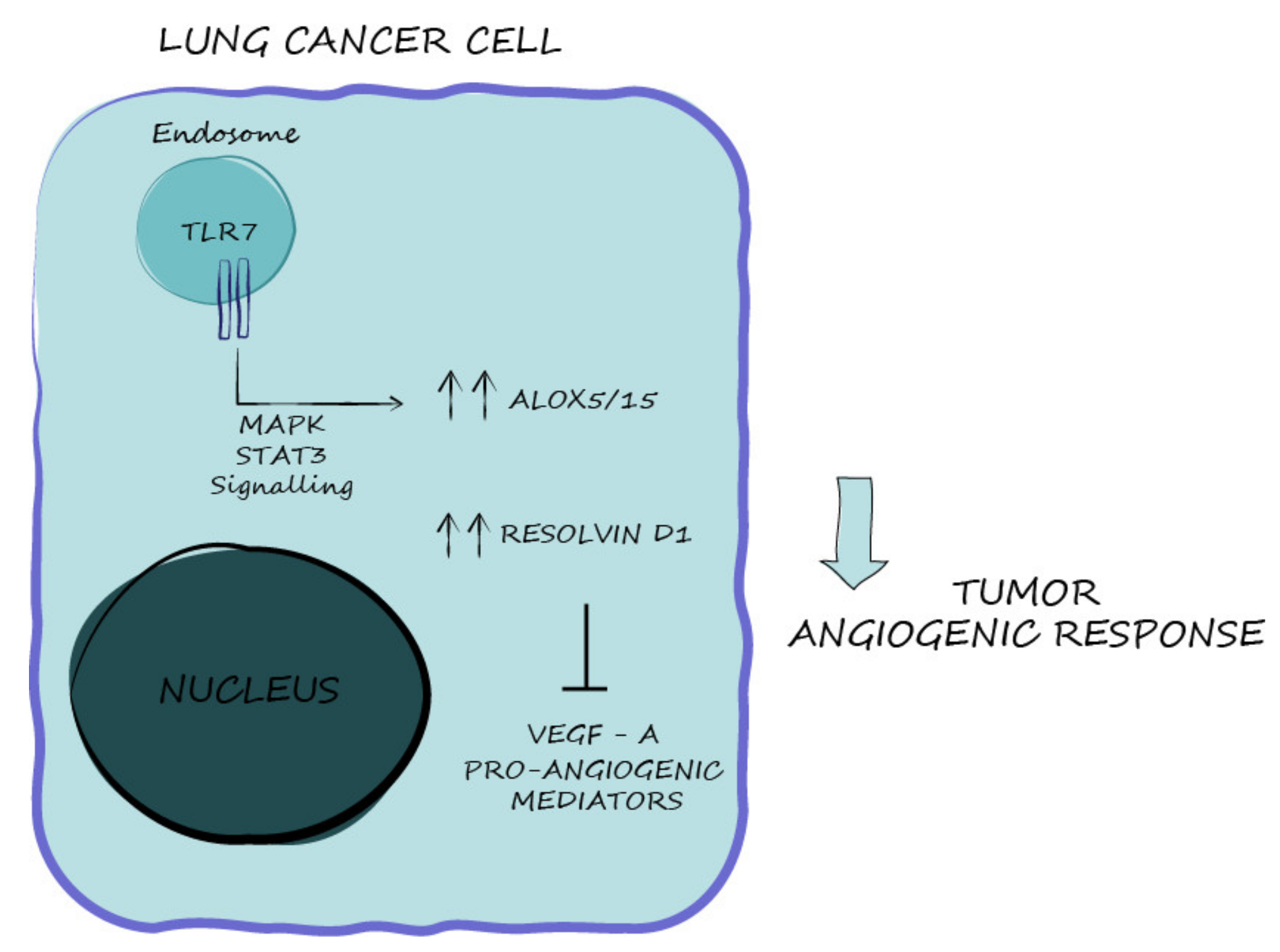
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Tubule Formation
4.4. S-Phase Entry
4.5. Endothelial Cell Migration
4.6. Protein Array
4.7. Flow Cytometry
4.8. ELISA and EIA Assays
4.9. Real-Time PCR
4.10. Protein Studies
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, M.R.; Kaminski, J.J.; Kurt-Jones, E.A.; Fitzgerald, K.A. Pattern recognition receptors and the innate immune response to viral infection. Viruses 2011, 3, 920–940. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Prevete, N.; Liotti, F.; Marone, G.; Melillo, R.M.; de Paulis, A. Formyl peptide receptors at the interface of inflammation, angiogenesis and tumor growth. Pharmacol. Res. 2015, 102, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Fukata, M.; Arditi, M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol. 2013, 6, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Prevete, N.; de Paulis, A.; Sgambato, D.; Melillo, R.M.; D’Argenio, G.; Romano, L.; Zagari, R.M.; Romano, M. Role of Formyl Peptide Receptors in Gastrointestinal Healing. Curr. Pharm. Des. 2018, 24, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Gravina, A.G.; Prevete, N.; Tuccillo, C.; De Musis, C.; Romano, L.; Federico, A.; de Paulis, A.; D’Argenio, G.; Romano, M. Peptide Hp(2–20) accelerates healing of TNBS-induced colitis in the rat. United Eur. Gastroenterol. J. 2018, 6, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Hacker, H.; Redecke, V.; Blagoev, B.; Kratchmarova, I.; Hsu, L.C.; Wang, G.G.; Kamps, M.P.; Raz, E.; Wagner, H.; Hacker, G.; et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature 2006, 439, 204–207. [Google Scholar] [CrossRef]
- Dufton, N.; Hannon, R.; Brancaleone, V.; Dalli, J.; Patel, H.B.; Gray, M.; D’Acquisto, F.; Buckingham, J.C.; Perretti, M.; Flower, R.J. Anti-inflammatory role of the murine formyl-peptide receptor 2: Ligand-specific effects on leukocyte responses and experimental inflammation. J. Immunol. 2010, 184, 2611–2619. [Google Scholar] [CrossRef]
- Prevete, N.; Liotti, F.; Amoresano, A.; Pucci, P.; de Paulis, A.; Melillo, R.M. New perspectives in cancer: Modulation of lipid metabolism and inflammation resolution. Pharmacol. Res. 2018, 128, 80–87. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-Resolving Lipid Mediators Are Leads for Resolution Physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Gronert, K.; Maheshwari, N.; Khan, N.; Hassan, I.R.; Dunn, M.; Laniado Schwartzman, M. A Role for the Mouse 12/15-Lipoxygenase Pathway in Promoting Epithelial Wound Healing and Host Defense. J. Biol. Chem. 2005, 280, 15267–15278. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, M.J.; Hellmann, J.; Kosuri, M.; Bhatnagar, A.; Spite, M. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes 2013, 62, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Miyata, J.; Fukunaga, K.; Iwamoto, R.; Isobe, Y.; Niimi, K.; Takamiya, R.; Takihara, T.; Tomomatsu, K.; Suzuki, Y.; Oguma, T.; et al. Dysregulated synthesis of protectin D1 in eosinophils from patients with severe asthma. J. Allergy Clin. Immunol. 2013, 131, 353–360.e1-2. [Google Scholar] [CrossRef] [PubMed]
- Leedom, A.J.; Sullivan, A.B.; Dong, B.; Lau, D.; Gronert, K. Endogenous LXA4 circuits are determinants of pathological angiogenesis in response to chronic injury. Am. J. Pathol. 2010, 176, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Prevete, N.; Liotti, F.; Illiano, A.; Amoresano, A.; Pucci, P.; de Paulis, A.; Melillo, R.M. Formyl peptide receptor 1 suppresses gastric cancer angiogenesis and growth by exploiting inflammation resolution pathways. Oncoimmunology 2017, 6, e1293213. [Google Scholar] [CrossRef]
- Gilligan, M.M.; Gartung, A.; Sulciner, M.L.; Norris, P.C.; Sukhatme, V.P.; Bielenberg, D.R.; Huang, S.; Kieran, M.W.; Serhan, C.N.; Panigrahy, D. Aspirin-triggered proresolving mediators stimulate resolution in cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 6292–6297. [Google Scholar] [CrossRef]
- Sulciner, M.L.; Gartung, A.; Gilligan, M.M.; Serhan, C.N.; Panigrahy, D. Targeting lipid mediators in cancer biology. Cancer Metastasis Rev. 2018, 37, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Zeromski, J.; Kaczmarek, M.; Boruczkowski, M.; Kierepa, A.; Kowala-Piaskowska, A.; Mozer-Lisewska, I. Significance and Role of Pattern Recognition Receptors in Malignancy. Arch. Immunol. Ther. Exp. 2019, 67, 133–141. [Google Scholar] [CrossRef]
- Prevete, N.; Liotti, F.; Visciano, C.; Marone, G.; Melillo, R.M.; de Paulis, A. The formyl peptide receptor 1 exerts a tumor suppressor function in human gastric cancer by inhibiting angiogenesis. Oncogene 2015, 34, 3826–3838. [Google Scholar] [CrossRef]
- Otani, T.; Ikeda, S.; Lwin, H.; Arai, T.; Muramatsu, M.; Sawabe, M. Polymorphisms of the formylpeptide receptor gene (FPR1) and susceptibility to stomach cancer in 1531 consecutive autopsy cases. Biochem. Biophys. Res. Commun. 2011, 405, 356–361. [Google Scholar] [CrossRef]
- Seifert, R.; Wenzel-Seifert, K. Defective Gi protein coupling in two formyl peptide receptor mutants associated with localized juvenile periodontitis. J. Biol. Chem. 2001, 276, 42043–42049. [Google Scholar] [CrossRef] [PubMed]
- Koltsida, O.; Karamnov, S.; Pyrillou, K.; Vickery, T.; Chairakaki, A.D.; Tamvakopoulos, C.; Sideras, P.; Serhan, C.N.; Andreakos, E. Toll-like receptor 7 stimulates production of specialized pro-resolving lipid mediators and promotes resolution of airway inflammation. EMBO Mol. Med. 2013, 5, 762–775. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Senger, D.R.; Davis, G.E. Angiogenesis. Cold Spring Harb. Perspect. Biol. 2011, 3, a005090. [Google Scholar] [CrossRef]
- Crozat, K.; Beutler, B. TLR7: A new sensor of viral infection. Proc. Natl. Acad. Sci. USA 2004, 101, 6835–6836. [Google Scholar] [CrossRef]
- Javaid, N.; Choi, S. Toll-like Receptors from the Perspective of Cancer Treatment. Cancers 2020, 12, 297. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin. Immunol. 2015, 27, 200–215. [Google Scholar] [CrossRef]
- Negishi, H.; Endo, N.; Nakajima, Y.; Nishiyama, T.; Tabunoki, Y.; Nishio, J.; Koshiba, R.; Matsuda, A.; Matsuki, K.; Okamura, T.; et al. Identification of U11snRNA as an endogenous agonist of TLR7-mediated immune pathogenesis. Proc. Natl. Acad. Sci. USA 2019, 116, 23653–23661. [Google Scholar] [CrossRef]
- Xing, J.; Liu, R.; Xing, M.; Trink, B. The BRAFT1799A mutation confers sensitivity of thyroid cancer cells to the BRAFV600E inhibitor PLX4032 (RG7204). Biochem. Biophys. Res. Commun. 2011, 404, 958–962. [Google Scholar] [CrossRef]
- Ball, D.W.; Jin, N.; Rosen, D.M.; Dackiw, A.; Sidransky, D.; Xing, M.; Nelkin, B.D. Selective growth inhibition in BRAF mutant thyroid cancer by the mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244. J. Clin. Endocrinol. Metab. 2007, 92, 4712–4718. [Google Scholar] [CrossRef]
- Lombardo, L.J.; Lee, F.Y.; Chen, P.; Norris, D.; Barrish, J.C.; Behnia, K.; Castaneda, S.; Cornelius, L.A.; Das, J.; Doweyko, A.M.; et al. Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J. Med. Chem. 2004, 47, 6658–6661. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.M.; Kim, M.H.; Kim, B.H.; Jung, S.H.; Kim, Y.S.; Park, H.J.; Hong, J.T.; Min, K.R.; Kim, Y. Inhibitory action of novel aromatic diamine compound on lipopolysaccharide-induced nuclear translocation of NF-kappaB without affecting IkappaB degradation. FEBS Lett. 2004, 571, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Hutzen, B.; Zuo, M.; Ball, S.; Deangelis, S.; Foust, E.; Pandit, B.; Ihnat, M.A.; Shenoy, S.S.; Kulp, S.; et al. Novel STAT3 phosphorylation inhibitors exhibit potent growth-suppressive activity in pancreatic and breast cancer cells. Cancer Res. 2010, 70, 2445–2454. [Google Scholar] [CrossRef]
- Visconti, R.; Morra, F.; Guggino, G.; Celetti, A. The between Now and Then of Lung Cancer Chemotherapy and Immunotherapy. Int. J. Mol. Sci. 2017, 18, 1374. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin(R)) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Zelnak, A.B.; O’Regan, R.M. Targeting angiogenesis in advanced breast cancer. BioDrugs 2007, 21, 209–214. [Google Scholar] [CrossRef]
- Lammers, P.E.; Horn, L. Targeting angiogenesis in advanced non-small cell lung cancer. J. Natl. Compr. Cancer Netw. 2013, 11, 1235–1247. [Google Scholar] [CrossRef]
- De Paulis, A.; Prevete, N.; Rossi, F.W.; Rivellese, F.; Salerno, F.; Delfino, G.; Liccardo, B.; Avilla, E.; Montuori, N.; Mascolo, M.; et al. Helicobacter pylori Hp(2–20) promotes migration and proliferation of gastric epithelial cells by interacting with formyl peptide receptors in vitro and accelerates gastric mucosal healing in vivo. J. Immunol. 2009, 183, 3761–3769. [Google Scholar] [CrossRef]
- Honey, K. TLR ligands from the natural world. Nat. Rev. Immunol. 2004, 4, 247. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, M.K.; Lee, E.J.; Lee, C.H. Resolvin D1 inhibits TGF-beta1-induced epithelial mesenchymal transition of A549 lung cancer cells via lipoxin A4 receptor/formyl peptide receptor 2 and GPR32. Int. J. Biochem. Cell Biol. 2013, 45, 2801–2807. [Google Scholar] [CrossRef]
- Sulciner, M.L.; Serhan, C.N.; Gilligan, M.M.; Mudge, D.K.; Chang, J.; Gartung, A.; Lehner, K.A.; Bielenberg, D.R.; Schmidt, B.; Dalli, J.; et al. Resolvins suppress tumor growth and enhance cancer therapy. J. Exp. Med. 2018, 215, 115–140. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, A.; Maggiora, M.; Martinasso, G.; Cotogni, P.; Canuto, R.A.; Muzio, G. Arachidonic and docosahexaenoic acids reduce the growth of A549 human lung-tumor cells increasing lipid peroxidation and PPARs. Chem. Biol. Interact. 2007, 165, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Nie, D. Tumor-suppressing 15-lipoxygenase-2: Time for prime time? Cell Cycle 2014, 13, 1836–1837. [Google Scholar] [CrossRef] [PubMed]
- Moussalli, M.J.; Wu, Y.; Zuo, X.; Yang, X.L.; Wistuba, I.I.; Raso, M.G.; Morris, J.S.; Bowser, J.L.; Minna, J.D.; Lotan, R.; et al. Mechanistic contribution of ubiquitous 15-lipoxygenase-1 expression loss in cancer cells to terminal cell differentiation evasion. Cancer Prev. Res. 2011, 4, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
- Cherfils-Vicini, J.; Platonova, S.; Gillard, M.; Laurans, L.; Validire, P.; Caliandro, R.; Magdeleinat, P.; Mami-Chouaib, F.; Dieu-Nosjean, M.C.; Fridman, W.H.; et al. Triggering of TLR7 and TLR8 expressed by human lung cancer cells induces cell survival and chemoresistance. J. Clin. Investig. 2010, 120, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Crozet, L.; Damotte, D.; Iribarren, K.; Schramm, C.; Alifano, M.; Lupo, A.; Cherfils-Vicini, J.; Goc, J.; Katsahian, S.; et al. TLR7 promotes tumor progression, chemotherapy resistance, and poor clinical outcomes in non-small cell lung cancer. Cancer Res. 2014, 74, 5008–5018. [Google Scholar] [CrossRef]
- Dajon, M.; Iribarren, K.; Petitprez, F.; Marmier, S.; Lupo, A.; Gillard, M.; Ouakrim, H.; Victor, N.; Vincenzo, D.B.; Joubert, P.E.; et al. Toll like receptor 7 expressed by malignant cells promotes tumor progression and metastasis through the recruitment of myeloid derived suppressor cells. Oncoimmunology 2019, 8, e1505174. [Google Scholar] [CrossRef]
- Bauer, A.K.; Upham, B.L.; Rondini, E.A.; Tennis, M.A.; Velmuragan, K.; Wiese, D. Toll-like receptor expression in human non-small cell lung carcinoma: Potential prognostic indicators of disease. Oncotarget 2017, 8, 91860–91875. [Google Scholar] [CrossRef]
- Bhagwani, A.; Thompson, A.A.R.; Farkas, L. When Innate Immunity Meets Angiogenesis-The Role of Toll-Like Receptors in Endothelial Cells and Pulmonary Hypertension. Front. Med. 2020, 7, 352. [Google Scholar] [CrossRef]
- Clark, A.R.; Dean, J.L.; Saklatvala, J. The p38 MAPK pathway mediates both antiinflammatory and proinflammatory processes: Comment on the article by Damjanov and the editorial by Genovese. Arthritis Rheum. 2009, 60, 3513–3514. [Google Scholar] [CrossRef]
- Gao, P.; Niu, N.; Wei, T.; Tozawa, H.; Chen, X.; Zhang, C.; Zhang, J.; Wada, Y.; Kapron, C.M.; Liu, J. The roles of signal transducer and activator of transcription factor 3 in tumor angiogenesis. Oncotarget 2017, 8, 69139–69161. [Google Scholar] [CrossRef]
- Valle-Mendiola, A.; Soto-Cruz, I. Energy Metabolism in Cancer: The Roles of STAT3 and STAT5 in the Regulation of Metabolism-Related Genes. Cancers 2020, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Lai, R. STAT3 in Cancer-Friend or Foe? Cancers 2014, 6, 1408–1440. [Google Scholar] [CrossRef] [PubMed]
- Larange, A.; Antonios, D.; Pallardy, M.; Kerdine-Romer, S. TLR7 and TLR8 agonists trigger different signaling pathways for human dendritic cell maturation. J. Leukoc. Biol. 2009, 85, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Frega, G.; Wu, Q.; Le Naour, J.; Vacchelli, E.; Galluzzi, L.; Kroemer, G.; Kepp, O. Trial Watch: Experimental TLR7/TLR8 agonists for oncological indications. Oncoimmunology 2020, 9, 1796002. [Google Scholar] [CrossRef] [PubMed]
- Vinod, N.; Hwang, D.; Azam, S.H.; Van Swearingen, A.E.D.; Wayne, E.; Fussell, S.C.; Sokolsky-Papkov, M.; Pecot, C.V.; Kabanov, A.V. High-capacity poly(2-oxazoline) formulation of TLR 7/8 agonist extends survival in a chemo-insensitive, metastatic model of lung adenocarcinoma. Sci. Adv. 2020, 6, eaba5542. [Google Scholar] [CrossRef] [PubMed]
- Morra, F.; Luise, C.; Visconti, R.; Staibano, S.; Merolla, F.; Ilardi, G.; Guggino, G.; Paladino, S.; Sarnataro, D.; Franco, R.; et al. New therapeutic perspectives in CCDC6 deficient lung cancer cells. Int. J. Cancer 2015, 136, 2146–2157. [Google Scholar] [CrossRef]
- Cerrato, A.; Morra, F.; Di Domenico, I.; Celetti, A. NSCLC Mutated Isoforms of CCDC6 Affect the Intracellular Distribution of the Wild Type Protein Promoting Cisplatinum Resistance and PARP Inhibitors Sensitivity in Lung Cancer Cells. Cancers 2019, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.F.; Hong, T.M.; Chang, C.C.; Hung, C.L.; Hsu, Y.L.; Chang, Y.L.; Wu, C.T.; Chang, G.C.; Chan, N.L.; Yu, S.L.; et al. Hypoxia-induced Slug SUMOylation enhances lung cancer metastasis. J. Exp. Clin. Cancer Res. 2019, 38, 5. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; De Rosa, M.; Sorriento, D.; Prevete, N.; Fiordelisi, A.; Ciccarelli, M.; Trimarco, B.; De Luca, N.; Iaccarino, G. Parathyroid Hormone Causes Endothelial Dysfunction by Inducing Mitochondrial ROS and Specific Oxidative Signal Transduction Modifications. Oxid. Med. Cell. Longev. 2018, 2018, 9582319. [Google Scholar] [CrossRef]
- Liotti, F.; De Pizzol, M.; Allegretti, M.; Prevete, N.; Melillo, R.M. Multiple anti-tumor effects of Reparixin on thyroid cancer. Oncotarget 2017, 8, 35946–35961. [Google Scholar] [CrossRef]
- Pellet-Many, C. Chemotactic Migration of Endothelial Cells Towards VEGF-A(1)(6)(5). Methods Mol. Biol. 2015, 1332, 151–157. [Google Scholar]
- Liotti, F.; Collina, F.; Pone, E.; La Sala, L.; Franco, R.; Prevete, N.; Melillo, R.M. Interleukin-8, but not the Related Chemokine CXCL1, Sustains an Autocrine Circuit Necessary for the Properties and Functions of Thyroid Cancer Stem Cells. Stem Cells 2017, 35, 135–146. [Google Scholar] [CrossRef]
- Collina, F.; La Sala, L.; Liotti, F.; Prevete, N.; La Mantia, E.; Chiofalo, M.G.; Aquino, G.; Arenare, L.; Cantile, M.; Liguori, G.; et al. Is a Novel Predictive Factor and Therapeutic Target for Radioactive Iodine Refractory Thyroid Cancer. Cancers 2019, 11, 785. [Google Scholar] [CrossRef] [PubMed]
- Morra, F.; Merolla, F.; Criscuolo, D.; Insabato, L.; Giannella, R.; Ilardi, G.; Cerrato, A.; Visconti, R.; Staibano, S.; Celetti, A. CCDC6 and USP7 expression levels suggest novel treatment options in high-grade urothelial bladder cancer. J. Exp. Clin. Cancer Res. 2019, 38, 90. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liotti, F.; Marotta, M.; Sorriento, D.; Pone, E.; Morra, F.; Melillo, R.M.; Prevete, N. Toll-Like Receptor 7 Mediates Inflammation Resolution and Inhibition of Angiogenesis in Non-Small Cell Lung Cancer. Cancers 2021, 13, 740. https://doi.org/10.3390/cancers13040740
Liotti F, Marotta M, Sorriento D, Pone E, Morra F, Melillo RM, Prevete N. Toll-Like Receptor 7 Mediates Inflammation Resolution and Inhibition of Angiogenesis in Non-Small Cell Lung Cancer. Cancers. 2021; 13(4):740. https://doi.org/10.3390/cancers13040740
Chicago/Turabian StyleLiotti, Federica, Maria Marotta, Daniela Sorriento, Emanuela Pone, Francesco Morra, Rosa Marina Melillo, and Nella Prevete. 2021. "Toll-Like Receptor 7 Mediates Inflammation Resolution and Inhibition of Angiogenesis in Non-Small Cell Lung Cancer" Cancers 13, no. 4: 740. https://doi.org/10.3390/cancers13040740
APA StyleLiotti, F., Marotta, M., Sorriento, D., Pone, E., Morra, F., Melillo, R. M., & Prevete, N. (2021). Toll-Like Receptor 7 Mediates Inflammation Resolution and Inhibition of Angiogenesis in Non-Small Cell Lung Cancer. Cancers, 13(4), 740. https://doi.org/10.3390/cancers13040740






