Long-Term Survival Outcomes of Cytoreductive Nephrectomy Combined with Targeted Therapy for Metastatic Renal Cell Carcinoma: A Systematic Review and Individual Patient Data Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Inclusion/Exclusion Criteria
- Participants: Patients of any age, sex, or race with mRCC.
- Intervention: Cytoreductive nephrectomy (CN), either before (upfront) or after the initiation of targeted therapy (deferred). We defined targeted therapy as systemic therapy with vascular endothelial growth factor (VEGF) receptor-directed tyrosine kinase inhibitors (TKIs) (e.g., sunitinib, axitinib, pazopanib, sorafenib, famitinib), anti-VEGF monoclonal antibodies (e.g., bevacizumab), or mammalian target of rapamycin (mTOR) inhibitors (e.g., everolimus, temsirolimus).
- Comparison: Targeted therapy without CN.
- Outcomes: Long-term survival outcomes, including overall survival (OS), progression-free survival (PFS), and cancer-specific survival (CSS) of the intention-to-treat populations, when applicable.
2.2. Literature Search Strategy
2.3. Data Tabulation and Extraction
2.4. Risk of Bias in Individual Studies
2.5. Statistical Analysis
2.5.1. Data Pooling
2.5.2. Reconstruction of Individual Patient Survival Data
2.5.3. One-Stage Survival Meta-Analysis
2.5.4. Two-Stage Survival Meta-Analyses
2.5.5. Bayesian Meta-Analysis
2.5.6. Subgroup Analysis According to Cytoreductive Nephrectomy Timing
3. Results
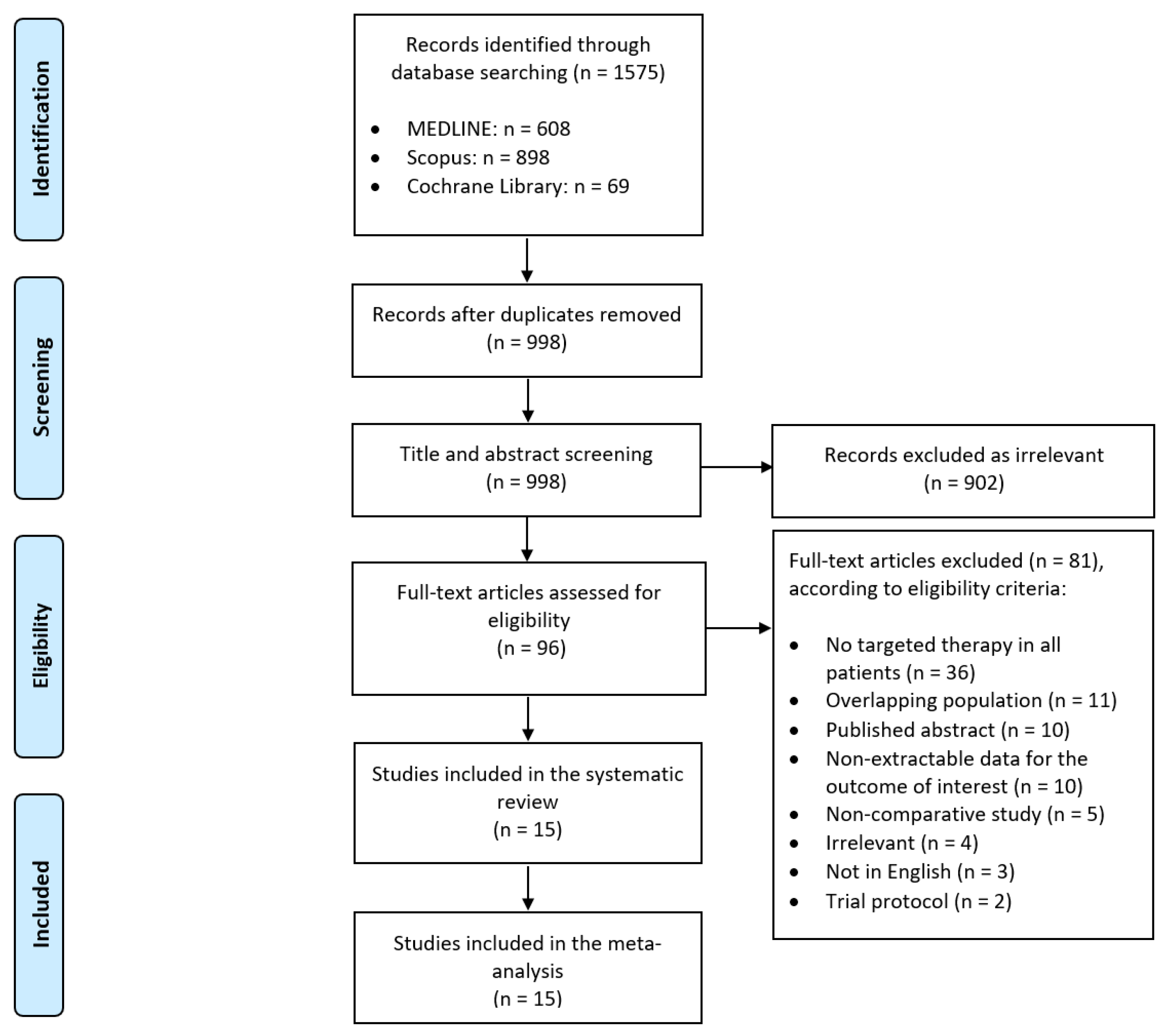
3.1. Study Selection and Characteristics
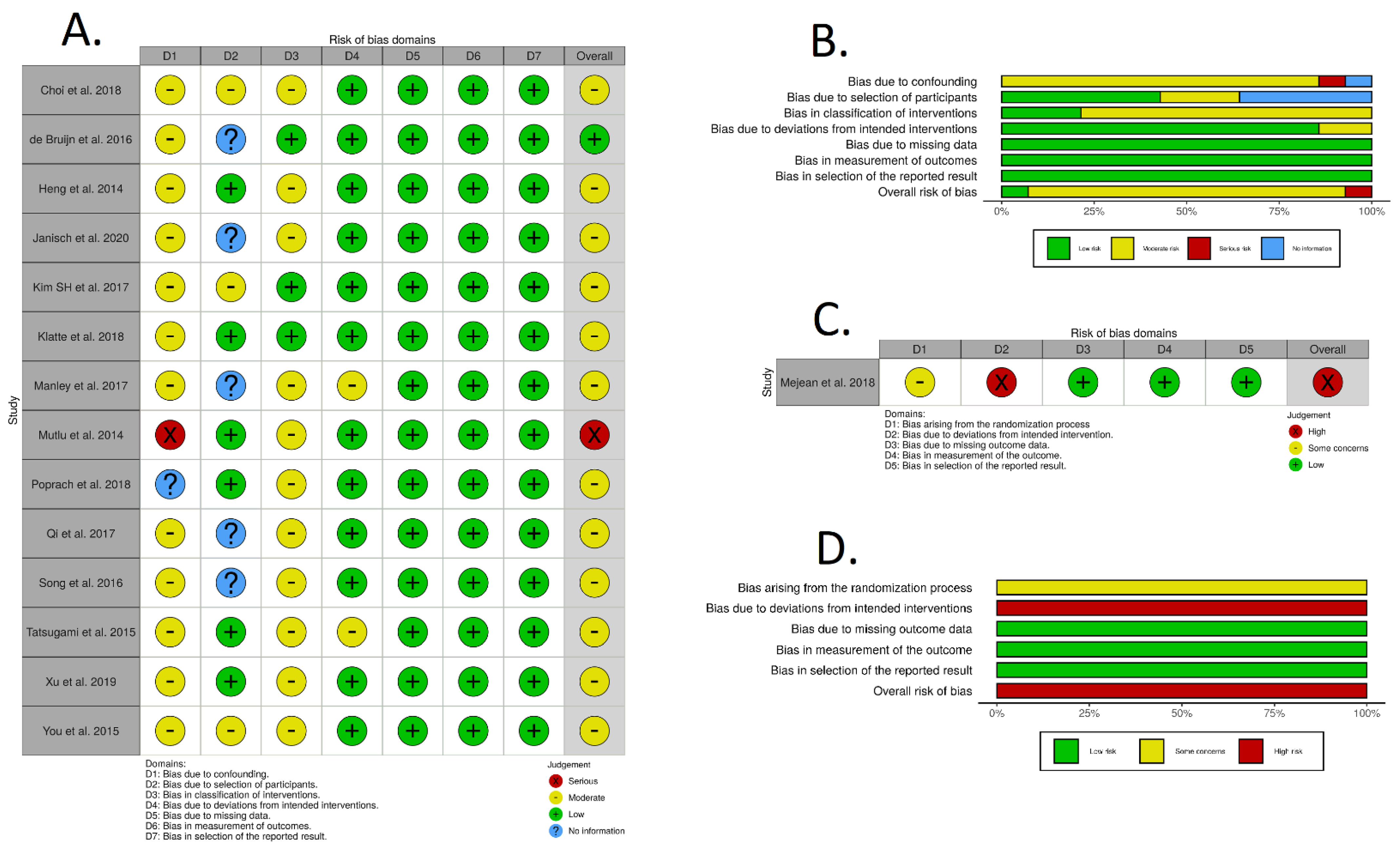
3.2. Risk of Bias Assessment Individual Patient Data and Survival Curve Reconstruction
3.3. Individual Patient Data and Survival Curve Reconstruction
3.4. One-Stage Frequentist Survival Meta-Analysis
3.4.1. Overall Survival
3.4.2. Progression-Free Survival
3.4.3. Cancer-Specific Survival
3.5. Two-Stage Frequentist Survival Meta-Analysis
3.6. Bayesian Meta-Analysis
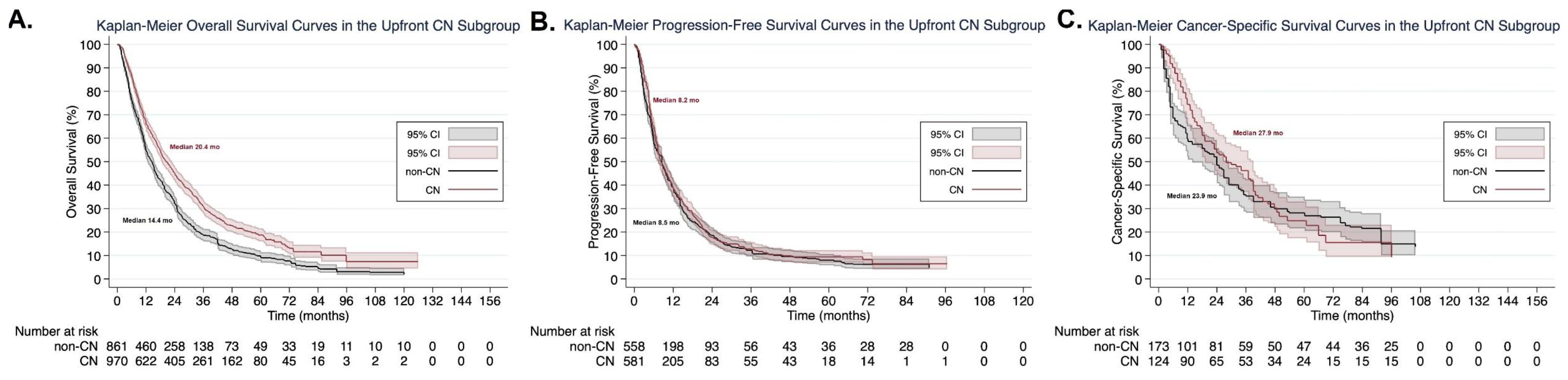
3.7. Subgroup Analysis According to Cytoreductive Nephrectomy Timing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Patel, H.D.; Gupta, M.; Joice, G.A.; Srivastava, A.; Alam, R.; Allaf, M.E.; Pierorazio, P.M. Clinical Stage Migration and Survival for Renal Cell Carcinoma in the United States. Eur. Urol. Oncol. 2019, 2, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, G.; Fisher, R.I.; Rosenberg, S.A.; Sznol, M.; Parkinson, D.R.; Louie, A.C. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J. Clin. Oncol. 1995, 13, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Biles, M.J.; Patel, H.D.; Allaf, M.E. Cytoreductive Nephrectomy in the Era of Tyrosine Kinase and Immuno-Oncology Checkpoint Inhibitors. Urol. Clin. N. Am. 2020, 47, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Adashek, J.J.; Genovese, G.; Tannir, N.M.; Msaouel, P. Recent advancements in the treatment of metastatic clear cell renal cell carcinoma: A review of the evidence using second-generation p-values. Cancer Treat. Res. Commun. 2020, 1, 23. [Google Scholar] [CrossRef]
- Garfield, D.H.; Kennedy, B.J. Regression of metastatic renal cell carcinoma following nephrectomy. Cancer 1972, 30, 190–196. [Google Scholar] [CrossRef]
- Marcus, S.G.; Choyke, P.L.; Reiter, R.; Jaffe, G.S.; Alexander, R.B.; Linehan, W.M.; Rosenberg, S.A.; Walther, M.M. Regression of Metastatic Renal Cell Carcinoma After Cytoreductive Nephrectomy. J. Urol. 1993, 150, 463–466. [Google Scholar] [CrossRef]
- Flanigan, R.C.; Salmon, S.E.; Blumenstein, B.A.; Bearman, S.I.; Roy, V.; McGrath, P.C.; Caton, J.R.; Munshi, N.; Crawford, E.D. Nephrectomy Followed by Interferon Alfa-2b Compared with Interferon Alfa-2b Alone for Metastatic Renal-Cell Cancer. N. Engl. J. Med. 2001, 345, 1655–1659. [Google Scholar] [CrossRef] [PubMed]
- Mickisch, G.H.J.; Garin, A.; Van Poppel, H.; De Prijck, L.; Sylvester, R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: A randomised trial. Lancet 2001, 358, 966–970. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Rixe, O.; Oudard, S.; Negrier, S.; Szczylik, C.; Kim, S.T.; et al. Sunitinib versus Interferon Alfa in Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2007, 356, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Eisen, T.; Stadler, W.M.; Szczylik, C.; Oudard, S.; Siebels, M.; Negrier, S.; Chevreau, C.; Solska, E.; Desai, A.A.; et al. Sorafenib in Advanced Clear-Cell Renal-Cell Carcinoma. N. Engl. J. Med. 2007, 356, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Hudes, G.; Carducci, M.; Tomczak, P.; Dutcher, J.; Figlin, R.; Kapoor, A.; Staroslawska, E.; Sosman, J.; McDermott, D.; Bodrogi, I.; et al. Temsirolimus, Interferon Alfa, or Both for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2007, 356, 2271–2281. [Google Scholar] [CrossRef]
- Psutka, S.P.; Kim, S.P.; Gross, C.P.; Van Houten, H.; Thompson, R.H.; Abouassaly, R.; Weight, C.; Boorjian, S.A.; Leibovich, B.C.; Shah, N.D. The impact of targeted therapy on management of metastatic renal cell carcinoma: Trends in systemic therapy and cytoreductive nephrectomy utilization. Urology 2015, 85, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Xie, W.; Kollmannsberger, C.; North, S.; Knox, J.J.; Lampard, J.G.; McDermott, D.F.; Rini, B.I.; Heng, D.Y. The Impact of Cytoreductive Nephrectomy on Survival of Patients With Metastatic Renal Cell Carcinoma Receiving Vascular Endothelial Growth Factor Targeted Therapy. J. Urol. 2011, 185, 60–66. [Google Scholar] [CrossRef]
- Choi, C.I.; Kang, M.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Jeon, S.S.; Lee, H.M.; Seo, S.I. Oncologic Outcomes of Cytoreductive Nephrectomy in Synchronous Metastatic Renal-Cell Carcinoma: A Single-Center Experience. Clin. Genitourin. Cancer 2018, 16, e1189–e1199. [Google Scholar] [CrossRef] [PubMed]
- Méjean, A.; Ravaud, A.; Thezenas, S.; Colas, S.; Beauval, J.-B.; Bensalah, K.; Geoffrois, L.; Thiery-Vuillemin, A.; Cormier, L.; Lang, H.; et al. Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Janisch, F.; Hillemacher, T.; Fuehner, C.; D’Andrea, D.; Meyer, C.P.; Klotzbücher, T.; Kienapfel, C.; Vetterlein, M.W.; Kimura, S.; Abufaraj, M.; et al. The impact of cytoreductive nephrectomy on survival outcomes in patients treated with tyrosine kinase inhibitors for metastatic renal cell carcinoma in a real-world cohort. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 739.e9–739.e15. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; A Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Wohlin, C. Guidelines for snowballing in systematic literature studies and a replication in software engineering. In Proceedings of the 18th International Conference on Evaluation and ASSESSMENT in software Engineering, London, UK, 13–14 May 2014. [Google Scholar]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0.; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Guyot, P.; E Ades, A.; Ouwens, M.J.N.M.; Welton, N.J. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2012, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Royston, P. Reconstructing time-to-event data from published Kaplan-Meier curves. Stata J. 2017, 17, 786–802. [Google Scholar] [CrossRef]
- Tierney, J.F.; A Stewart, L.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef]
- Royston, P.; Parmar, M.K. The use of restricted mean survival time to estimate the treatment effect in randomized clinical trials when the proportional hazards assumption is in doubt. Stat. Med. 2011, 30, 2409–2421. [Google Scholar] [CrossRef]
- Dehbi, H.-M.; Royston, P.; Hackshaw, A. Life expectancy difference and life expectancy ratio: Two measures of treatment effects in randomised trials with non-proportional hazards. BMJ 2017, 357, j2250. [Google Scholar] [CrossRef] [PubMed]
- Royston, P.; Parmar, M.K.B. Restricted mean survival time: An alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med. Res. Methodol. 2013, 13, 152. [Google Scholar] [CrossRef]
- Lueza, B.; Rotolo, F.; Bonastre, J.; Pignon, J.-P.; Michiels, S. Bias and precision of methods for estimating the difference in restricted mean survival time from an individual patient data meta-analysis. BMC Med. Res. Methodol. 2016, 16, 37. [Google Scholar]
- Riley, R.D.; Lambert, P.C.; Abo-Zaid, G. Meta-analysis of individual participant data: Rationale, conduct, and reporting. BMJ 2010, 340, c221. [Google Scholar] [CrossRef]
- Tibshirani, R. Noninformative priors for one parameter of many. Biometrika 1989, 76, 604–608. [Google Scholar] [CrossRef]
- Qi, N.; Wu, P.; Chen, J.; Li, T.; Ning, X.; Wang, J.; Gong, K. Cytoreductive nephrectomy with thrombectomy before targeted therapy improves survival for metastatic renal cell carcinoma with venous tumor thrombus: A single-center experience. World J. Surg. Oncol. 2017, 15, 4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, Y.; Du, C.-X.; Zhang, W.; Sun, Y.-K.; Yang, L.; Cui, C.-X.; Chi, Y.-B.; Shou, J.-Z.; Zhou, A.-P.; Li, C.-L.; et al. Impact of Cytoreductive Nephrectomy on Survival in Patients with Metastatic Renal Cell Carcinoma Treated by Targeted Therapy. Chin. Med. J. 2016, 129, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.H.; Wang, J.; Huo, D.Z.; Yin, G.C.; Cao, D.L.; Shi, G.H.; Qu, Y.Y.; Ye, D.W.; Zhang, H.L. C-reactive protein levels and survival following cytoreductive nephrectomy in 118 patients with metastatic renal cell carcinoma treated with sunitinib: A retrospective study. Med. Sci. Monit. 2019, 25, 8984–8994. [Google Scholar] [CrossRef]
- Tatsugami, K.; Shinohara, N.; Kondo, T.; Yamasaki, T.; Eto, M.; Tsushima, T.; Terachi, T.; Naito, S.; Japanese Society of Renal Cancer. Role of cytoreductive nephrectomy for Japanese patients with primary renal cell carcinoma in the cytokine and targeted therapy era. Int. J. Urol. 2015, 22, 736–740. [Google Scholar] [CrossRef]
- You, D.; Jeong, I.G.; Song, C.; Lee, J.-L.; Hong, B.; Hong, J.H.; Ahn, H.; Kim, C.-S. Analysis of pre-operative variables for identifying patients who might benefit from upfront cytoreductive nephrectomy for metastatic renal cell carcinoma in the targeted therapy era. Jpn. J. Clin. Oncol. 2014, 45, 96–102. [Google Scholar] [CrossRef]
- Klatte, T.; Fife, K.; Welsh, S.J.; Sachdeva, M.; Armitage, J.N.; Riddick, A.C.; Matakidou, A.; Eisen, T.; Stewart, G.D. Prognostic effect of cytoreductive nephrectomy in synchronous metastatic renal cell carcinoma: A comparative study using inverse probability of treatment weighting. World J. Urol. 2018, 36, 417–425. [Google Scholar] [CrossRef]
- de Bruijn, R.E.; Nijkamp, J.; Noe, A.; Horenblas, S.; Haanen, J.B.; Prevoo, W.; Bex, A. Baseline tumor volume in assessing prognosis of patients with intermediate-risk synchronous metastatic renal cell carcinoma. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 258.e7–258.e13. [Google Scholar] [CrossRef]
- Heng, D.Y.C.; Wells, J.C.; Rini, B.I.; Beuselinck, B.; Lee, J.-L.; Knox, J.J.; Bjarnason, G.A.; Pal, S.K.; Kollmannsberger, C.K.; Yuasa, T.; et al. Cytoreductive Nephrectomy in Patients with Synchronous Metastases from Renal Cell Carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur. Urol. 2014, 66, 704–710. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.K.; Park, B.; Joo, J.; Joung, J.Y.; Seo, H.K.; Lee, K.H.; Chung, J. Effect of renal embolization in patients with synchronous metastatic renal cell carcinoma: A retrospective comparison of cytoreductive nephrectomy and systemic medical therapy. Oncotarget 2017, 8, 49615–49624. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manley, B.J.; Kim, E.H.; Vetter, J.M.; Potretzke, A.M.; Strope, S.A. Validation of preoperative variables and stratification of patients to help predict benefit of cytoreductive nephrectomy in the targeted therapy ERA. Int. Braz. J. Urol. 2017, 43, 432–439. [Google Scholar] [CrossRef]
- Mutlu, H.; Gündüz, Ş.; Büyükçelik, A.; Yıldız, Ö.; Uysal, M.; Tural, D.; Bozcuk, H.; Coşkun, H.Ş. The necessity of cytoreductive nephrectomy in patients with metastatic renal cell carcinoma using antiangiogenic targeted therapy after interferon alfa-2b. Clin. Genitourin. Cancer 2014, 12, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Poprach, A.; Fiala, O.; Chloupkova, R.; Melichar, B.; Lakomy, R.; Petrakova, K.; Zemanova, M.; Kopeckova, K.; Capoor, M.N.; Studentova, H.; et al. Pazopanib for Metastatic Renal Cell Carcinoma: A Registry-based Analysis of 426 Patients. Anticancer. Res. 2018, 38, 449–456. [Google Scholar] [CrossRef] [PubMed]
- You, D.; Jeong, I.G.; Ahn, J.-H.; Lee, D.H.; Lee, J.-L.; Hong, J.H.; Ahn, H.; Kim, C.-S. The Value of Cytoreductive Nephrectomy for Metastatic Renal Cell Carcinoma in the Era of Targeted Therapy. J. Urol. 2011, 185, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Coinu, A.; Vavassori, I.; Cabiddu, M.; Borgonovo, K.; Ghilardi, M.; Lonati, V.; Barni, S. Cytoreductive Nephrectomy in Metastatic Renal Cell Carcinoma Treated With Targeted Therapies: A Systematic Review With a Meta-Analysis. Clin. Genitourin. Cancer 2016, 14, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Li, F.; Tang, K.; Pang, S.; Lin, G.; Li, S.; Bao, J.; Tan, W.-L. Prognostic value of cytoreductive nephrectomy combined with targeted therapy for metastatic renal cell carcinoma: A meta-analysis. Int. Urol. Nephrol. 2016, 48, 967–975. [Google Scholar] [CrossRef]
- Massari, F.; Di Nunno, V.; Gatto, L.; Santoni, M.; Schiavina, R.; Cosmai, L.; Brunocilla, E.; Ardizzoni, A.; Porta, C. Should CARMENA Really Change our Attitude Towards Cytoreductive Nephrectomy in Metastatic Renal Cell Carcinoma? A Systematic Review and Meta-Analysis Evaluating Cytoreductive Nephrectomy in the Era of Targeted Therapy. Target Oncol. 2018, 13, 705–714. [Google Scholar] [CrossRef]
- García-Perdomo, H.A.; Zapata-Copete, J.A.; Castillo-Cobaleda, D.F. Role of cytoreductive nephrectomy in the targeted therapy era: A systematic review and meta-analysis. Investig. Clin. Urol. 2018, 59, 2–9. [Google Scholar] [CrossRef]
- Bhindi, B.; Abel, E.J.; Albiges, L.; Bensalah, K.; Boorjian, S.A.; Daneshmand, S.; Karam, J.A.; Mason, R.J.; Powles, T.; Bex, A. Systematic Review of the Role of Cytoreductive Nephrectomy in the Targeted Therapy Era and Beyond: An Individualized Approach to Metastatic Renal Cell Carcinoma. Eur. Urol. 2019, 75, 111–128. [Google Scholar] [CrossRef]
- Conti, S.L.; Thomas, I.-C.; Hagedorn, J.C.; Chung, B.I.; Chertow, G.M.; Wagner, T.H.; Brooks, J.D.; Srinivas, S.; Leppert, J.T. Utilization of cytoreductive nephrectomy and patient survival in the targeted therapy era. Int. J. Cancer 2013, 134, 2245–2252. [Google Scholar] [CrossRef]
- Bhindi, B.; Habermann, E.B.; Mason, R.J.; Costello, B.A.; Pagliaro, L.C.; Thompson, R.H.; Leibovich, B.C.; Boorjian, S.A. Comparative Survival following Initial Cytoreductive Nephrectomy versus Initial Targeted Therapy for Metastatic Renal Cell Carcinoma. J. Urol. 2018, 200, 528–534. [Google Scholar] [CrossRef]
- Stewart, C.L.; Warner, S.; Ito, K.; Raoof, M.; Wu, G.X.; Kessler, J.; Kim, J.Y.; Fong, Y. Cytoreduction for colorectal metastases: Liver, lung, peritoneum, lymph nodes, bone, brain. When does it palliate, prolong survival, and potentially cure? Curr. Probl. Surg. 2018, 55, 330–379. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A.; Gawlinski, E.T.; Tangen, C.M.; Flanigan, R.C.; Crawford, E.D. The possible role of postoperative azotemia in enhanced survival of patients with metastatic renal cancer after cytoreductive nephrectomy. Cancer Res. 2002, 62. [Google Scholar]
- Klatte, T.; Böhm, M.; Nelius, T.; Filleur, S.; Reiher, F.; Allhoff, E.P. Evaluation of peri-operative peripheral and renal venous levels of pro- and anti-angiogenic factors and their relevance in patients with renal cell carcinoma. BJU Int. 2007, 100, 209–214. [Google Scholar] [CrossRef]
- Tatsumi, T.; Herrem, C.J.; Olson, W.C.; Finke, J.H.; Bukowski, R.M.; Kinch, M.S.; Ranieri, E.; Storkus, W.J. Disease stage variation in CD4+ and CD8+ T-cell reactivity to the receptor tyrosine kinase EphA2 in patients with renal cell carcinoma. Cancer Res. 2003, 63, 4481–4489. [Google Scholar] [PubMed]
- Orris, B.G.; Flanigan, R.C. Nephrectomy in Patients with Metastatic Renal Cell Carcinoma: Clinical and Biologic Effects. In Clinical Management of Renal Tumors; Springer Nature: London, UK, 2008; pp. 335–353. [Google Scholar]
- Wald, G.; Barnes, K.T.; Bing, M.T.; Kresowik, T.P.; Tomanek-Chalkley, A.; Kucaba, T.A.; Griffith, T.S.; Brown, J.A.; A Norian, L. Minimal changes in the systemic immune response after nephrectomy of localized renal masses11This work was supported by the University of Iowa Carver College of Medicine/Department of Urology Investigator Start-up Funds, NIH Grant CA181088-01 (to L.A.N.), and NIH Grant CA109446 (to T.S.G.). Urol. Oncol. Semin. Orig. Investig. 2014, 32, 589–600. [Google Scholar] [CrossRef]
- Nakayama, T.; Saito, K.; Kumagai, J.; Nakajima, Y.; Kijima, T.; Yoshida, S.; Kihara, K.; Fujii, Y. Higher Serum C-reactive Protein Level Represents the Immunosuppressive Tumor Microenvironment in Patients With Clear Cell Renal Cell Carcinoma. Clin. Genitourin. Cancer 2018, 16, e1151–e1158. [Google Scholar] [CrossRef] [PubMed]
- Westerman, M.E.; Shapiro, D.D.; Tannir, N.M.; Campbell, M.T.; Matin, S.F.; Karam, J.A.; Wood, C.G. Survival following cytoreductive nephrectomy: A comparison of existing prognostic models. BJU Int. 2020, 126, 745–753. [Google Scholar] [CrossRef]
- Abdollah, F.; Sun, M.; Thuret, R.; Schmitges, J.; Shariat, S.F.; Perrotte, P.; Montorsi, F.; Karakiewicz, P.I. Mortality and Morbidity After Cytoreductive Nephrectomy for Metastatic Renal Cell Carcinoma: A Population-Based Study. Ann. Surg. Oncol. 2011, 18, 2988–2996. [Google Scholar] [CrossRef]
- Kutikov, A.; Uzzo, R.G.; Caraway, A.; Reese, C.T.; Egleston, B.L.; Chen, D.Y.; Viterbo, R.; Greenberg, R.E.; Wong, Y.N.; Raman, J.D.; et al. Use of systemic therapy and factors affecting survival for patients undergoing cytoreductive nephrectomy. BJU Int. 2009, 106, 218–223. [Google Scholar] [CrossRef]
- O’Malley, R.L.; Brewer, K.A.; Hayn, M.H.; Kim, H.L.; Underwood, W., III; Pili, R.; Schwaab, T. Impact of cytoreductive nephrectomy on eligibility for systemic treatment and effects on survival: Are surgical complications or disease related factors responsible? Urology 2011, 78, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Bex, A.; Albiges, L.; Ljungberg, B.; Bensalah, K.; Dabestani, S.; Giles, R.H.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; Lam, T.B.; et al. Updated European Association of Urology Guidelines for Cytoreductive Nephrectomy in Patients with Synchronous Metastatic Clear-cell Renal Cell Carcinoma. Eur. Urol. 2018, 74, 805–809. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, A.G.; Umbreit, E.C.; Bs, L.C.H.; Gu, C.; Tannir, N.M.; Matin, S.F.; Karam, J.A.; Culp, S.H.; Wood, C.G.; Holland, L.C.; et al. Optimizing patient selection for cytoreductive nephrectomy based on outcomes in the contemporary era of systemic therapy. Cancer 2020, 126, 3950–3960. [Google Scholar] [CrossRef] [PubMed]
- Marchioni, M.; Kriegmair, M.; Heck, M.; Amiel, T.; Porpiglia, F.; Ceccucci, E.; Campi, R.; Minervini, A.; Mari, A.; Van Bruwaene, S.; et al. Development of a Novel Risk Score to Select the Optimal Candidate for Cytoreductive Nephrectomy Among Patients with Metastatic Renal Cell Carcinoma. Results from a Multi-institutional Registry (REMARCC). Eur. Urol. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Heng, D.Y.; Xie, W.; Regan, M.M.; Harshman, L.C.; Bjarnason, G.A.; Vaishampayan, U.N.; Mackenzie, M.; Wood, L.; Donskov, F.; Tan, M.H.; et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A population-based study. Lancet Oncol. 2013, 14, 141–148. [Google Scholar] [CrossRef]
- Mejean, A.; Thezenas, S.; Chevreau, C.; Bensalah, K.; Geoffrois, L.; Thiery-Vuillemin, A.; Cormier, L.; Lang, H.; Guy, L.; Gravis, G.; et al. Cytoreductive nephrectomy (CN) in metastatic renal cancer (mRCC): Update on Carmena trial with focus on intermediate IMDC-risk population. J. Clin. Oncol. 2019, 37, 4508. [Google Scholar] [CrossRef]
- Bex, A.; Mulders, P.; Jewett, M.; Wagstaff, J.; Van Thienen, J.V.; Blank, C.U.; Van Velthoven, R.; Laguna, M.D.P.; Wood, L.; Van Melick, H.H.E.; et al. Comparison of Immediate vs Deferred Cytoreductive Nephrectomy in Patients With Synchronous Metastatic Renal Cell Carcinoma Receiving Sunitinib: The SURTIME Randomized Clinical Trial. JAMA Oncol. 2019, 5, 164–170. [Google Scholar] [CrossRef]
- de Bruijn, R.; Wimalasingham, A.; Szabados, B.; Stewart, G.D.; Welsh, S.J.; Kuusk, T.; Blank, C.; Haanen, J.; Klatte, T.; Staehler, M.; et al. Deferred Cytoreductive Nephrectomy Following Presurgical Vascular Endothelial Growth Factor Receptor-targeted Therapy in Patients with Primary Metastatic Clear Cell Renal Cell Carcinoma: A Pooled Analysis of Prospective Trial Data. Eur. Urol. Oncol. 2020, 3, 168–173. [Google Scholar] [CrossRef]
- Bhindi, B.; Graham, J.; Wells, J.C.; Bakouny, Z.; Donskov, F.; Fraccon, A.; Pasini, F.; Lee, J.L.; Basappa, N.S.; Hansen, A.; et al. Deferred Cytoreductive Nephrectomy in Patients with Newly Diagnosed Metastatic Renal Cell Carcinoma. Eur. Urol. 2020, 78, 615–623. [Google Scholar] [CrossRef]
- Suissa, S.; Dell’Aniello, S. Time-related biases in pharmacoepidemiology. Pharmacoepidemiol. Drug Saf. 2020, 29, 1101–1110. [Google Scholar] [CrossRef]

| Author | Year | Center and Country | Study Period | CN Patients | Non-CN Patients | Age (years) | Male Sex | Clear Cell Histology | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CN | Non-CN | CN | Non-CN | CN | Non-CN | ||||||
| Choi et al. [15] | 2018 | Samsung Medical Center, Seoul, South Korea | January 2005 to December 2015 | 189 | 105 | 60.7 ± 14.2 | 56.3 ± 9.8 | 149 | 82 | 166 | 76 |
| de Bruijn et al. [41] | 2016 | The Netherlands Cancer Institute, Amsterdam, The Netherlands | January 2006 to December 2012 | 39 | 29 | NA | NA | NA | NA | 39 | 29 |
| Heng et al. [42] | 2014 | IMDC database (20 international centers) | NA | 982 | 676 | 59.8 ± 10.9 | 61.5 ± 11.4 | 721 | 488 | 841 | 450 |
| Janisch et al. [17] | 2020 | University Medical Center Hamburg-Eppendorf, Hamburg, Germany | 2000 to 2016 | 104 | 158 | 60.7 ± 9.8 | 61.3 ± 11.2 | 80 | 113 | 89 | 130 |
| Kim et al. [43] | 2017 | Research Institute and Hospital of National Cancer Center, Goyang, South Korea | January 2000 to December 2015 | 27 | 84 | NA | NA | NA | NA | NA | NA |
| Klatte et al. [40] | 2018 | Cambridge Oncology Registry, UK | 2006 to 2017 | 97 | 164 | 58.6 ± 12.4 | 64.4 ± 9.9 | 65 | 116 | 80 | 123 |
| Manley et al. [44] | 2017 | Washington University School of Medicine, Division of Urology, St. Louis, Missouri, USA | 2005 to 2013 | 88 | 35 | 57.4 ± 10.4 | 57.8 ± 10.4 | NA | NA | NA | NA |
| Mejean et al. [16] | 2018 | Multicenter (79 centers from France, Norway, England, Scotland, Sweden) | September 2009 to September 2017 | 226 | 224 | 60.7 ± 8.5 | 60.2 ± 9.5 | 169 | 167 | 226 | 224 |
| Mutlu et al. [45] | 2014 | Akdeniz University, Antalya, Afyon Kocatepe University, Afyon and Medipol University, Istanbul, Turkey | NA | 28 | 24 | 53.6 ± 9.8 | 67.5 ± 10.5 | 22 | 16 | NA | NA |
| Poprach et al. [46] | 2018 | Renal Cell Carcinoma Information System (RENIS) registry, Czech Republic | August 2011 to December 2015 | 114 | 71 | NA | NA | NA | NA | NA | NA |
| Qi et al. [35] | 2017 | Peking University First Hospital, Institute of Urology, Beijing, China | April 2008 to October 2014 | 20 | 15 | NA | NA | 15 | 10 | 16 | 12 |
| Song et al. [36] | 2016 | Cancer Hospital (Institute), Chinese Academy of Medical Sciences, Beijing, China | NA | 51 | 23 | NA | NA | 37 | 19 | NA | NA |
| Tatsugami et al. [38] | 2015 | 7 centers from Japan | January 2001 to December 2010 | 103 | 25 | NA | NA | NA | NA | NA | NA |
| Xu et al [37]. | 2019 | Fudan University Shanghai Cancer Center (FUSCC), Shanghai, China | May 2009 to June 2018 | 70 | 48 | NA | NA | 46 | 38 | 55 | 37 |
| You et al. [39] | 2015 | Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea | 2006 to 2012 | 96 | 75 | 56.5 ± 10.4 | 60.2 ± 12.8 | 66 | 51 | 92 | 64 |
| Total | 2000–2018 | 2234 | 1756 | 59.6 ± 11.1 | 61.2 ± 11.0 | 1370/1853 (73.9) | 1100/1512 (72.8) | 1604/1793 (89.5) | 1145/1334 (85.8) | ||
| Clinical Characteristic | CN Group | Non-CN Group |
|---|---|---|
| IMDC Risk Score | ||
| 0 | 81/1108 (7.3) | 8/910 (0.9) |
| 1–2 | 720/1108 (65.0) | 459/910 (50.4) |
| 3–6 | 307/1108 (27.7) | 443/910 (48.7) |
| MSKCC Risk Score | ||
| 0 | 19/384 (5.0) | 48/490 (9.8) |
| 1–2 | 232/384 (60.4) | 308/490 (62.9) |
| 3–6 | 133/384 (34.6) | 134/490 (27.4) |
| ECOG ≥ 2 | 22/539 (4.1) | 19/502 (3.8) |
| Karnofsky ≥ 80% | 976/1187 (82.2) | 606/980 (61.8) |
| T1/2 Stage | 365/901 (40.5) | 174/490 (35.5) |
| N1 Stage | 149/372 (40.1) | 121/233 (51.9) |
| >2 Metastases | 76/321 (23.7) | 136/393 (34.6) |
| Brain Metastases | 98/1221 (8.0) | 118/1137 (10.4) |
| Bone Metastases | 565/1519 (37.2) | 622/1388 (44.8) |
| Liver Metastases | 239/1326 (18.0) | 274/1178 (23.3) |
| Lung Metastases | 426/566 (75.3) | 504/698 (72.2) |
| Lymph Node Metastases | 188/418 (45.0) | 276/543 (50.8) |
| Type of Targeted Therapy | ||
| Sunitinib | 1110/1572 (70.6) | 1029/1249 (82.4) |
| Pazopanib | 166/1351 (12.3) | 108/1019 (10.6) |
| Axitinib | 31/1219 (2.5) | 32/921 (3.5) |
| Sorafenib | 285/1463 (19.5) | 110/1172 (9.4) |
| Famitinib | 5/51 (9.8) | 9/23 (39.1) |
| Bevacizumab | 42/965 (4.4) | 10/673 (1.5) |
| Everolimus | 34/1191 (2.9) | 45/897 (5.0) |
| One-Stage Meta-Analysis | Overall Survival | Progression-Free Survival | Cancer-Specific Survival | ||||
|---|---|---|---|---|---|---|---|
| Relative Effect of CN versus Non-CN (95% Cl/CrI) | p-Value for Relative Effect | Relative Effect of CN versus Non-CN (95% Cl/CrI) | p-Value for Relative Effect | Relative Effect of CN versus Non-CN (95% Cl/CrI) | p-Value for Relative Effect | ||
| Frequentist Approach | Cox Proportional Hazards Model | 0.58 a (0.54–0.62) | <0.0001 | 0.90 b (0.80–1.02) | 0.093 | 0.63 c (0.53–0.75) | <0.0001 |
| Life Expectancy Difference (up to 3 years) | 6.0 months (5.2–6.8) | <0.0001 | 1.1 months [(−0.2)–(2.3)] | 0.100 | 6.2 months (4.2–8.3) | <0.0001 | |
| Life Expectancy Ratio (up to 3 years) | 1.36 (1.30–1.42) | <0.0001 | 1.09 (0.98–1.20) | 0.100 | 1.32 (1.20–1.46) | <0.0001 | |
| Life Expectancy Difference (up to 5 years) | 9.4 months (8.1–10.7) | <0.0001 | 1.4 months [(−0.5)–(3.3)] | 0.150 | 9.4 months (6.1–12.8) | <0.0001 | |
| Life Expectancy Ratio (up to 5 years) | 1.48 (1.40–1.56) | <0.0001 | 1.10 (0.97–1.25) | 0.150 | 1.39 (1.23–1.57) | <0.0001 | |
| Bayesian Approach | Cox Proportional Hazards Model | 0.59 (0.55–0.63) | N/A | 0.91 (0.80–1.02) | N/A | 0.63 (0.53–0.75) | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esagian, S.M.; Ziogas, I.A.; Kosmidis, D.; Hossain, M.D.; Tannir, N.M.; Msaouel, P. Long-Term Survival Outcomes of Cytoreductive Nephrectomy Combined with Targeted Therapy for Metastatic Renal Cell Carcinoma: A Systematic Review and Individual Patient Data Meta-Analysis. Cancers 2021, 13, 695. https://doi.org/10.3390/cancers13040695
Esagian SM, Ziogas IA, Kosmidis D, Hossain MD, Tannir NM, Msaouel P. Long-Term Survival Outcomes of Cytoreductive Nephrectomy Combined with Targeted Therapy for Metastatic Renal Cell Carcinoma: A Systematic Review and Individual Patient Data Meta-Analysis. Cancers. 2021; 13(4):695. https://doi.org/10.3390/cancers13040695
Chicago/Turabian StyleEsagian, Stepan M., Ioannis A. Ziogas, Dimitrios Kosmidis, Mohammad D. Hossain, Nizar M. Tannir, and Pavlos Msaouel. 2021. "Long-Term Survival Outcomes of Cytoreductive Nephrectomy Combined with Targeted Therapy for Metastatic Renal Cell Carcinoma: A Systematic Review and Individual Patient Data Meta-Analysis" Cancers 13, no. 4: 695. https://doi.org/10.3390/cancers13040695
APA StyleEsagian, S. M., Ziogas, I. A., Kosmidis, D., Hossain, M. D., Tannir, N. M., & Msaouel, P. (2021). Long-Term Survival Outcomes of Cytoreductive Nephrectomy Combined with Targeted Therapy for Metastatic Renal Cell Carcinoma: A Systematic Review and Individual Patient Data Meta-Analysis. Cancers, 13(4), 695. https://doi.org/10.3390/cancers13040695







