Efficacy of Afatinib in the Treatment of Patients with Non-Small Cell Lung Cancer and Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
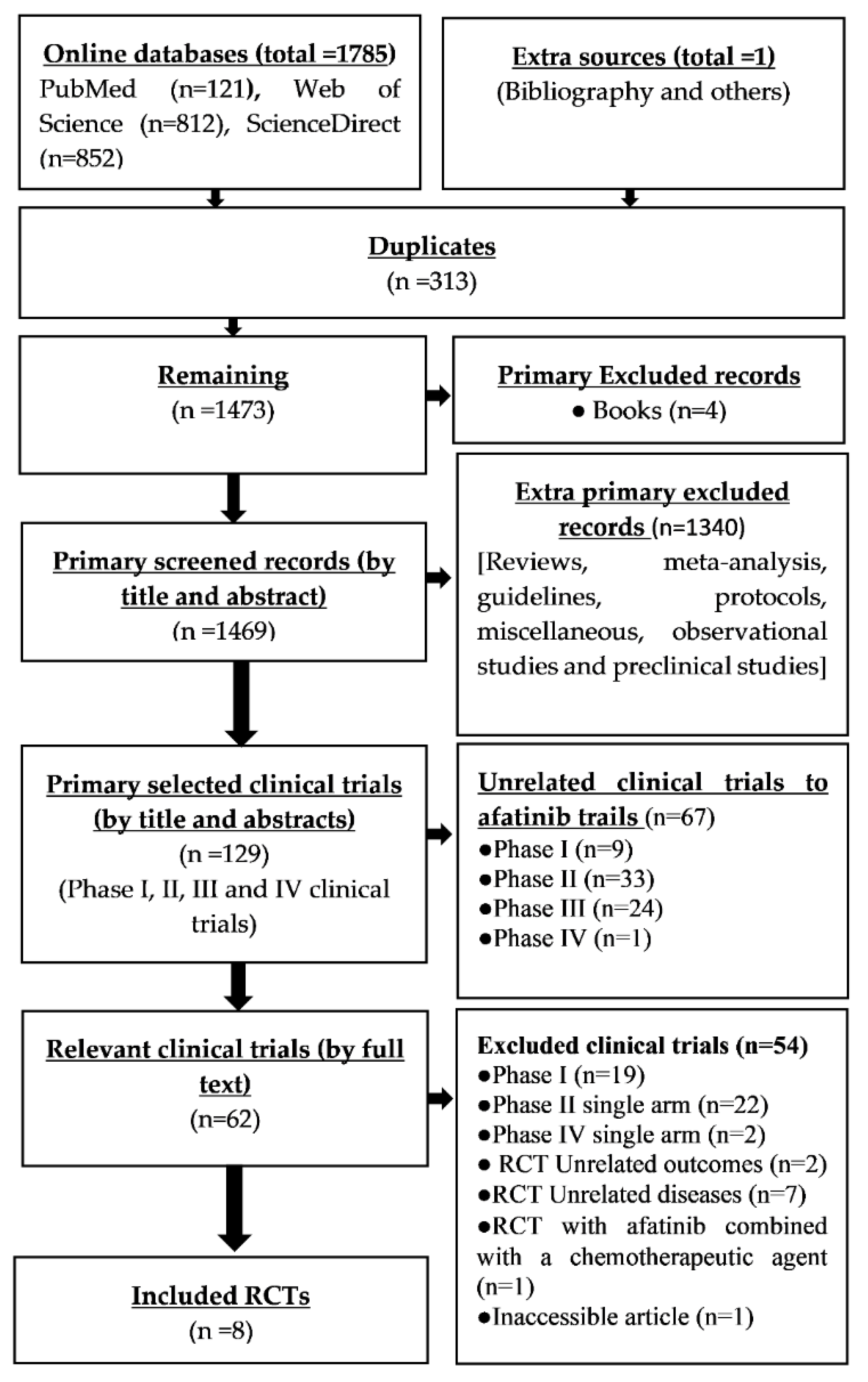
2.2. Selection
2.3. Quality Assessment
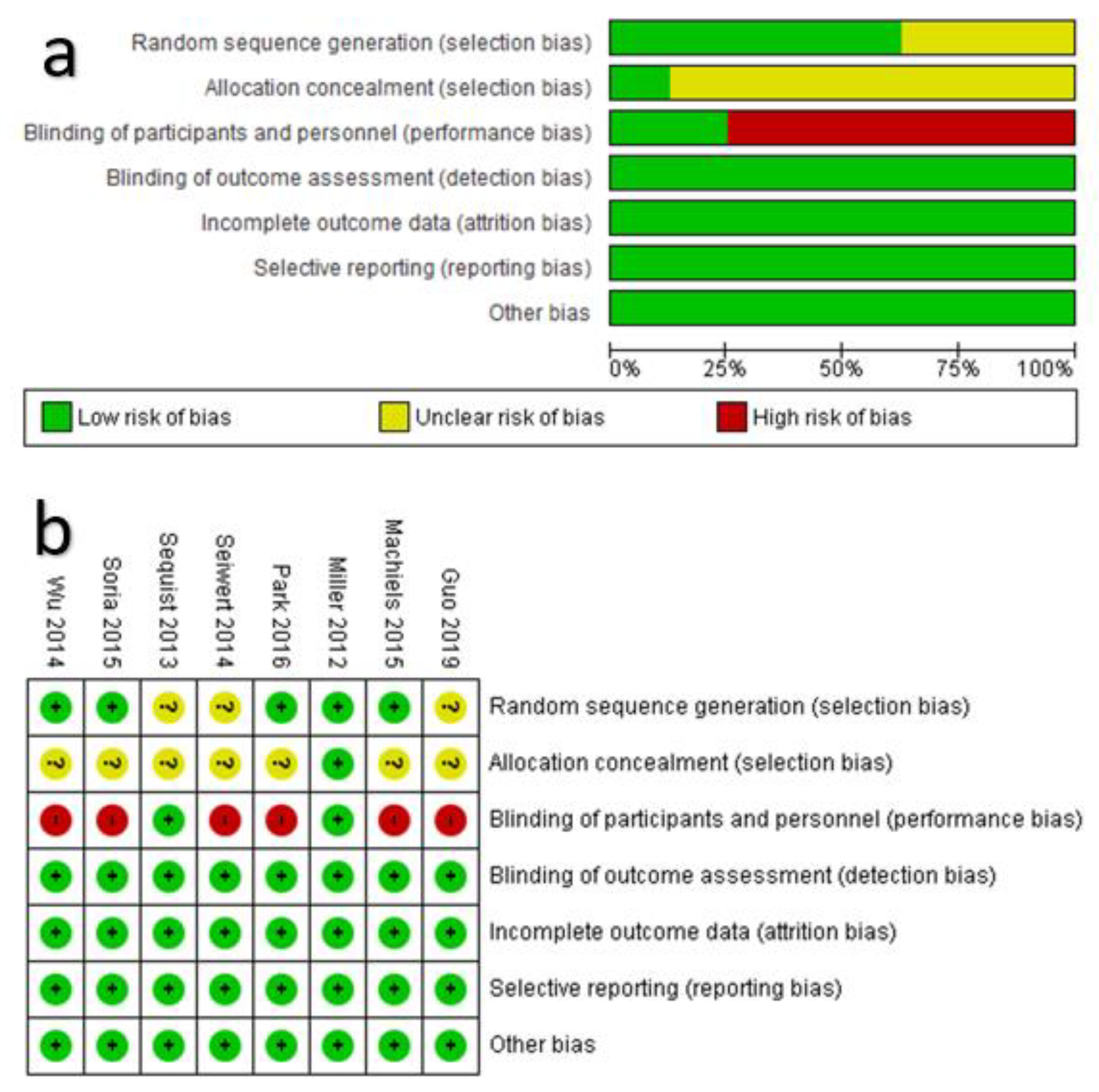
2.3.1. Assessment of the Risk of Bias in the Included RCTs
- Selection bias was assessed in terms of “random generation sequences” and “allocation concealment”. If a random generation sequence method (e.g., computer-generated random numbers) was explicitly reported, the result of appraisal was “low risk”. Otherwise, the appraisal was “unclear risk” if the randomization was undertaken without reporting the random generation sequence method or “high risk” if randomization was not undertaken at all. Regarding “allocation concealment”, if it was directly or indirectly reported that the allocation of participants was adequately addressed from time of randomization to the cutoff date of the trial, the appraisal was “low risk”. If it was directly reported that the allocation was concealed (e.g., open-label or single-blind), the appraisal was “high risk” [unless there was a rationale justification]. If not reported directly or indirectly, the appraisal was “unclear risk”.
- Performance bias was assessed in terms of “blinding of participants and personnel”. Therefore, If the masking was double-blind, the appraisal was “low risk”. Alternatively, if it was explicitly reported that the assignment of the trail was an open-label or single-blind, the appraisal was “high risk” [unless there was a rationale justification].
- Detection bias was assessed in terms of “blinding of outcome assessment”. If the outcome assessors and data analysis were reported that performed by an independent review committee, the appraisal was “low risk”. Otherwise, the appraisal was “high risk”.
- Attrition bias was assessed in terms of “incomplete outcome data”. Therefore, if the statistical analysis addressed the dropout, withdrawal, missing data, and discontinuation, the appraisal was “low risk”. Otherwise, the appraisal was “high risk”.
- Reporting bias was assessed in terms of “selective reporting”. Thus, if the primary and secondary outcomes were reported in accord with the objectives of the trial in the published manuscript and prospective protocol of the trial, the appraisal was “low risk”. Otherwise, the appraisal was “high risk” [unless rationally justified].
- Other sources of bias were assessed in terms of “adherence to the prospective protocol”. Therefore, if it was reported that the prospective protocol was not violated during the trial or after interim analysis, the appraisal was “low risk”. Alternatively, if there was direct evidence indicated switching the primary and secondary outcomes, the appraisal was “high risk” [unless rationally justified].
2.3.2. Quality of the Pooled Evidence from Meta-Analysis
2.4. Data Collection and Data Extraction Strategy
- Characteristics of study design: Study ID (first author with the year), registered name, prospective protocol ID, interventional model (all the assignment models.), phase, allocation ratio, assignment model, number of centers and countries where the trial was conducted).
- Population: The total number (sample size of participants; age, gender, and ethnicity of participants.
- Characteristics of the health condition of interest: Type and stage of cancer.
- Intervention characteristics: Line of treatment, dose, frequency of dose, route of administration, and the number of participants.
- Comparator characteristics: Type of control (standard therapy or placebo), dose, frequency of dose, route of administration, and number of participants.
- Characteristics of outcomes.
- Primary clinical endpoints: Measure the efficacy of afatinib in terms of participants survival included overall survival (OS) and progression-free survival (PFS), and the data that were extracted included the difference in the effect size (significant or non-significant), hazard ratio with the corresponding 95% confidence interval and P-value, the median of difference and duration of median follow-up of the outcome in weeks or months in the intervention and control groups. OS with median and median follow-up duration [Defined as time from randomization to death for any reason in months and calculated as HR along with its corresponding 95% confidence intervals and P-value]. PFS with median and median follow-up duration [the time from randomization to progression in months and calculated as HR along with its corresponding 95% confidence interval and p-value].
- Secondary clinical endpoint was adverse events, which were recruited for evaluating safety (defined as the percentage of severe grade-3 adverse events (AEs) among participants in the intervention as compared to comparator). The data that were extracted included adverse events and the percentage of incidence.
2.5. Data Synthesis
2.5.1. Qualitative Synthetic Literature
2.5.2. Meta-Analysis
- Equivalent efficacy of afatinib to that of the comparator, if hazard ratio = 1.
- Superior efficacy of afatinib to that of the comparator, if hazard ratio < 1.
- Inferior efficacy of afatinib to that of the comparator, if hazard ratio > 1.
3. Results
3.1. Identification and Selection of Records
3.2. Appriasal of the Risk of Bias within Each RCT and across RCTs
3.3. Grading the Quality of Pooled Evidence from Meta-Analysis
3.4. Study Characteristics
3.5. Qualitative Literature Review
3.5.1. Efficacy of Afatinib
3.5.2. Adverse Events
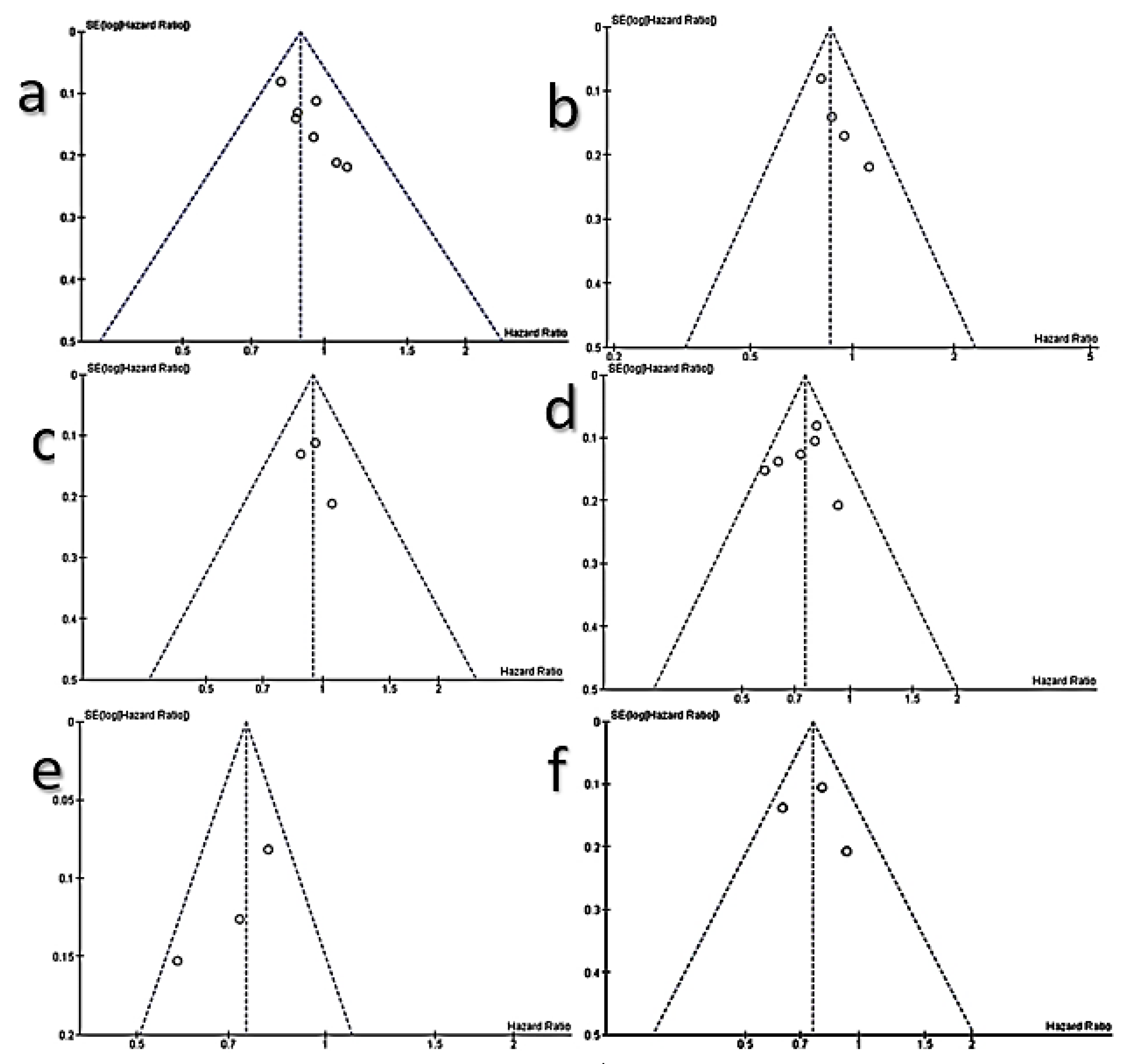
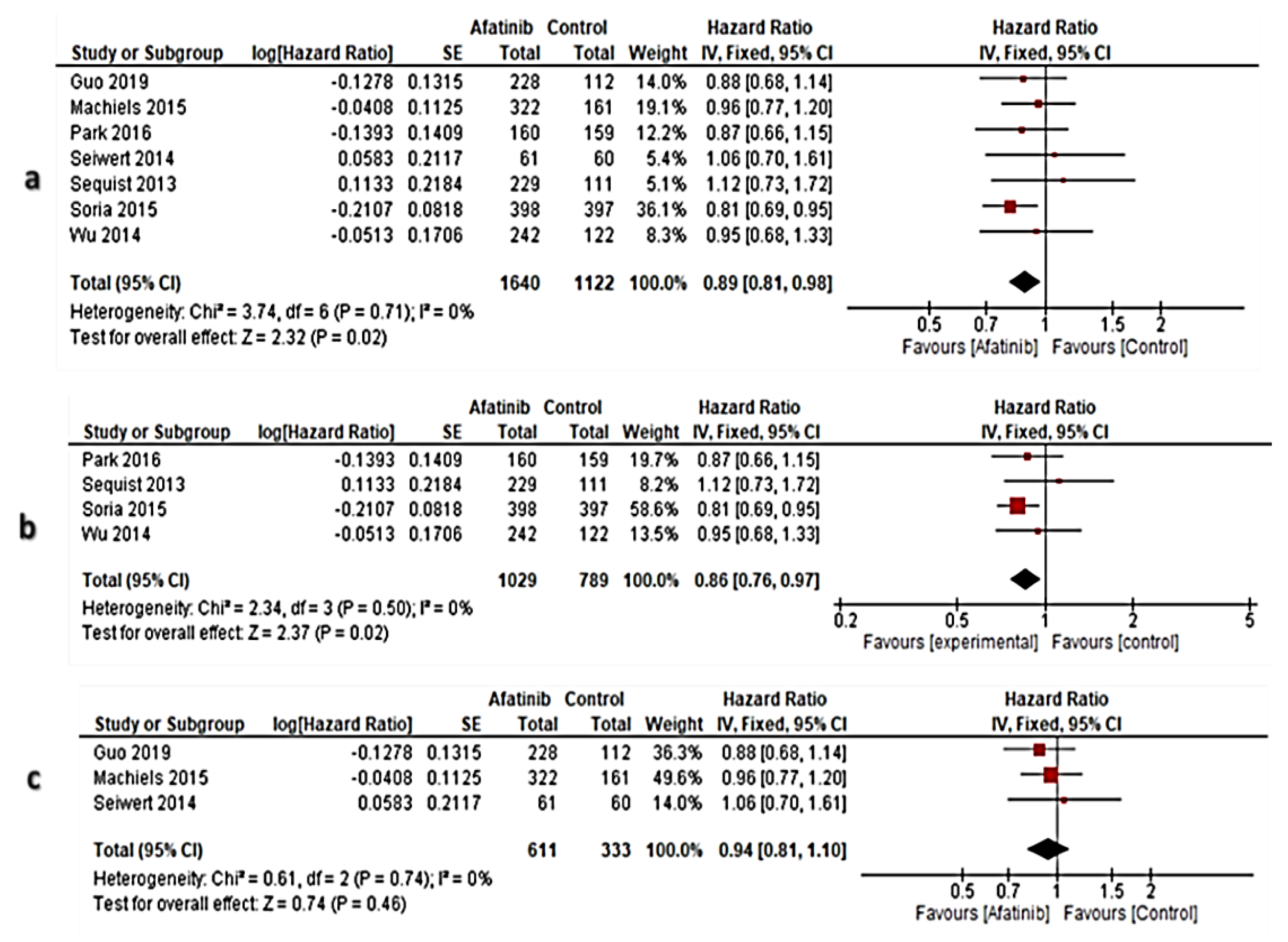
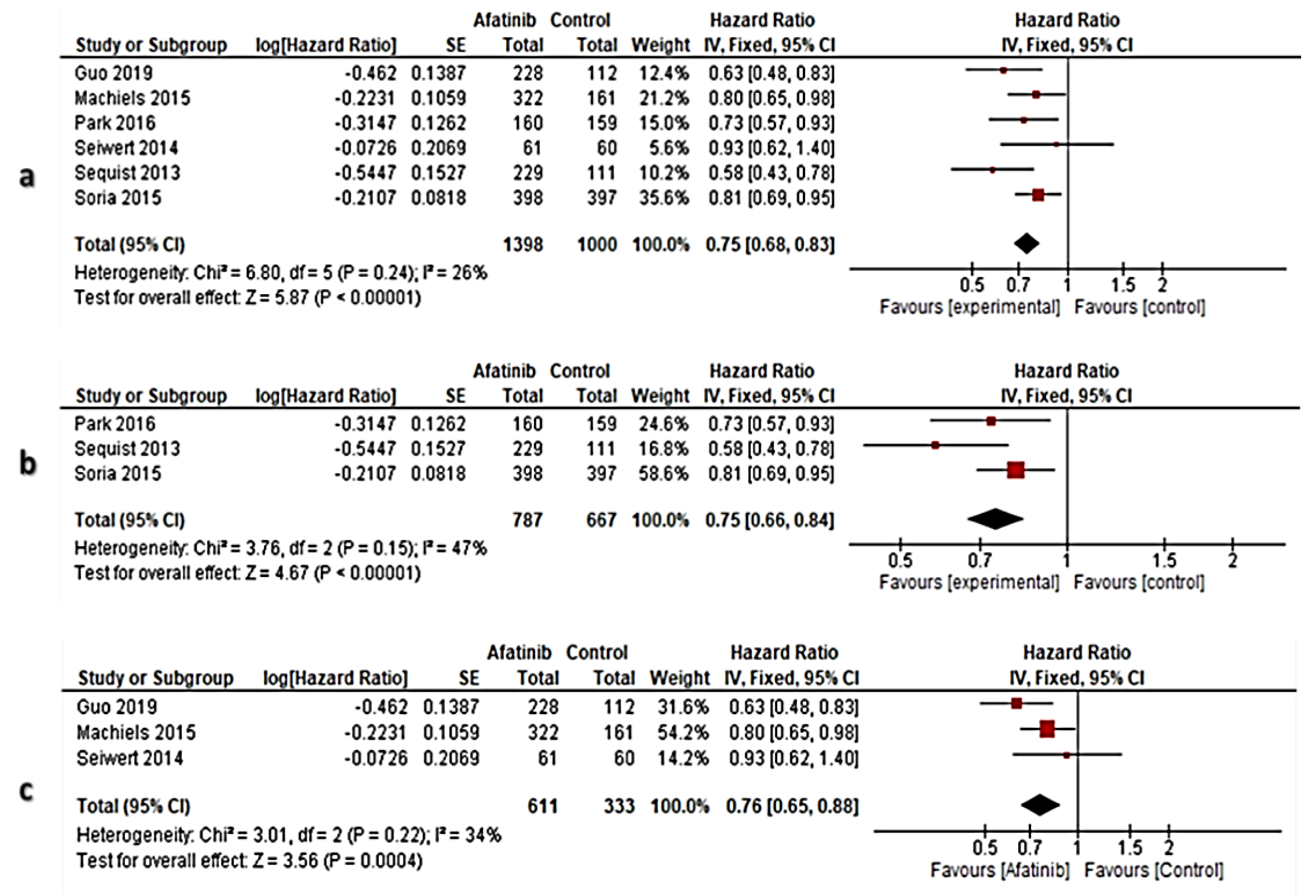
3.6. Meta-Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| ID | Study Design | Health Condition | Population | Intervention | Comparator | Outcomes | ||
|---|---|---|---|---|---|---|---|---|
| Overall Survival | Progression-Free Survival | Grade 3 Adverse Events (AE) | ||||||
| [22] |
|
|
|
|
|
|
|
|
| [23] |
|
|
|
|
|
|
|
|
| [30] |
|
|
|
|
|
|
|
|
| [24] |
|
|
|
|
|
|
|
|
| [32] |
|
|
|
|
|
|
|
|
| [25] |
|
|
|
|
|
|
|
|
| [26] |
|
|
|
|
|
|
|
|
| [31] |
|
|
|
|
|
|
|
|
Appendix B
| Participants | Overall Survival | Progression–Free Survival | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Study ID | Total | Afatinib | Control | Hazard Ratio | 95% Confidence Interval | p–Value | Hazard Ratio | 95% Confidence Interval | p–Value |
| [22] | 585 | 390 | 195 | 1.08 | 0.86–1.35 | 0.74 | 0.38 | 0.31–0.48 | 0.0001 |
| [23] | 345 | 229 | 111 | 1.12 | 0.73–1.73 | 0.60 | 0.58 | 0.43–0.78 | 0.0010 |
| [30] | 121 | 61 | 60 | 1.06 | 0.70–1.62 | 0.78 | 0.93 | 0.62–1.38 | 0.7100 |
| [24] | 364 | 242 | 122 | 0.95 | 0.68–1.33 | 0.76 | 0.26 | 0.19–0.36 | 0.0001 |
| [32] | 483 | 322 | 161 | 0.96 | 0.77–1.19 | 0.70 | 0.80 | 0.65–0.98 | 0.0300 |
| [25] | 795 | 398 | 397 | 0.81 | 0.69–0.95 | 0.01 | 0.81 | 0.69–0.96 | 0.0103 |
| [26] | 319 | 160 | 159 | 0.87 | 0.66–1.15 | 0.33 | 0.73 | 0.57–0.95 | 0.0170 |
| [31] | 340 | 228 | 112 | 0.88 | 0.68–1.13 | 0.32 | 0.63 | 0.48–0.82 | 0.0005 |
References
- Hata, A.; Katakami, N.; Kaji, R.; Yokoyama, T.; Kaneda, T.; Tamiya, M.; Inoue, T.; Kimura, H.; Yano, Y.; Tamura, D. Afatinib plus bevacizumab combination after acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer: Multicenter, single-arm, phase 2 trial (ABC Study). Cancer 2018, 124, 3830–3838. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Sun, J.-M.; Lim, S.H.; Kim, H.S.; Yoo, K.H.; Jung, K.S.; Song, H.-N.; Ku, B.M.; Koh, J.; Bae, Y.-H. A phase Ib/II study of afatinib in combination with nimotuzumab in non–small cell lung cancer patients with acquired resistance to gefitinib or erlotinib. Clin. Cancer Res. 2016, 22, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.K.; Wang, L.; Han, B.e.; Li, W.; Yu, P.; Liu, Y.; Ding, C.; Song, X.; Ma, Z.; Ren, X. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): A phase 3, open-label, randomized study. Ann. Oncol. 2017, 28, 2443–2450. [Google Scholar] [CrossRef] [PubMed]
- Katakami, N.; Felip, E.; Spigel, D.R.; Kim, J.H.; Olivo, M.; Guo, M.; Nokihara, H.; Yang, J.C.H.; Iannotti, N.; Satouchi, M.; et al. A randomized, open-label, multicenter, phase 3 study to compare the efficacy and safety of eribulin to treatment of physician’s choice in patients with advanced non-small cell lung cancer. Ann. Oncol. 2017, 28, 2241–2247. [Google Scholar] [CrossRef]
- Tanaka, H.; Taima, K.; Tanaka, Y.; Itoga, M.; Ishioka, Y.; Nakagawa, H.; Baba, K.; Hasegawa, Y.; Takanashi, S.; Tasaka, S. A phase I study of afatinib for patients aged 75 or older with advanced non-small cell lung cancer harboring EGFR mutations. Med. Oncol. 2018, 35, 34. [Google Scholar] [CrossRef]
- Clement, P.M.; Gauler, T.; Machiels, J.P.; Haddad, R.I.; Fayette, J.; Licitra, L.F.; Tahara, M.; Cohen, E.E.; Cupissol, D.; Grau, J.J.; et al. Afatinib versus methotrexate in older patients with second-line recurrent and/or metastatic head and neck squamous cell carcinoma: Subgroup analysis of the LUX-Head & Neck 1 trial. Ann. Oncol. 2016, 27, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Jurmeister, P.; Bockmayr, M.; Seegerer, P.; Bockmayr, T.; Treue, D.; Montavon, G.; Vollbrecht, C.; Arnold, A.; Teichmann, D.; Bressem, K. Machine learning analysis of DNA methylation profiles distinguishes primary lung squamous cell carcinomas from head and neck metastases. Sci. Transl. Med. 2019, 11, 1–10. [Google Scholar] [CrossRef]
- Shiono, S.; Kawamura, M.; Sato, T.; Okumura, S.; Nakajima, J.; Yoshino, I.; Ikeda, N.; Horio, H.; Akiyama, H.; Kobayashi, K. Pulmonary metastasectomy for pulmonary metastases of head and neck squamous cell carcinomas. Ann. Thorac. Surg. 2009, 88, 856–860. [Google Scholar] [CrossRef]
- Reardon, D.A.; Nabors, L.B.; Mason, W.P.; Perry, J.R.; Shapiro, W.; Kavan, P.; Mathieu, D.; Phuphanich, S.; Cseh, A.; Fu, Y.; et al. Phase I/randomized phase II study of afatinib, an irreversible ErbB family blocker, with or without protracted temozolomide in adults with recurrent glioblastoma. Neuro. Oncol. 2015, 17, 430–439. [Google Scholar] [CrossRef]
- Bahleda, R.; Hollebecque, A.; Varga, A.; Gazzah, A.; Massard, C.; Deutsch, E.; Amellal, N.; Farace, F.; Ould-Kaci, M.; Roux, F.; et al. Phase I study of afatinib combined with nintedanib in patients with advanced solid tumours. Br. J. Cancer 2015, 113, 1413–1420. [Google Scholar] [CrossRef]
- De Gresve, J.; Van Meerbeeck, J.; Vansteenkiste, J.F.; Decoster, L.; Meert, A.P.; Vuylsteke, P.; Focan, C.; Canon, J.L.; Humblet, Y.; Berchem, G.; et al. Prospective Evaluation of First-Line Erlotinib in Advanced Non-Small Cell Lung Cancer (NSCLC) Carrying an Activating EGFR Mutation: A Multicenter Academic Phase II Study in Caucasian Patients (FIELT). PLoS ONE 2016, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Tanaka, H.; Saito, R.; Suzuki, A.; Harada, T.; Inoue, S.; Yamada, T.; Nakagawa, T.; Jingu, D.; Sugawara, S. Phase II Study of Low-Dose Afatinib Maintenance Treatment among Patients with EGFR-Mutated Non-Small Cell Lung Cancer: North Japan Lung Cancer Study Group Trial 1601 (NJLCG1601). Oncologist 2020, 25, e1451–e1456. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Rottey, S.; Ehrnrooth, E.; Pelling, K.; Lahogue, A.; Wind, S.; Machiels, J.P. A phase Ib, open-label study to assess the safety of continuous oral treatment with afatinib in combination with two chemotherapy regimens: Cisplatin plus paclitaxel and cisplatin plus 5-fluorouracil, in patients with advanced solid tumors. Ann. Oncol. 2013, 24, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. 2014 FDA drug approvals. Nat. Rev. Drug Discov. 2015, 14, 77–81. [Google Scholar] [CrossRef]
- Dungo, R.T.; Keating, G.M. Afatinib: First global approval. Drugs 2013, 73, 1503–1515. [Google Scholar] [CrossRef]
- Thongprasert, S.; Geater, S.L.; Clement, D.; Abdelaziz, A.; Reyes-Igama, J.; Jovanovic, D.; Alexandru, A.; Schenker, M.; Sriuranpong, V.; Serwatowski, P.; et al. Afatinib in locally advanced/metastatic NSCLC harboring common EGFR mutations, after chemotherapy: A Phase IV study. Lung Cancer Manag. 2019, 8, 10. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Gold, K.A. Afatinib in the treatment of head and neck squamous cell carcinoma. Expert Opin. Investig. Drugs 2014, 23, 135–143. [Google Scholar] [CrossRef]
- Oberndorfer, F.; Müllauer, L. Molecular pathology of lung cancer: Current status and perspectives. Curr. Opin. Oncol. 2018, 30, 69–76. [Google Scholar] [CrossRef]
- Chiang, K.H.; Shieh, J.M.; Shen, C.J.; Chang, T.W.; Wu, P.T.; Hsu, J.Y.; Tsai, J.P.; Chang, W.C.; Chen, B.K. Epidermal growth factor-induced COX-2 regulates metastasis of head and neck squamous cell carcinoma through upregulation of angiopoietin-like 4. Cancer Sci. 2020, 111, 2004. [Google Scholar] [CrossRef]
- Frederick, B.A.; Helfrich, B.A.; Coldren, C.D.; Zheng, D.; Chan, D.; Bunn, P.A.; Raben, D. Epithelial to mesenchymal transition predicts gefitinib resistance in cell lines of head and neck squamous cell carcinoma and non–small cell lung carcinoma. Mol. Cancer Ther. 2007, 6, 1683–1691. [Google Scholar] [CrossRef]
- Burtness, B.; Haddad, R.; Dinis, J.; Trigo, J.; Yokota, T.; de Souza Viana, L.; Romanov, I.; Vermorken, J.; Bourhis, J.; Tahara, M.; et al. Afatinib vs Placebo as Adjuvant Therapy After Chemoradiotherapy in Squamous Cell Carcinoma of the Head and Neck: A Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.A.; Hirsh, V.; Cadranel, J.; Chen, Y.-M.; Park, K.; Kim, S.-W.; Zhou, C.; Su, W.-C.; Wang, M.; Sun, Y.; et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): A phase 2b/3 randomised trial. Lancet Oncol. 2012, 13, 528–538. [Google Scholar] [CrossRef]
- Sequist, L.V.; Yang, J.C.; Yamamoto, N.; O’Byrne, K.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.; Tsai, C.M.; Boyer, M.; et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 2013, 31, 3327–3334. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Zhou, C.; Hu, C.P.; Feng, J.; Lu, S.; Huang, Y.; Li, W.; Hou, M.; Shi, J.H.; Lee, K.Y.; et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol. 2014, 15, 213–222. [Google Scholar] [CrossRef]
- Soria, J.C.; Felip, E.; Cobo, M.; Lu, S.; Syrigos, K.; Lee, K.H.; Göker, E.; Georgoulias, V.; Li, W.; Isla, D.; et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): An open-label randomised controlled phase 3 trial. Lancet Oncol. 2015, 16, 897–907. [Google Scholar] [CrossRef]
- Park, K.; Tan, E.H.; O’Byrne, K.; Zhang, L.; Boyer, M.; Mok, T.; Hirsh, V.; Yang, J.C.; Lee, K.H.; Lu, S.; et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016, 17, 577–589. [Google Scholar] [CrossRef]
- Schuler, M.; Yang, J.C.H.; Park, K.; Kim, J.H.; Bennouna, J.; Chen, Y.M.; Chouaid, C.; De Marinis, F.; Feng, J.F.; Grossi, F.; et al. Afatinib beyond progression in patients with non-small-cell lung cancer following chemotherapy, erlotinib/gefitinib and afatinib: Phase III randomized LUX-Lung 5 trial. Ann. Oncol. 2016, 27, 417–423. [Google Scholar] [CrossRef]
- Cortot, A.B.; Madroszyk, A.; Leprieur, E.G.; Molinier, O.; Quoix, E.A.; Berard, H.; Otto, J.; Rault, I.; Raimbourg, J.; Hureaux, J.; et al. Phase II randomized trial of afatinib with or without cetuximab as first-line treatment for EGFR mutated non-small cell lung cancer (NSCLC) patients (IFCT-1503 ACE-Lung). J. Clin. Oncol. 2019, 37, 2. [Google Scholar] [CrossRef]
- Machiels, J.P.; Bossi, P.; Menis, J.; Lia, M.; Fortpied, C.; Liu, Y.; Lhommel, R.; Lemort, M.; Schmitz, S.; Canevari, S.; et al. Activity and safety of afatinib in a window preoperative EORTC study in patients with squamous cell carcinoma of the head and neck (SCCHN). Ann. Oncol. 2018, 29, 985–991. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Fayette, J.; Cupissol, D.; Del Campo, J.M.; Clement, P.M.; Hitt, R.; Degardin, M.; Zhang, W.; Blackman, A.; Ehrnrooth, E.; et al. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck. Ann. Oncol. 2014, 25, 1813–1820. [Google Scholar] [CrossRef]
- Guo, Y.; Ahn, M.J.; Chan, A.; Wang, C.H.; Kang, J.H.; Kim, S.B.; Bello, M.; Arora, R.S.; Zhang, Q.; He, X.; et al. Afatinib versus methotrexate as second-line treatment in Asian patients with recurrent or metastatic squamous cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 3): An open-label, randomised phase III trial. Ann. Oncol. 2019, 30, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Machiels, J.P.; Haddad, R.I.; Fayette, J.; Licitra, L.F.; Tahara, M.; Vermorken, J.B.; Clement, P.M.; Gauler, T.; Cupissol, D.; Grau, J.J.; et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): An open-label, randomised phase 3 trial. Lancet Oncol. 2015, 16, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Y.; Ke, L.; Cao, L.; Fan, P.; Wu, Z.; Wu, Q. The Impact of Afatinib on Survival in Advanced Non-Small Cell Lung Cancer: A Meta-Analysis of Randomized Controlled Trials. J. Cancer 2019, 10, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Szajewska, H.; Horvath, A.; Koletzko, B. Effect of n− 3 long-chain polyunsaturated fatty acid supplementation of women with low-risk pregnancies on pregnancy outcomes and growth measures at birth: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2006, 83, 1337–1344. [Google Scholar] [CrossRef]
- Barraclough, H.; Simms, L.; Govindan, R. Biostatistics primer: What a clinician ought to know: Hazard ratios. J. Thorac. Oncol. 2011, 6, 978–982. [Google Scholar] [CrossRef]
- Fleming, P.S.; Seehra, J.; Polychronopoulou, A.; Fedorowicz, Z.; Pandis, N. A PRISMA assessment of the reporting quality of systematic reviews in orthodontics. Angle Orthod. 2013, 83, 158–163. [Google Scholar] [CrossRef]
- Kessler, T.M.; Bachmann, L.M.; Minder, C.; Löhrer, D.; Umbehr, M.; Schünemann, H.J.; Kessels, A.G. Adverse event assessment of antimuscarinics for treating overactive bladder: A network meta-analytic approach. PLoS ONE 2011, 6, e16718. [Google Scholar] [CrossRef]
- Moye, L.A. Fundamentals of Clinical Trial Design; Springer: New York, NY, USA, 2003; pp. 21–46. [Google Scholar]
- Taylor, G.J.; Wainwright, P. Open label extension studies: Research or marketing? BMJ 2005, 331, 572–574. [Google Scholar] [CrossRef][Green Version]
- Kahan, B.C.; Cro, S.; Doré, C.J.; Bratton, D.J.; Rehal, S.; Maskell, N.A.; Rahman, N.; Jairath, V. Reducing bias in open-label trials where blinded outcome assessment is not feasible: Strategies from two randomised trials. Trials 2014, 15, 456. [Google Scholar] [CrossRef]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations Using the GRADE Approach. Updated October 2013. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 30 November 2020).
| Items | Inclusion | Exclusion |
|---|---|---|
| Study design |
|
|
| Health problem |
|
|
| Population |
|
|
| Intervention |
|
|
| Comparator |
|
|
| Outcomes |
|
|
| Article types |
|
|
| Keyword | PubMed | Web of Science | Science Direct |
|---|---|---|---|
| “Afatinib” AND “Non-Small-Cell Lung Cancer” | 59 | 782 | 788 |
| “Afatinib” AND “Randomized Controlled Trial” | 52 | 27 | 60 |
| “Afatinib” AND “Squamous Cell Carcinoma of Head and Neck” | 10 | 3 | 4 |
| Total | 121 | 812 | 852 |
| No of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Certainty |
|---|---|---|---|---|---|---|---|
| Overall survival in all 1 RCTs (assessed with: 2 HR) | |||||||
| 7 | randomized trials | not serious | not serious | not serious | not serious | none | ⨁⨁⨁⨁(HIGH) |
| Overall survival in 3 NSCLC RCTs (assessed with: HR) | |||||||
| 4 | randomized trials | not serious | not serious | not serious | not serious | none | ⨁⨁⨁⨁(HIGH) |
| Overall survival in 4 HNSCC RCTs (assessed with: HR) | |||||||
| 3 | randomized trials | not serious | not serious | not serious | not serious | none | ⨁⨁⨁⨁(HIGH) |
| Progression-free survival in all RCTs (assessed with: HR) | |||||||
| 6 | randomized trials | not serious | not serious | not serious | not serious | none | ⨁⨁⨁⨁(HIGH) |
| Progression-free survival in all RCTs (assessed with: HR) | |||||||
| 3 | randomized trials | not serious | not serious | not serious | not serious | none | ⨁⨁⨁⨁(HIGH) |
| Progression-free survival in HNSCC RCTs (assessed with: HR) | |||||||
| 3 | randomized trials | not serious | not serious | not serious | not serious | none | ⨁⨁⨁⨁(HIGH) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maarof, N.N.N.; Alsalahi, A.; Abdulmalek, E.; Fakurazi, S.; Tejo, B.A.; Abdul Rahman, M.B. Efficacy of Afatinib in the Treatment of Patients with Non-Small Cell Lung Cancer and Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 688. https://doi.org/10.3390/cancers13040688
Maarof NNN, Alsalahi A, Abdulmalek E, Fakurazi S, Tejo BA, Abdul Rahman MB. Efficacy of Afatinib in the Treatment of Patients with Non-Small Cell Lung Cancer and Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers. 2021; 13(4):688. https://doi.org/10.3390/cancers13040688
Chicago/Turabian StyleMaarof, Nian N. N., Abdulsamad Alsalahi, Emilia Abdulmalek, Sharida Fakurazi, Bimo Ario Tejo, and Mohd Basyaruddin Abdul Rahman. 2021. "Efficacy of Afatinib in the Treatment of Patients with Non-Small Cell Lung Cancer and Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis" Cancers 13, no. 4: 688. https://doi.org/10.3390/cancers13040688
APA StyleMaarof, N. N. N., Alsalahi, A., Abdulmalek, E., Fakurazi, S., Tejo, B. A., & Abdul Rahman, M. B. (2021). Efficacy of Afatinib in the Treatment of Patients with Non-Small Cell Lung Cancer and Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers, 13(4), 688. https://doi.org/10.3390/cancers13040688







