The Prognostic Value of the New Combined Hemo-Eosinophil Inflammation Index (HEI Index): A Multicenter Analysis of Anal Cancer Patients Treated with Concurrent Chemo-Radiation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Statistical Analysis
3. Results
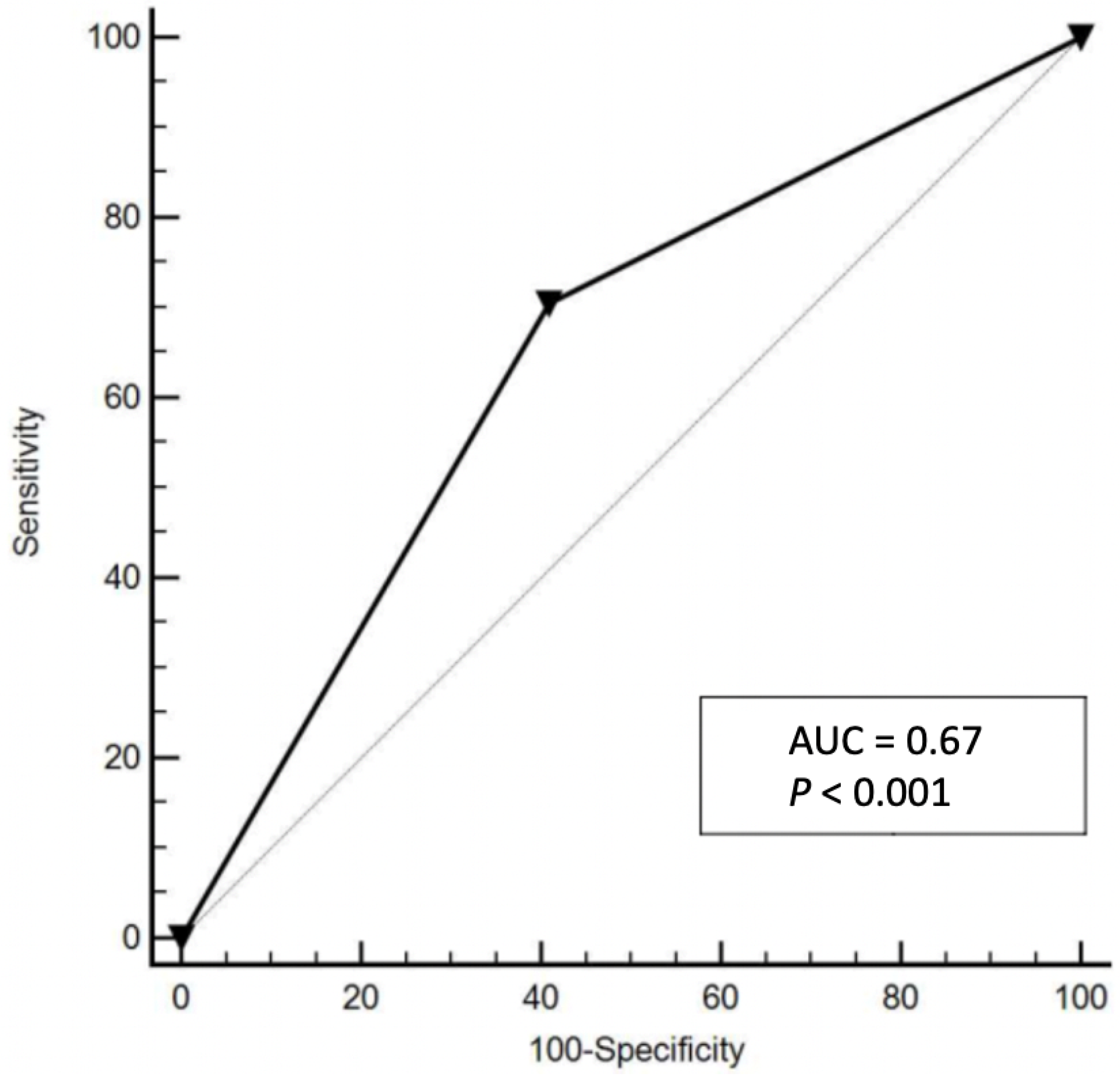
3.1. The Hemo-Eosinophil Inflammation Index
3.2. Univariate and Multivariate Analysis
3.3. Toxicities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- UKCCCR Anal cancer Trial Working Party. UK Co-ordination Committee on Cancer Research: Epidermoid anal cancer: Results from the UKCCCR randomized trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. Lancet 1996, 348, 1049–1054. [Google Scholar] [CrossRef]
- Bartelink, H.; Roelofsen, F.; Eschwege, F.; Rougier, P.; Bosset, J.F.; Gonzalez, D.G.; Peiffert, D.; Van Glabbeke, M.; Pierart, M. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: Results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J. Clin. Oncol. 1997, 15, 2040–2049. [Google Scholar] [CrossRef]
- Flam, M.; John, M.; Pajak, T.F.; Petrelli, N.; Myerson, R.; Doggett, S.; Quivey, J.; Rotman, M.; Kerman, H.; Coia, L.; et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: Results of a phase III randomized intergroup study. J. Clin. Oncol. 1996, 14, 2527–2539. [Google Scholar] [CrossRef]
- Ajani, J.A.; Winter, K.A.; Gunderson, L.L.; Pedersen, J.; Benson, A.B., 3rd; Thomas, C.R., Jr.; Mayer, R.J.; Haddock, M.G.; Rich, T.A.; Willet, C. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and ra-diotherapy for carcinoma of the anal canal: A randomized controlled trial. JAMA 2008, 299, 1914–1921. [Google Scholar] [CrossRef]
- James, R.D.; Glynne-Jones, R.; Meadows, H.M.; Cunningham, D.; Mynt, A.S.; Saunders, M.P.; Maughan, T.; McDonald, A.; Essapen, S.; Leslie, M.; et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACTII): A randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol. 2013, 14, 516–524. [Google Scholar] [CrossRef]
- Glynne-Jones, R.; Sebag-Montefiore, D.; Adams, R.; Gollins, S.; Harrison, M.; Meadows, H.M.; Jitlal, M. Prognostic factors for recurrence and survival in anal cancer: Generating hypotheses from the mature outcomes of the first Coordinating Committee on Cancer Research Anal Cancer Trial Working Party Prognostic factors for recurrence and survival in anal cancer. Cancer 2012, 119, 748–755. [Google Scholar] [CrossRef]
- Franco, P.; Montagnani, F.; Arcadipane, F.; Casadei, C.; Andrikou, K.; Martini, S.; Iorio, G.C.; Scartozzi, M.; Mistrangelo, M.; Fornaro, L.; et al. The prognostic role of hemoglobin levels in patients undergoing concurrent chemo-radiation for anal cancer. Radiat. Oncol. 2018, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Serup-Hansen, E.; Linnemann, D.; Skovrider-Ruminski, W.; Høgdall, E.V.; Geertsen, P.F.; Havsteen, H. Human Papillomavirus Genotyping and p16 Expression As Prognostic Factors for Patients With American Joint Committee on Cancer Stages I to III Carcinoma of the Anal Canal. J. Clin. Oncol. 2014, 32, 1812–1817. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; Winter, K.A.; Gunderson, L.L.; Pedersen, J.; Benson, A.B.; Thomas, C.R.; Mayer, R.J.; Haddock, M.G.; Rich, T.A.; Willett, C.G. Prognostic factors derived from a prospective database dictate clinical biology of anal cancer. Cancer 2010, 116, 4007–4013. [Google Scholar] [CrossRef] [PubMed]
- Gardini, A.C.; Scarpi, E.; Faloppi, L.; Scartozzi, M.; Silvestris, N.; Santini, D.; De Stefano, G.; Marisi, G.; Negri, F.V.; Foschi, F.G.; et al. Immune inflammation indicators and implication for immune modulation strategies in advanced hepatocellular carcinoma patients receiving sorafenib. Oncotarget 2016, 7, 67142–67149. [Google Scholar] [CrossRef]
- Bruix, J.; Cheng, A.-L.; Meinhardt, G.; Nakajima, K.; De Sanctis, Y.; Llovet, J.M. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J. Hepatol. 2017, 67, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Casadei-Gardini, A.; Scarpi, E.; Ulivi, P.; Palladino, M.A.; Accettura, C.; Bernardini, I.; Spallanzani, A.; Gelsomino, F.; Corbelli, J.; Marisi, G.; et al. Erratum: Prognostic Role of a New Inflammatory Index with Neutrophil-to-Lymphocyte Ratio and Lactate Dehydrogenase (CII: Colon Inflammatory Index) in Patients with Metastatic Colorectal Cancer: Results from the Randomized Italian Trial in Advanced Colorectal Cancer (ITACa) Study [Corrigendum]. Cancer Manag. Res. 2020, 12, 541–542. [Google Scholar] [CrossRef]
- Casadei-Gardini, A.; Montagnani, F.; Casadei, C.; Arcadipane, F.; Andrikou, K.; Aloi, D.; Prete, A.A.; Zampino, M.G.; Argentiero, A.; Pugliese, G.; et al. Immune inflammation indicators in anal cancer patients treated with concurrent chemoradiation: Training and validation cohort with online calculator (ARC: Anal Cancer Response Classifier). Cancer Manag. Res. 2019, 11, 3631–3642. [Google Scholar] [CrossRef] [PubMed]
- Olivero, F.; Franco, P.; Ferreri, F.; Andrikou, K.; Arcadipane, F.; De Luca, V.; Gastino, A.; Ricardi, U.; Cascinu, S.; Casadei-Gardini, A. Prognostic value of eosinophil levels in oropharingeal and anal cancer: A retrospective multicentric study. ESTRO 2020, PO-0806. [Google Scholar]
- AJCC Cancer Staging Manual-7th Edition. Available online: www.cancerstaging.org (accessed on 24 January 2021).
- Franco, P.; Arcadipane, F.; Ragona, R.; Mistrangelo, D.M.; Cassoni, P.; Munoz, F.; Rondi, N.; Morino, M.; Racca, P.; Ricardi, U. Volumetric modulated arc therapy (VMAT) in the combined modality treatment of anal cancer patients. Br. J. Radiol. 2016, 89, 20150832. [Google Scholar] [CrossRef]
- Arcadipane, F.; Franco, P.; Ceccarelli, M.; Furfaro, G.; Rondi, N.; Trino, E.; Martini, S.; Iorio, G.C.; Mistrangelo, M.; Cassoni, P.; et al. Image-guided IMRT with simultaneous integrated boost as per RTOG 0529 for the treatment of anal cancer. Asia-Pac. J. Clin. Oncol. 2018, 14, 217–223. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Available online: www.evs.nci.nih.gov (accessed on 12 September 2020).
- Feng, J.-F.; Chen, S.; Yang, X. Systemic immune-inflammation index (SII) is a useful prognostic indicator for patients with squamous cell carcinoma of the esophagus. Medicine 2017, 96, e5886. [Google Scholar] [CrossRef]
- Hong, X.; Cui, B.; Wang, M.; Yang, Z.; Wang, L.; Xu, Q. Systemic Immune-inflammation Index, Based on Platelet Counts and Neutrophil-Lymphocyte Ratio, Is Useful for Predicting Prognosis in Small Cell Lung Cancer. Tohoku J. Exp. Med. 2015, 236, 297–304. [Google Scholar] [CrossRef]
- Diakos, C.; A Charles, K.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Schernberg, A.; Escande, A.; Del Campo, E.R.; Ducreux, M.; Nguyen, F.; Goere, D.; Chargari, C.; Deutsch, E. Leukocytosis and neutrophilia predicts outcome in anal cancer. Radiother. Oncol. 2017, 122, 137–145. [Google Scholar] [CrossRef]
- Schernberg, A.; Huguet, F.; Moureau-Zabotto, L.; Chargari, C.; Del Campo, E.R.; Schlienger, M.; Escande, A.; Touboul, E.; Deutsch, E. External validation of leukocytosis and neutrophilia as a prognostic marker in anal carcinoma treated with definitive chemoradiation. Radiother. Oncol. 2017, 124, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Seruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocana, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Shi, L.; Wang, B.; Yang, J.; Xiao, Z.; Du, P.; Wang, Q.; Yang, W. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: A systematic review and meta-analysis of 66 cohort studies. Cancer Treat. Rev. 2017, 58, 1–13. [Google Scholar] [CrossRef]
- Toh, E.; Wilson, J.; Sebag-Montefiore, D.; Botterill, I. Neutrophil:lymphocyteratio as a simple and novel biomarker for pre-diction of locoregionalrecurrence after chemoradiotherapy for squamous cell carcinoma of the anus. Colorectal Dis. 2014, 16, O90–O97. [Google Scholar] [CrossRef]
- De Felice, F.; Rubini, F.L.; Romano, L.; Bulzonetti, N.; Caiazzo, R.; Musio, D.; Tombolini, V. Prognostic significance of inflammatory-related parameters in patients with anal canal cancer. Int. J. Color. Dis. 2019, 34, 519–525. [Google Scholar] [CrossRef]
- Knight, K.; Choong, J.; McKee, R.; Anderson, J.; Horgan, P.; McMillan, D.; McDonald, A.; Roxburgh, C. The Influence of Systemic Inflammation on Treatment Response and Survival in Anal Squamous Cell Cancer. Clin. Oncol. 2021, 33, e22–e30. [Google Scholar] [CrossRef]
- Roldán, G.B.; Chan, A.K.P.; Buckner, M.; Magliocco, A.M.; Doll, C.M. The Prognostic Value of Hemoglobin in Patients With Anal Cancer Treated With Chemoradiotherapy. Dis. Colon Rectum 2010, 53, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Kapacee, Z.A.; Susnerwala, S.; Wise, M.; Biswas, A.; Danwata, F.; Scott, N. Chemoradiotherapy for squamous cell anal carcinoma: A review of prognostic factors. Color. Dis. 2016, 18, 1080–1086. [Google Scholar] [CrossRef]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Lucarini, V.; Marone, G.; Mattei, F.; Marone, G.; Schiavoni, G. Eosinophils: The unsung heroes in cancer? OncoImmunology 2018, 7, e1393134. [Google Scholar] [CrossRef]
- Zahoor, H.; Barata, P.C.; Jia, X.; Martin, A.; Allman, K.D.; Wood, L.S.; Gilligan, T.D.; Grivas, P.; Ornstein, M.C.; Garcia, J.A.; et al. Patterns, predictors and subsequent outcomes of disease progression in metastatic renal cell carcinoma patients treated with nivolumab. J. Immunother. Cancer 2018, 6, 107. [Google Scholar] [CrossRef]
- Rosner, S.; Kwong, E.; Shoushtari, A.N.; Friedman, C.F.; Betof, A.S.; Brady, M.S.; Coit, D.G.; Callahan, M.K.; Wolchok, J.D.; Chapman, P.B.; et al. Peripheral blood clinical laboratory variables associated with outcomes following combination nivolumab and ipilimumab immunotherapy in melanoma. Cancer Med. 2018, 7, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, X.; Wang, G.; Zhou, Y.; Luo, M.; Wang, S.; Hong, C. The impacts of pretreatment circulating eosinophils and basophils on prognosis of stage Ⅰ-Ⅲ colorectal cancer. Asia-Pac. J. Clin. Oncol. 2018, 14, e243–e251. [Google Scholar] [CrossRef] [PubMed]
- Tanizaki, J.; Haratani, K.; Hayashi, H.; Chiba, Y.; Nakamura, Y.; Yonesaka, K.; Kudo, K.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; et al. Peripheral Blood Biomarkers Associated with Clinical Outcome in Non–Small Cell Lung Cancer Patients Treated with Nivolumab. J. Thorac. Oncol. 2018, 13, 97–105. [Google Scholar] [CrossRef]
- Holub, K.; Biete, A. Impact of systemic inflammation biomarkers on the survival outcomes of cervical cancer patients. Clin. Transl. Oncol. 2018, 21, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Holub, K.; Conill, C. Unveiling the mechanisms of immune evasion in pancreatic cancer: May it be a systemic inflammation responsible for dismal survival? Clin. Transl. Oncol. 2019, 22, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Ownby, H.E.; Roi, L.D.; Isenberg, R.R.; Brennan, M.J. Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast cancer. Cancer 2006, 52, 126–130. [Google Scholar] [CrossRef]
- Utsunomiya, A.; Ishida, T.; Inagaki, A.; Ishii, T.; Yano, H.; Komatsu, H.; Iida, S.; Yonekura, K.; Takeuchi, S.; Takatsuka, Y.; et al. Clinical significance of a blood eosinophilia in adult T-cell leukemia/lymphoma: A blood eosinophilia is a significant unfavorable prognostic factor. Leuk. Res. 2007, 31, 915–920. [Google Scholar] [CrossRef]
- Hude, I.; Sasse, S.; Bröckelmann, P.J.; Von Tresckow, B.; Momotow, J.; Engert, A.; Borchmann, S. Leucocyte and eosinophil counts predict progression-free survival in relapsed or refractory classical Hodgkin Lymphoma patients treated with PD1 inhibition. Br. J. Haematol. 2018, 181, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Orsi, G.; Tovoli, F.; Dadduzio, V.; Vivaldi, C.; Brunetti, O.; Ielasi, L.; Conti, F.; Rovesti, G.; Gramantieri, L.; Rizzato, M.D.; et al. Prognostic role of blood eosinophil count in sorafenib-treated hepatocellular carcinoma patients: Time to reconsider the minorities. Target Oncol. 2020, 15, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Daling, J.R.; Madeleine, M.M.; Johnson, L.G.; Schwartz, S.M.; Shera, K.A.; Wurscher, M.A.; Carter, J.J.; Porter, P.L.; Galloway, D.A.; McDougall, J.K. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer 2004, 101, 270–280. [Google Scholar] [CrossRef]
- Frisch, M.; Glimelius, B.; Brule, A.J.V.D.; Wohlfahrt, J.; Meijer, C.J.; Walboomers, J.M.; Goldman, S.; Svensson, C.; Adami, H.-O.; Melbye, M. Sexually Transmitted Infection as a Cause of Anal Cancer. N. Engl. J. Med. 1997, 337, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Carretero, R.; Sektioglu, I.M.; Garbi, N.; Salgado, O.C.; Beckhove, P.; Hämmerling, G.J. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8+ T cells. Nat. Immunol. 2015, 16, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Winter, O.; Hartig, C.; Siebels, S.; Szyska, M.; Tiburzy, B.; Meng, L.; Kulkarni, U.; Fähnrich, A.; Bommert, K.; et al. Eosinophils and Megakaryocytes Support the Early Growth of Murine MOPC315 Myeloma Cells in Their Bone Marrow Niches. PLoS ONE 2014, 9, e109018. [Google Scholar] [CrossRef]
- Ndiaye, C.; Mena, M.; Alemany, L.; Arbyn, M.; Castellsagué, X.; Laporte, L.; Bosch, F.X.; De Sanjosé, S.; Trottier, H. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: A systematic review and meta-analysis. Lancet Oncol. 2014, 15, 1319–1331. [Google Scholar] [CrossRef]
- Bonomi, M.; Patsias, A.; Posner, M.; Sikora, A. The Role of Inflammation in Head and Neck Cancer. Adv. Exp. Med. Biol. 2014, 816, 107–127. [Google Scholar] [CrossRef]
- Franco, P.; Ragona, R.; Arcadipane, F.; Mistrangelo, M.; Cassoni, P.; Rondi, N.; Morino, M.; Racca, P.; Ricardi, U. Dosimetric predictors of acute hematologic tocixity during concurrent intensity-modulated radiotherapy and chemotherapy in anal cancer. Clin Transl Oncol. 2017, 19, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Arcadipane, F.; Silvetti, P.; Olivero, F.; Gastino, A.; De Luca, V.; Mistrangelo, M.; Cassoni, P.; Racca, P.; Gallio, E.; Lesca, A.; et al. Bone marrow-sparing IMRT in anal cancer patients undergoing concurrent chemo-radiation: Results of the first phase of a prospective phase II trial. Cancers 2020, 12, 3306. [Google Scholar] [CrossRef]
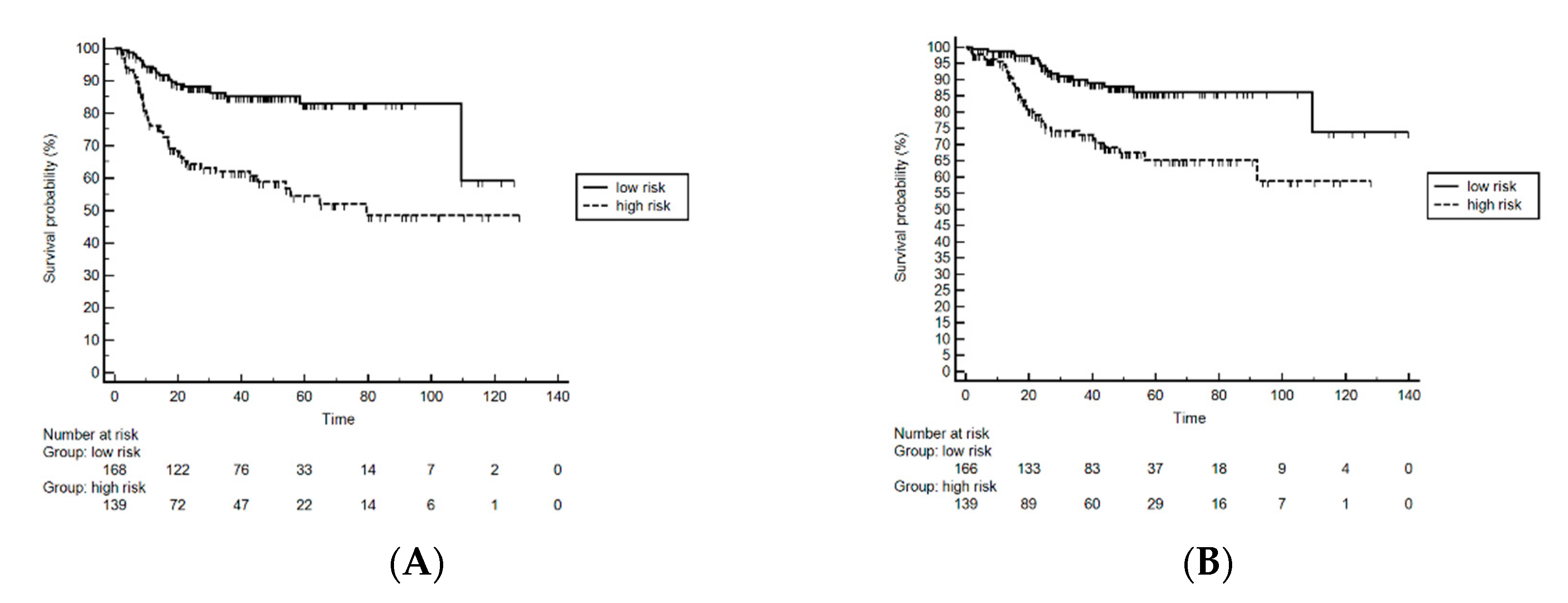
| Variable | N (%) |
|---|---|
| Age | - |
| Median | 64 |
| Range | 33–92 |
| Gender | - |
| Female | 231 (75.0) |
| Male | 77 (25.0) |
| T-stage | - |
| T1–T2 | 187 (61) |
| T3–T4 | 120 (38.5) |
| Not available | 1 (0.5) |
| N-stage | - |
| N0 | 151 (49.0) |
| N1–N3 | 155 (50.3) |
| NA | 2 (0.7) |
| Global Stage | - |
| I–II | 142 (46) |
| III | 165 (53.5) |
| Not available | 1 (0.5) |
| Grading | - |
| 1–2 | 164 (53.2) |
| 3 | 77 (25.3) |
| Not available | 67 (21.5) |
| Pre-treatment Hb (g/dL) | - |
| Mean ± SD | 13 ± 1.6 |
| Hb < 12 g/dL | 251 (81.5) |
| Hb ≥ 12 g/dL | 57 (18.5) |
| Pre-treatment SII | - |
| Mean ± SD | 779 ± 681 |
| Pre-treatment Eosinophil count (103 cells/µL) | - |
| Mean ± SD | 181 ± 162 |
| Variable | Low Risk n (%) | High Risk n (%) | p |
|---|---|---|---|
| Gender Male Female | - 45 (27) 123 (73) | - 32 (23) 108 (77) | - 0.51 |
| Age <70 ≥70 | - 83 (49) 85 (51) | - 62 (44) 73 (56) | - 0.56 - |
| T-stage T3–T4 T1–T2 | - 47 (29) 120 (71) | - 73 (52) 67 (48) | - <0.001 |
| n-stage N1–3 N0 | - 17 (10) 24 (14) | - 16 (11) 11 (8) | - 0.22 |
| Global Stage I–II III | - 98 (57) 72 (43) | - 46 (32) 95 (68) | <0.001 |
| Grading 1–2 3 | - 87 (52) 51 (30) | - 46 (33) 16 (11) | - 0.15 |
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| - | HR (95% CI) | p | HR (95% CI) | p |
| Age (≥70 vs. <70) | 0.89 (0.56–1.40) | 0.6031 | 2.25 (1.19–4.26) | 0.0120 |
| Gender (Male vs. Female) | 1.20 (0.70–2.04) | 0.5136 | 1.19 (1.43–4.72) | 0.5711 |
| Chemotherapy (CCDP-based vs. MMC-based) | 0.58 (0.33–0.99) | 0.0458 | 0.34 (0.15–0.76) | 0.0092 |
| HB (< 12 vs. ≥12 g/dL) | 2.30 (1.21–4.37) | 0.0102 | - | - |
| SII (>560 vs. ≤560) | 1.97 (1.26–3.10) | 0.0031 | - | - |
| Eosinophil (≥100 vs. <100/µL) | 1.82 (1.07–3.09) | 0.0265 | - | - |
| Nodal status (N3 vs. N0–N2) | 3.06 (1.50–6.24) | 0.0021 | - | - |
| T stage (T4 vs. T1–T3) | 3.06 (1.50–6.25) | 0.0021 | - | - |
| Stage (III vs. I–II) | 1.95 (1.24–3.06) | 0.0036 | 1.39 (0.76–2.54) | 0.2836 |
| HEI Index (High Risk vs. Low Risk) | 3.22 (2.04–5.10) | <0.0001 | 2.59 (1.42–4.72) | <0.0001 |
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| - | HR (95% CI) | p | HR (95% CI) | p |
| Age (≥70 vs. <70) | 1.05 (0.61–1.80) | 0.8737 | 1.92 (0.88–4.16) | 0.0972 |
| Gender (Male vs. Female) | 3.34 (1.75–6.36) | 0.0002 | 1.79 (0.89–3.58) | 0.010 |
| Chemotherapy (CCDP-based vs. MMC-based) | 0.47 (0.25–0.90) | 0.0237 | 0.25 (0.08–0.79) | 0.0186 |
| HB (<12 vs. ≥12 g/dL) | 6.68 (3.10–14.41) | <0.0001 | - | - |
| SII (>560 vs. ≤560) | 2.11 (1.24–3.60) | 0.0062 | - | - |
| Eosinophil (≥100 vs. <100/µL) | 1.44 (0.76–2.71) | 0.2612 | - | - |
| Nodal status (N3 vs. N0–N2) | 1.80 (1.06–3.08) | 0.0309 | - | - |
| T status (T4 vs. T1–T3) | 2.06 (0.92–4.63) | 0.0788 | - | - |
| Stage (III vs. I–II) | 2.02 (1.18–3.46) | 0.0108 | 1.97 (0.87–4.42) | 0.1018 |
| HEI Index (High Risk vs. Low Risk) | 3.01 (1.75–5.17) | 0.0001 | 2.97 (1.36–6.50) | 0.0063 |
| Toxicity (Any Grade) | Low Risk n (%) | High Risk n (%) | p |
|---|---|---|---|
| Overall Toxicities Yes No | - 158 (94) 10 (6) | - 125 (89) 15 (11) | - 0.15 |
| Hematological | |||
| Neutropenia Yes No | - 72 (43) 96 (57) | - 56 (40) 84 (60) | - 0.64 |
| Thrombocytopenia Yes No | - 48 (29) 120 (71) | - 31 (22) 109 (78) | - 0.24 |
| Anemia Yes No | - 41 (24) 127 (76) | - 47 (34) 93 (66) | - 0.1 |
| Non-Hematological | |||
| Genitourinary Yes No | - 92 (55) 76 (45) | - 61 (44) 79 (56) | - 0.05 |
| Gastrointestinal Yes No | - 55 (33) 113 (67) | - 44 (31) 96 (69) | - 0.9 |
| Skin Yes No | - 131 (78) 37 (22) | - 88 (63) 52 (37) | - 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rimini, M.; Franco, P.; De Bari, B.; Zampino, M.G.; Vagge, S.; Frassinetti, G.L.; Arcadipane, F.; Bacigalupo, A.; Valgiusti, M.; Aloi, D.; et al. The Prognostic Value of the New Combined Hemo-Eosinophil Inflammation Index (HEI Index): A Multicenter Analysis of Anal Cancer Patients Treated with Concurrent Chemo-Radiation. Cancers 2021, 13, 671. https://doi.org/10.3390/cancers13040671
Rimini M, Franco P, De Bari B, Zampino MG, Vagge S, Frassinetti GL, Arcadipane F, Bacigalupo A, Valgiusti M, Aloi D, et al. The Prognostic Value of the New Combined Hemo-Eosinophil Inflammation Index (HEI Index): A Multicenter Analysis of Anal Cancer Patients Treated with Concurrent Chemo-Radiation. Cancers. 2021; 13(4):671. https://doi.org/10.3390/cancers13040671
Chicago/Turabian StyleRimini, Margherita, Pierfrancesco Franco, Berardino De Bari, Maria Giulia Zampino, Stefano Vagge, Giovanni Luca Frassinetti, Francesca Arcadipane, Almalina Bacigalupo, Martina Valgiusti, Deborah Aloi, and et al. 2021. "The Prognostic Value of the New Combined Hemo-Eosinophil Inflammation Index (HEI Index): A Multicenter Analysis of Anal Cancer Patients Treated with Concurrent Chemo-Radiation" Cancers 13, no. 4: 671. https://doi.org/10.3390/cancers13040671
APA StyleRimini, M., Franco, P., De Bari, B., Zampino, M. G., Vagge, S., Frassinetti, G. L., Arcadipane, F., Bacigalupo, A., Valgiusti, M., Aloi, D., Gervaso, L., Corvò, R., Bartolini, G., Gerardi, M. A., Cascinu, S., & Casadei-Gardini, A. (2021). The Prognostic Value of the New Combined Hemo-Eosinophil Inflammation Index (HEI Index): A Multicenter Analysis of Anal Cancer Patients Treated with Concurrent Chemo-Radiation. Cancers, 13(4), 671. https://doi.org/10.3390/cancers13040671








