Deep Learning for the Preoperative Diagnosis of Metastatic Cervical Lymph Nodes on Contrast-Enhanced Computed ToMography in Patients with Oral Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
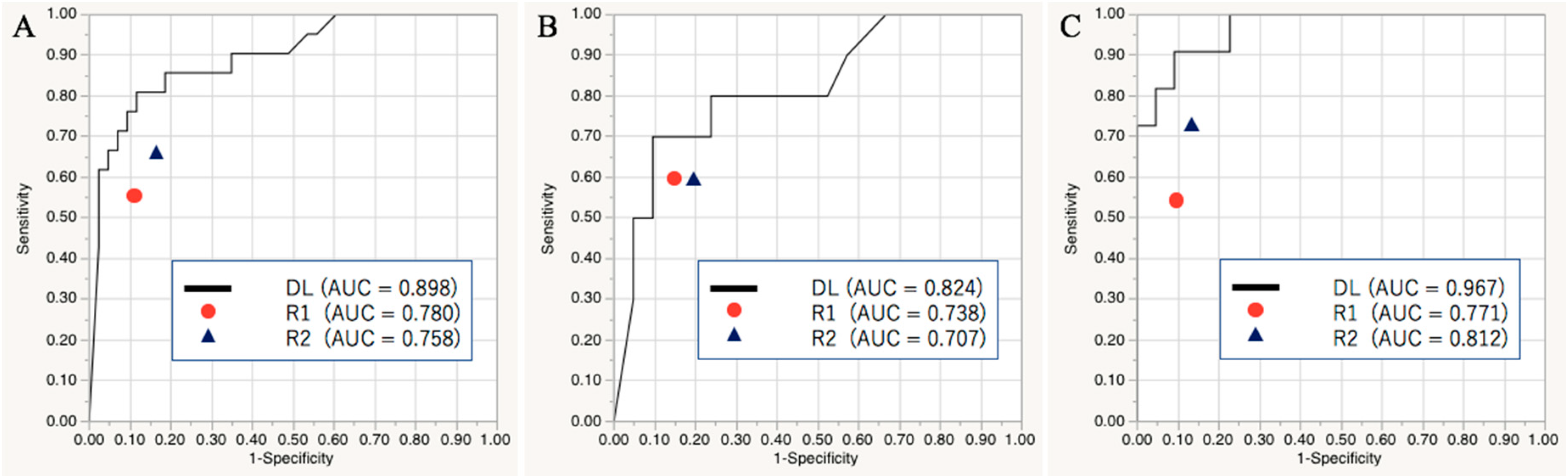
2.1. Diagnostic Performance of the Deep Learning Model in the Validation and Test Sets
2.2. Diagnostic Performance of the Readers in the Test Set
3. Discussion
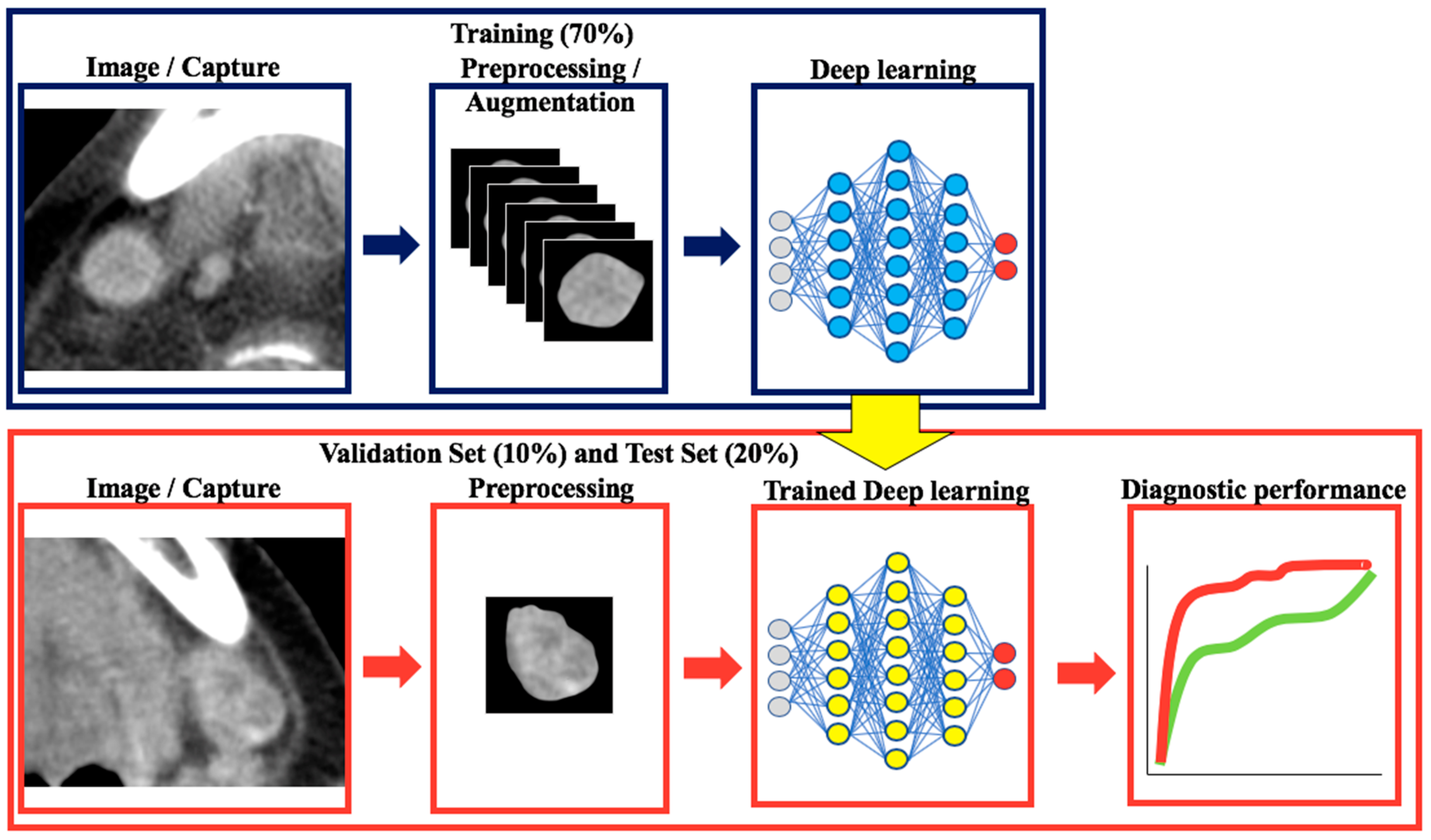
4. Materials and Methods
4.1. Ethical Statement
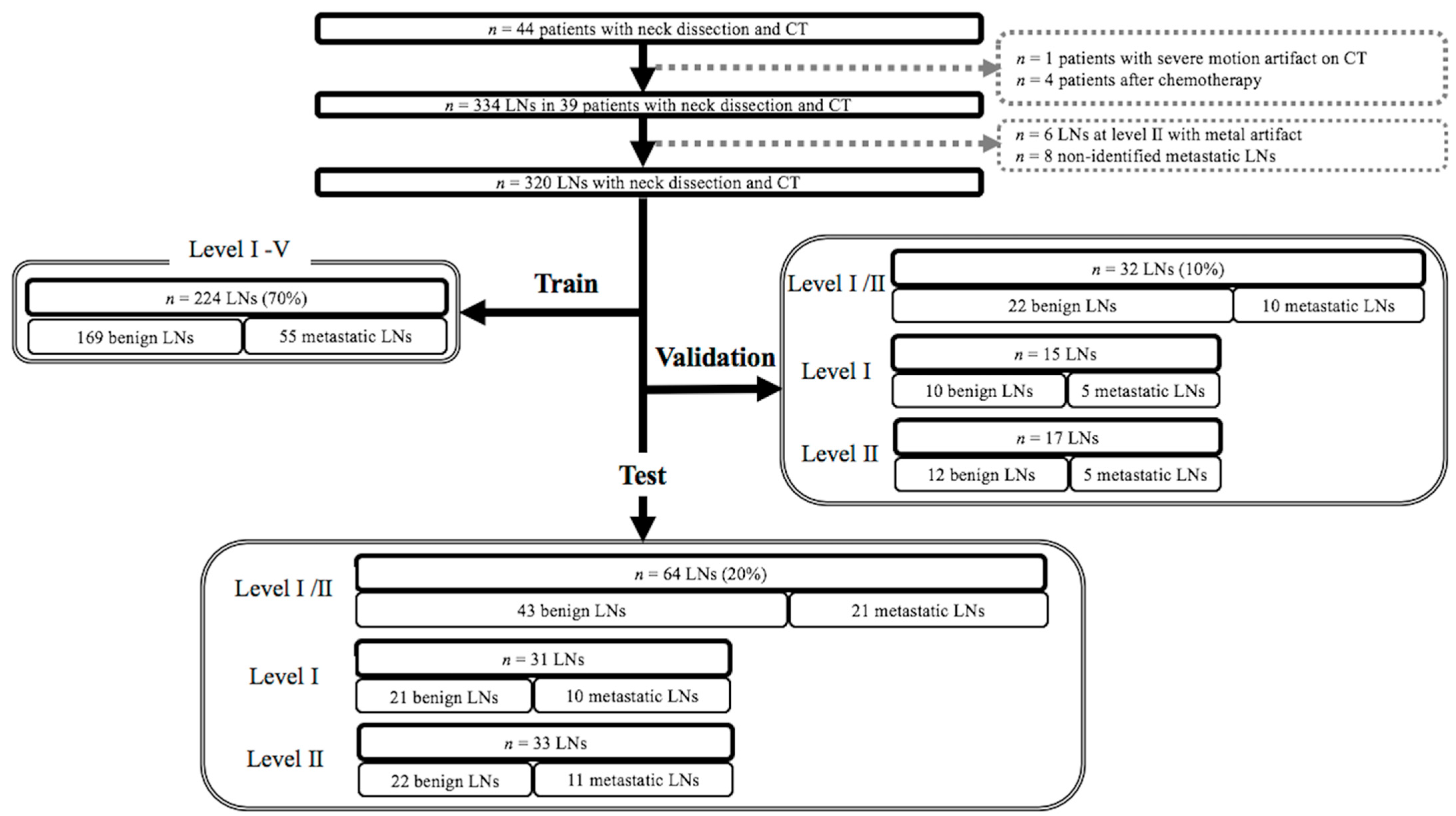
4.2. Subjects
4.3. Computed Tomography
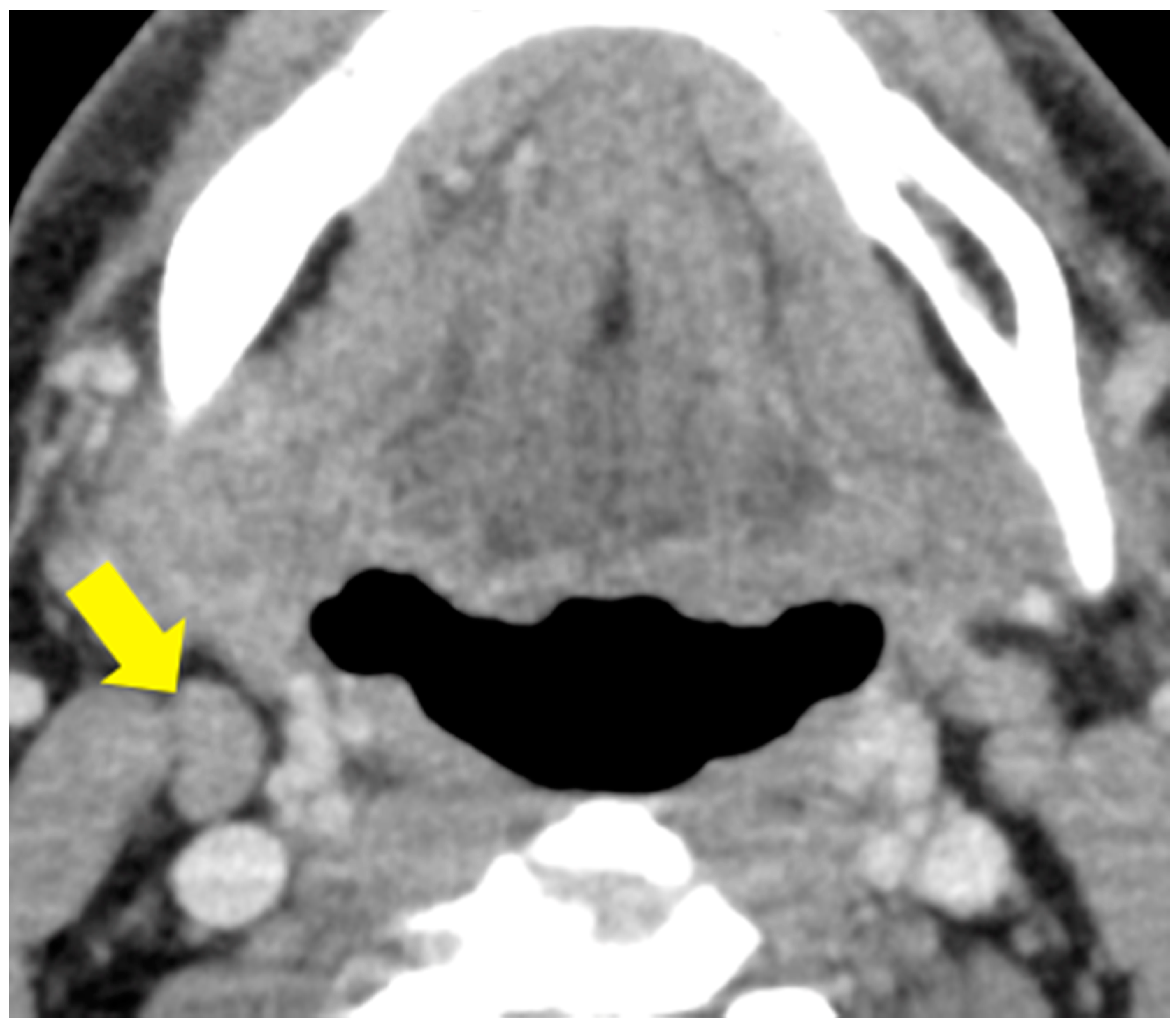
4.4. Labeling of Cervical Lymph Nodes and Targeted Lymph Node
4.5. Image Preprocessing for Deep Learning
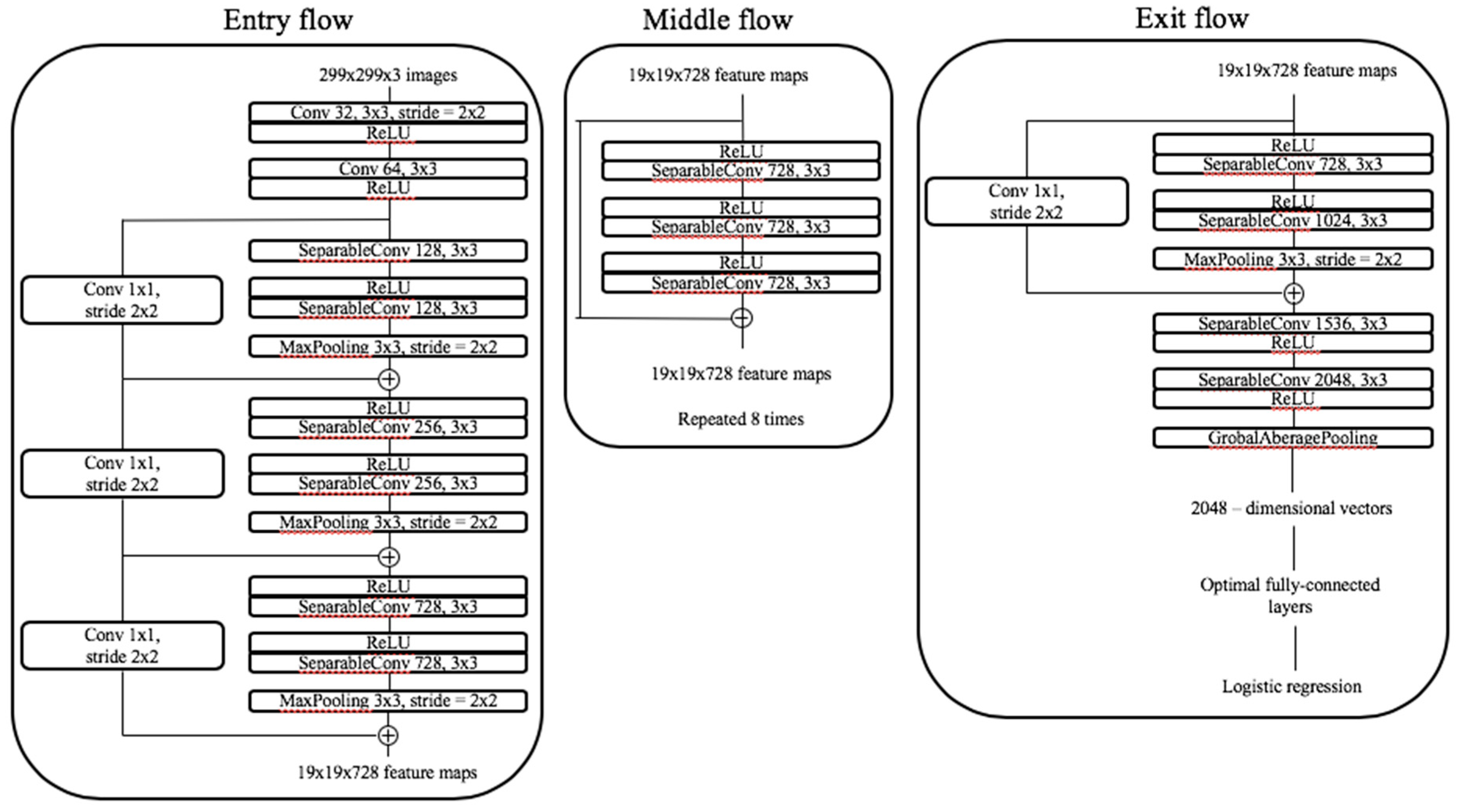
4.6. Classification with Convolutional Neural Networks and Transfer Learning
4.7. Visual Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Roh, J.L.; Park, J.P.; Kim, J.S.; Lee, J.H.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. 18F Fluorodeoxyglucose PET/CT in Head and Neck Squamous Cell Carcinoma with Negative Neck Palpation Findings: A Prospective Study. Radiology 2014, 271, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Park, J.T.; Roh, J.L.; Kim, J.S.; Lee, J.H.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. (18)F FDG PET/CT Versus CT/MR Imaging and the Prognostic Value of Contralateral Neck Metastases in Patients with Head and Neck Squamous Cell Carcinoma. Radiology 2016, 279, 481–491. [Google Scholar] [CrossRef]
- Shin, N.Y.; Lee, J.H.; Kang, W.J.; Koh, Y.W.; Sohn, B.; Kim, J. Clinical Usefulness of [18F]FDG PET-CT and CT/MRI for Detecting Nodal Metastasis in Patients with Hypopharyngeal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2015, 22, 994–999. [Google Scholar] [CrossRef]
- Sumi, M.; Ohki, M.; Nakamura, T. Comparison of Sonography and CT for Differentiating Benign from Malignant Cervical Lymph Nodes in Patients with Squamous Cell Carcinoma of the Head and Neck. Am. J. Roentgenol. 2001, 176, 1019–1024. [Google Scholar] [CrossRef]
- Foust, A.M.; Ali, R.M.; Nguyen, X.V.; Agrawal, A.; Prevedello, L.M.; Bourekas, E.C.; Boulter, D.J. Dual-Energy CT-Derived Iodine Content and Spectral Attenuation Analysis of Metastatic Versus Nonmetastatic Lymph Nodes in Squamous Cell Carcinoma of the Oropharynx. Tomography 2018, 4, 66–71. [Google Scholar]
- Liu, X.; Ouyang, D.; Li, H.; Zhang, R.; Lv, Y.; Yang, A.; Xie, C. Papillary Thyroid Cancer: Dual-Energy Spectral CT Quantitative Parameters for Preoperative Diagnosis of Metastasis to the Cervical Lymph Nodes. Radiology 2015, 275, 167–176. [Google Scholar] [CrossRef]
- Takamochi, K.; Yoshida, J.; Murakami, K.; Niho, S.; Ishii, G.; Nishimura, M.; Nishiwaki, Y.; Suzuki, K.; Nagai, K. Pitfalls in Lymph Node Staging with Positron Emission Tomography in Non-Small Cell Lung Cancer Patients. Lung Cancer 2005, 47, 235–242. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Saitoh, M.; Notani, K.; Tei, K.; Totsuka, Y.; Takinami, S.; Kanegae, K.; Inubushi, M.; Tamaki, N.; Kitagawa, Y. Assessment of Cervical Lymph Node Metastases using FDG-PET in Patients with Head and Neck Cancer. Ann. Nucl. Med. 2008, 22, 177–184. [Google Scholar] [CrossRef]
- Mahieu, R.; de Maar, J.S.; Nieuwenhuis, E.R.; Deckers, R.; Moonen, C.; Alic, L.; Ten Haken, B.; de Keizer, B.; Bree, R. New Developments in Imaging for Sentinel Lymph Node Biopsy in Early-Stage Oral Cavity Squamous Cell Carcinoma. Cancers 2020, 12, 3055. [Google Scholar] [CrossRef]
- den Toom, I.J.; Boeve, K.; Lobeek, D.; Bloemena, E.; Donswijk, M.L.; de Keizer, B.; Klop, W.M.C.; Leemans, C.R.; Willems, S.M.; Takes, R.P.; et al. Elective Neck Dissection Or Sentinel Lymph Node Biopsy in Early Stage Oral Cavity Cancer Patients: The Dutch Experience. Cancers 2020, 12, 1783. [Google Scholar] [CrossRef]
- Yanagawa, M.; Niioka, H.; Hata, A.; Kikuchi, N.; Honda, O.; Kurakami, H.; Morii, E.; Noguchi, M.; Watanabe, Y.; Miyake, J.; et al. Application of Deep Learning (3-Dimensional Convolutional Neural Network) for the Prediction of Pathological Invasiveness in Lung Adenocarcinoma: A Preliminary Study. Medicine 2019, 98, e16119. [Google Scholar] [CrossRef]
- Peng, J.; Kang, S.; Ning, Z.; Deng, H.; Shen, J.; Xu, Y.; Zhang, J.; Zhao, W.; Li, X.; Gong, W.; et al. Residual Convolutional Neural Network for Predicting Response of Transarterial Chemoembolization in Hepatocellular Carcinoma from CT Imaging. Eur. Radiol. 2019, 30, 413–424. [Google Scholar] [CrossRef]
- Yasaka, K.; Akai, H.; Abe, O.; Kiryu, S. Deep Learning with Convolutional Neural Network for Differentiation of Liver Masses at Dynamic Contrast-Enhanced CT: A Preliminary Study. Radiology 2018, 286, 887–896. [Google Scholar] [CrossRef]
- Aldoj, N.; Lukas, S.; Dewey, M.; Penzkofer, T. Semi-Automatic Classification of Prostate Cancer on Multi-Parametric MR Imaging using a Multi-Channel 3D Convolutional Neural Network. Eur. Radiol. 2019, 30, 1243–1253. [Google Scholar] [CrossRef]
- Zhuge, Y.; Krauze, A.V.; Ning, H.; Cheng, J.Y.; Arora, B.C.; Camphausen, K.; Miller, R.W. Brain Tumor Segmentation using Holistically Nested Neural Networks in MRI Images. Med. Phys. 2017, 44, 5234–5243. [Google Scholar] [CrossRef] [PubMed]
- Ariji, Y.; Fukuda, M.; Kise, Y.; Nozawa, M.; Yanashita, Y.; Fujita, H.; Katsumata, A.; Ariji, E. Contrast-Enhanced Computed Tomography Image Assessment of Cervical Lymph Node Metastasis in Patients with Oral Cancer by using a Deep Learning System of Artificial Intelligence. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 458–463. [Google Scholar] [CrossRef]
- Kann, B.H.; Aneja, S.; Loganadane, G.V.; Kelly, J.R.; Smith, S.M.; Decker, R.H.; Yu, J.B.; Park, H.S.; Yarbrough, W.G.; Malhotra, A.; et al. Pretreatment Identification of Head and Neck Cancer Nodal Metastasis and Extranodal Extension using Deep Learning Neural Networks. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Kann, B.H.; Hicks, D.F.; Payabvash, S.; Mahajan, A.; Du, J.; Gupta, V.; Park, H.S.; Yu, J.B.; Yarbrough, W.G.; Burtness, B.A.; et al. Multi-Institutional Validation of Deep Learning for Pretreatment Identification of Extranodal Extension in Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2019, 38, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ni, D.; Qin, J.; Li, S.; Yang, X.; Wang, T.; Heng, P.A. Standard Plane Localization in Fetal Ultrasound Via Domain Transferred Deep Neural Networks. IEEE J. Biomed. Health. Inform. 2015, 19, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Ariji, Y.; Sugita, Y.; Nagao, T.; Nakayama, A.; Fukuda, M.; Kise, Y.; Nozawa, M.; Nishiyama, M.; Katumata, A.; Ariji, E. CT Evaluation of Extranodal Extension of Cervical Lymph Node Metastases in Patients with Oral Squamous Cell Carcinoma using Deep Learning Classification. Oral Radiol. 2020, 36, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Pinto, A.; Morales, S.; Naranjo, V.; Kohler, T.; Mossi, J.M.; Navea, A. CNNs for Automatic Glaucoma Assessment using Fundus Images: An Extensive Validation. Biomed. Eng. Online 2019, 18, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.R.; Riaz, N.; McBride, S.; Morris, L.G.; Patel, S.; Ganly, I.; Wong, R.J.; Palmer, F.; Schoder, H.; Lee, N. Postoperative PET/CT and Target Delineation before Adjuvant Radiotherapy in Patients with Oral Cavity Squamous Cell Carcinoma. Head Neck 2016, 38 (Suppl. 1), E1285–E1293. [Google Scholar] [CrossRef] [PubMed]
- Chollet, F. Xception: Deep Learning with Depthwise Separable Convolutions. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Honolulu, HI, USA, 21–26 July 2017; pp. 1251–1258. [Google Scholar]
- Szegedy, C.; Liu, W.; Jia, Y.; Sermanet, P.; Reed, S.; Anguelov, D.; Erhan, D.; Vanhoucke, V.; Rabinovich, A. Going Deeper with Convolutions. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Boston, MA, USA, 7–12 June 2015; pp. 1–9. [Google Scholar]
- Ng, S.H.; Yen, T.C.; Liao, C.T.; Chang, J.T.; Chan, S.C.; Ko, S.F.; Wang, H.M.; Wong, H.F. 18F-FDG PET and CT/MRI in Oral Cavity Squamous Cell Carcinoma: A Prospective Study of 124 Patients with Histologic Correlation. J. Nucl. Med. 2005, 46, 1136–1143. [Google Scholar] [PubMed]
| Characteristics | Patient-Based | |
|---|---|---|
| n = 39 | ||
| Age mean ± SD | 64.0 ± 14.0 | |
| Gender male/female | 23/16 | |
| Primary tumor sites | Oral tongue | 26 |
| Gingiva | 8 | |
| Floor of mouse | 5 | |
| T stage | T1 | 7 |
| T2 | 17 | |
| T3 | 7 | |
| T4 | 8 | |
| N stage | N0 | 7 |
| N1 | 11 | |
| N2a | 0 | |
| N2b | 13 | |
| N2c | 6 | |
| N3a | 0 | |
| N3b | 2 | |
| LevelⅠ | 121 | |
| Ipsilateral LevelⅠ | 84 | |
| LevelⅡ | 132 | |
| Ipsilateral LevelⅡ | 72 | |
| LevelⅢ | 37 | |
| Ipsilateral LevelⅢ | 19 | |
| LevelⅣ | 4 | |
| Ipsilateral LevelⅣ | 4 | |
| LevelⅤ | 26 | |
| Ipsilateral LevelⅤ | 13 | |
| Level | LN-Based | |||||
|---|---|---|---|---|---|---|
| Train Cohort (n = 224) | Validation Cohort (n = 32) | Test Cohort (n = 64) | ||||
| Benign (n = 169) | Metastasis (n = 55) | Benign (n = 22) | Metastasis (n = 10) | Benign (n = 43) | Metastasis (n = 21) | |
| LevelI | 51 | 24 | 10 | 5 | 21 | 10 |
| Ipsilateral LevelI | 29 | 20 | 7 | 5 | 15 | 8 |
| LevelII | 64 | 18 | 12 | 5 | 22 | 11 |
| Ipsilateral LevelII | 26 | 14 | 4 | 5 | 12 | 11 |
| LevelIII | 27 | 10 | - | - | - | - |
| Ipsilateral LevelIII | 12 | 7 | - | - | - | - |
| LevelIV | 1 | 3 | - | - | - | - |
| Ipsilateral LevelIV | 0 | 3 | - | - | - | - |
| LevelV | 26 | 0 | - | - | - | - |
| Ipsilateral LevelV | 13 | 0 | - | - | - | - |
| AUC [95% Confidence Interval] | Accuracy | Sensitivity | Specificity | |
|---|---|---|---|---|
| Level I/II | ||||
| Deep learning | 0.898 [0.778, 0.956] | 85.9 | 66.7 | 95.4 |
| Reader 1 | 0.780 [0.559, 0.864] * | 78.1 | 57.1 | 88.4 |
| Reader 2 | 0.758 [0.587, 0.873] * | 78.1 | 66.7 | 83.7 |
| Level I | ||||
| Deep learning | 0.824 [0.600, 0.936] | 80.6 | 60.0 | 90.5 |
| Reader 1 | 0.738 [0.497, 0.889] | 77.4 | 60.0 | 85.7 |
| Reader 2 | 0.707 [0.443, 0.880] | 74.2 | 60.0 | 81.0 |
| Level II | ||||
| Deep learning | 0.967 [0.854, 0.993] | 90.9 | 72.7 | 100.0 |
| Reader 1 | 0.771 [0.546, 0.904] * | 78.8 | 54.6 | 90.9 |
| Reader 2 | 0.812 [0.574, 0.933] * | 81.8 | 72.7 | 86.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomita, H.; Yamashiro, T.; Heianna, J.; Nakasone, T.; Kobayashi, T.; Mishiro, S.; Hirahara, D.; Takaya, E.; Mimura, H.; Murayama, S.; et al. Deep Learning for the Preoperative Diagnosis of Metastatic Cervical Lymph Nodes on Contrast-Enhanced Computed ToMography in Patients with Oral Squamous Cell Carcinoma. Cancers 2021, 13, 600. https://doi.org/10.3390/cancers13040600
Tomita H, Yamashiro T, Heianna J, Nakasone T, Kobayashi T, Mishiro S, Hirahara D, Takaya E, Mimura H, Murayama S, et al. Deep Learning for the Preoperative Diagnosis of Metastatic Cervical Lymph Nodes on Contrast-Enhanced Computed ToMography in Patients with Oral Squamous Cell Carcinoma. Cancers. 2021; 13(4):600. https://doi.org/10.3390/cancers13040600
Chicago/Turabian StyleTomita, Hayato, Tsuneo Yamashiro, Joichi Heianna, Toshiyuki Nakasone, Tatsuaki Kobayashi, Sono Mishiro, Daisuke Hirahara, Eichi Takaya, Hidefumi Mimura, Sadayuki Murayama, and et al. 2021. "Deep Learning for the Preoperative Diagnosis of Metastatic Cervical Lymph Nodes on Contrast-Enhanced Computed ToMography in Patients with Oral Squamous Cell Carcinoma" Cancers 13, no. 4: 600. https://doi.org/10.3390/cancers13040600
APA StyleTomita, H., Yamashiro, T., Heianna, J., Nakasone, T., Kobayashi, T., Mishiro, S., Hirahara, D., Takaya, E., Mimura, H., Murayama, S., & Kobayashi, Y. (2021). Deep Learning for the Preoperative Diagnosis of Metastatic Cervical Lymph Nodes on Contrast-Enhanced Computed ToMography in Patients with Oral Squamous Cell Carcinoma. Cancers, 13(4), 600. https://doi.org/10.3390/cancers13040600







