1. Introduction
Non-thermal plasma (NTP), also referred to as cold atmospheric plasma, has been emerging as a therapeutic modality against cancer [
1]. NTP, a partially ionized gas generated at atmospheric pressure and room temperature, is composed of several physical and chemical components (e.g., electric fields, charged particles, reactive chemical species) [
2,
3]. It is now well established that the anti-cancer capacity of NTP is derived from the reactive oxygen and nitrogen species (RONS) it generates, including hydrogen peroxide (H
2O
2), nitrite (NO
2−), peroxynitrite (ONOO
−), and other radical species. Reports of NTP inducing apoptosis and necrosis and inhibiting cancer cell growth, without adverse side effects, makes it attractive for cancer therapy.
In recent years, it was proposed that NTP has cancer immunotherapeutic properties via induction of immunogenic cell death (ICD). ICD is a form of cell death that renders tumor cells more detectable to immune cells through emission of damage-associated molecular patterns (DAMPs), such as surface calreticulin (CRT), released high mobility group box 1, and secreted adenosine triphosphate, and stimulates the anti-cancer immune response [
4,
5]. Preclinical studies have demonstrated the ability of NTP to induce emission of several DAMPs in vitro [
6,
7,
8,
9] and in vivo [
9,
10,
11].
Our lab has previously established an ICD-inducing regime of NTP using a vaccination assay, currently considered the ‘gold standard’ for identifying ICD agents [
8]. We determined that the chemical species responsible for eliciting this effect in that regime were short-lived RONS, particularly hydroxyl radials (•OH), atomic oxygen (O), ozone (O
3), and nitric oxide (•NO), while the more persistent species, with lifetimes greater than a second (e.g., H
2O
2, NO
2−, NO
3−, and ONOO
−), had no effect [
8]. Since NTP-generated RONS would first interact with cell membranes during treatment, we hypothesized that they will also oxidize and destroy membrane-bound proteins. This is advantageous for cancer immunotherapy as immunosuppressive surface proteins and checkpoints are often overexpressed on cancerous cells.
Advances in cancer immunotherapy in the past decade have been spurred by research into inhibitory immune checkpoints [
12]. CD47, a transmembrane protein overexpressed on malignant cells in several types of tumors, is a checkpoint of the innate immune system that has garnered attention [
13,
14,
15]. Upon binding with signal-regulatory protein alpha (SIRPα), found on innate immune cells [
16], activation of pro-phagocytic receptors is prevented, and subsequently phagocytosis is sequestered [
17,
18]. Thus, CD47 is often known as a ‘don’t eat me’ signal and contributes to immune evasion of cancerous cells [
19]. Therefore, disruption of the CD47–SIRPα pathway is of great interest.
In this study, we investigated the potential of NTP, operated in our previously defined ICD-inducing regime, to alter surface CD47 on cancer cells. The effect of NTP on CD47-expressing cancer cells was identified in vitro and in vivo, and the underlying mechanism of action was investigated in silico. In vitro, three different cancer cell lines were exposed to NTP in 3D tumor models. CD47 was evaluated immediately and 24 h after treatment. Melanoma tumors, established subcutaneously in syngeneic mice, were also treated with NTP, resected, and evaluated for CD47. Molecular dynamics (MD) simulations were performed to determine the structural changes of CD47 after oxidation and its subsequent influence on its binding affinity with SIRPα. Our in vitro results revealed that CD47 was modulated immediately after NTP treatment. In our 3D model of melanoma, this effect persisted up to 24 h after treatment. NTP treatment not only reduced tumor size, but also caused a slight reduction in CD47. The MD simulations indicated that oxidation of CD47 induced conformational changes to the protein, disrupts the Lys39-Asp100 and Lys41-Asp100 salt bridges in the CD47–SIRPα system, and subsequently reduced the binding affinity. Taken together, our in silico results and validation assays complement each other in evidencing the ability of NTP treatment to oxidize immunosuppressive CD47 on cancerous cells, which may affect downstream binding to innate immune cells. This study provides crucial, fundamental insight into NTP effects on cancer cells, and potentially other immune checkpoints, while supporting its role for cancer immunotherapy.
2. Materials and Methods
2.1. Cell Culture
Human glioblastoma (U87), melanoma (A375), and head and neck squamous cell carcinoma (SC263) cell lines were used in this study. All cell lines were cultured in Dulbecco’s modified Eagle medium containing 10% fetal bovine serum (Gibco, Loughborough, United Kingdom) and 100 U/mL penicillin, 100 μg/mL streptomycin (Gibco). L-Glutamine was also supplemented in the media: 2 × 10−3 M for U87 and SC263 and 4 × 10−3 M for A375. Cells were cultured in a humidified environment with 5% CO2 at 37 °C before seeding for experiments.
2.2. 3D Spheroid Model
For spheroid culture, U87 and SC236 cells were seeded into specialized round-bottomed, hydrogel coated 96-well plates (ultra-low attachment plates, ULA, Corning® 7007, Corning, Amsterdam, The Netherlands). Cell suspensions were prepared with a concentrations of 5 × 104 cells/mL, supplemented with 2% Matrigel (8.6 mg/mL, Corning) to enhance spheroid formation. Cells were seeded at a concentration of 5000 cells/well in 100 µL of culture medium and centrifuged for 10 min at 1000 RPM. Spheroids were formed in three days of undisturbed incubation at 37 °C and 5% CO2.
2.3. In Ovo Model
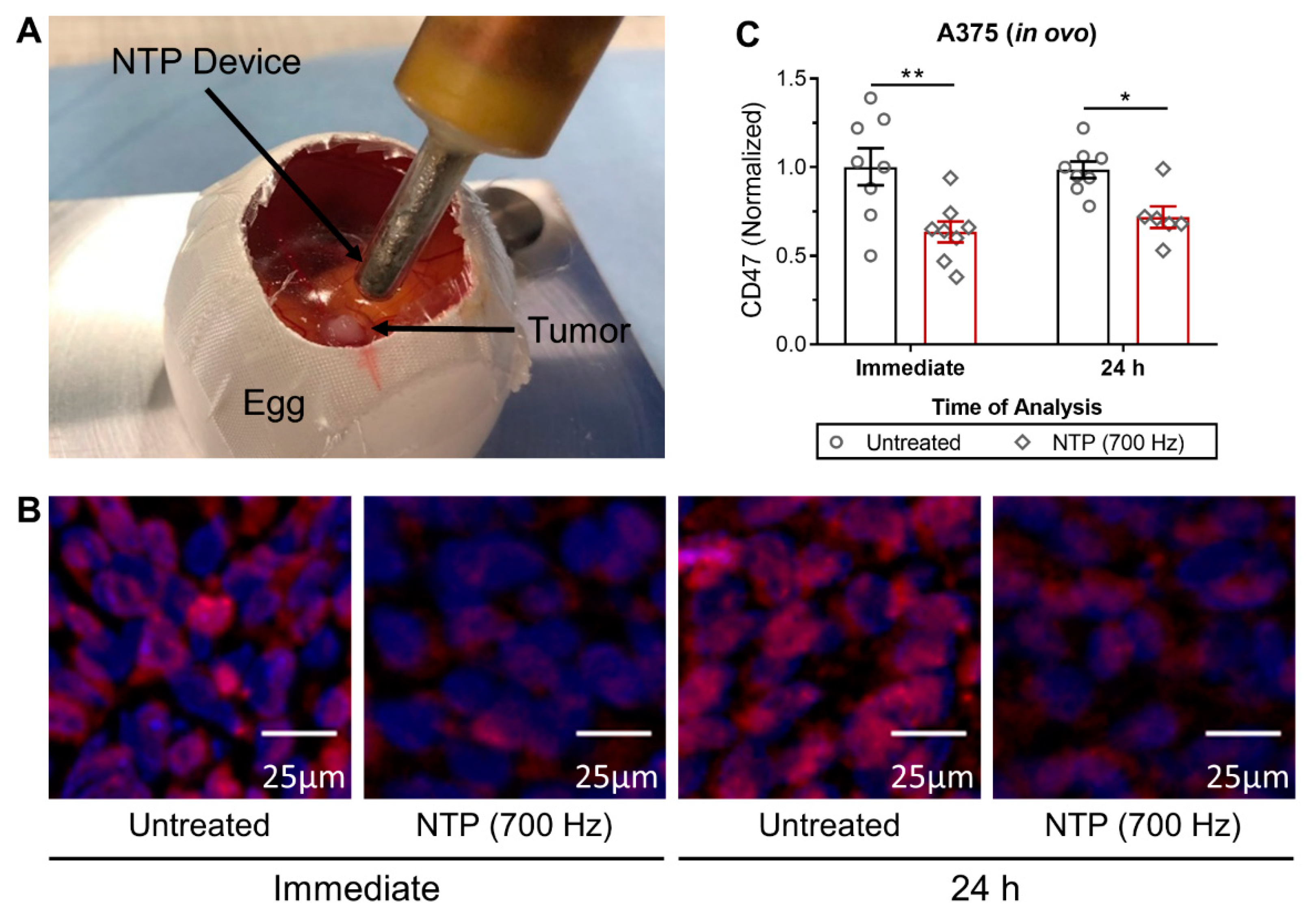
Fertilized chicken eggs (4-day old) were incubated for 1 day at 37.7 °C and 65% humidity in an egg incubator with automatic turning function (Ova-Easy 100, Brinsea, Veenendaal, The Netherlands). On day 5, the upper pole was disinfected and pierced with a 20 G sterile needle (BD) and sealed with medical tape (Leukosilk S, Covamed Farma BVBA, Marke, Belgium) to promote the relocation of the air chamber to the upper pole. The eggs were incubated in vertical position (without turning) until day 7. The eggshell was cut to expose the CAM and a 1 × 1 mm filter paper soaked in diethyl ether (Fisher Scientific, Merelbeke, Belgium) was briefly applied on a vascularized region of the CAM. A sterile silicone ring (ID = 5 mm, OD = 6 mm, 1 mm thickness) was placed on the CAM and 2 × 106 A375 cells mixed with 15 µL growth factor reduced Matrigel (8.6 mg/mL, Corning, Amsterdam, The Netherlands) were loaded into the ring. The eggs were sealed with Tegaderm (3D) and placed back in the incubator for four days. On day 11, the Tegaderm was cut and the tumors were treated with NTP. Tumors were excised immediately or 24 h after treatment for CD47 analysis. All steps outside the incubator were carried out using a heat block (Corning, Lasne, Belgium) set at 37.7 °C with a custom-made egg-shaped aluminum adapter.
2.4. NTP Treatment
A microsecond-pulsed DBD system was used to generate NTP for treatment of 3D spheroids and in ovo tumors (30 kV output, 1–1.5 µs rise time, and 2 µs pulse width). Right before NTP treatment of the spheroids, spheroids were transferred to an empty flat-bottomed 96-well plate in 3 µL of culture medium. The DBD electrode (3 mm diameter) was lowered into the well, using a z-axis positioner, 1–2 mm above the cells. The NTP pulse frequency and treatment time was fixed at 500 Hz and 10 s, respectively. Immediately after treatment, 150 µL of fresh complete medium was added to the well and the spheroids were either collected immediately or placed back into incubation until further analysis. The DBD electrode was held by hand for treatment of A375 tumors in the in ovo model. The treatment was fixed at one position for 10 s, and the pulse frequency was increased to 700 Hz, to account for the larger tumor size. This setting is similar to what we used previously in subcutaneous tumors in vivo [
10]. The tumors were resected immediately for analysis or incubated further for 24 h before collection.
2.5. In Vivo Model and Treatment
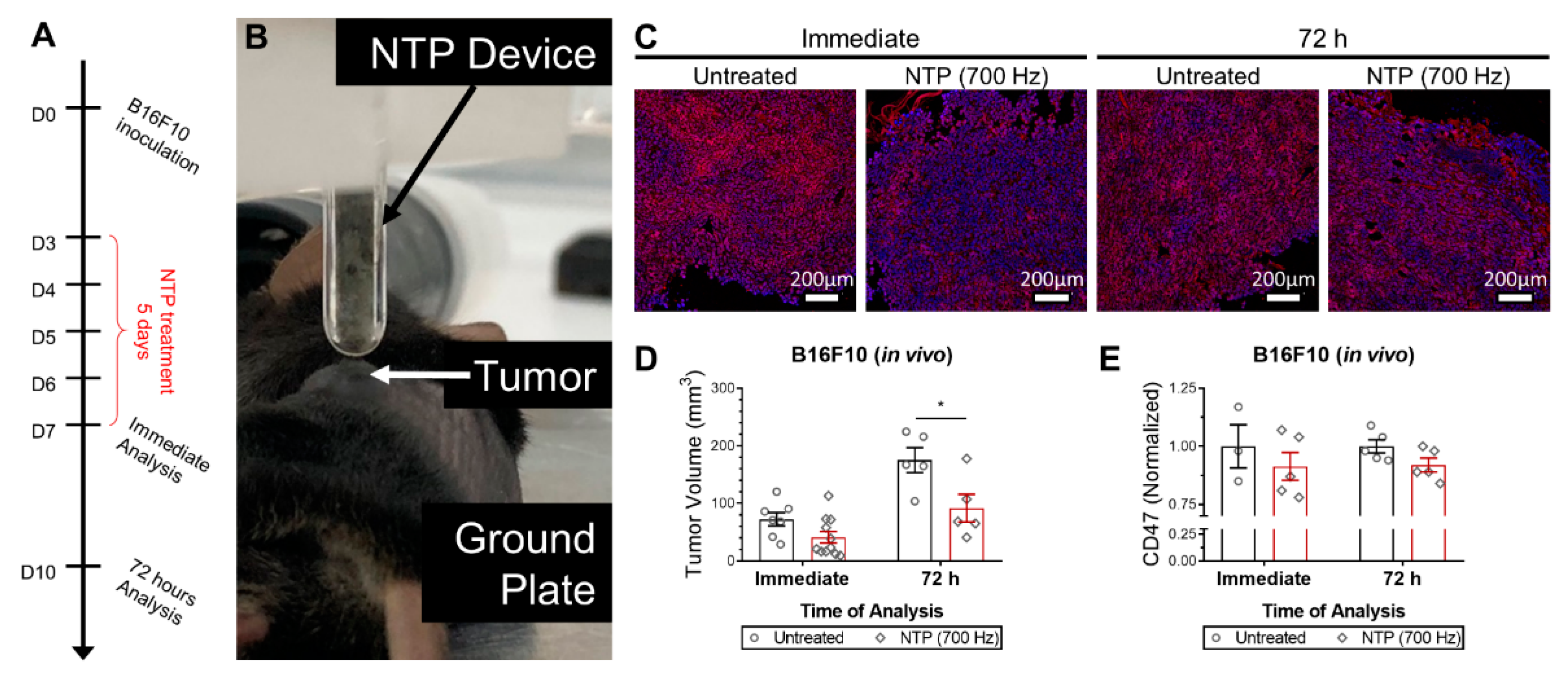
Female C57BL/6J mice, 8-week old, were purchased from Charles River (Charles River Laboratories, Wilmington, MA, USA) and housed in a pathogen-free room at the Animal Center of the University of Antwerp. 106 B16F10 melanoma cells were subcutaneously injected into the left flank of each mouse. NTP treatment was initiated when tumors became palpable (18.2 ± 4.8 mm3), 3 days after melanoma cell injection. The NTP device was held ~1 mm above the tumor and treatment was performed for 10 s at 700 Hz. Mice were treated once a day for 5 consecutive days. Tumors were collected immediately after the last treatment or 72 h after the last treatment for analysis. Two orthogonal diameters were measured on the tumor (length and width) using digital calipers and volumes were calculated using 0.5 × length × width2. All animal experiments were approved by the University of Antwerp Animal Research Ethical Committee (ECD-dossier 2017-53).
2.6. Flow Cytometry Analysis of CD47 on Cell Lines
CD47 was measured using dual staining of 7-aminoactinomycin D (7AAD), a viability stain, and a monoclonal CD47 antibody. Prior to staining, the cells were washed with PBS, detached with 200 µL of accutase, and washed twice with 2 mL of FACS buffer (500 mL sheath fluid (342003, BD Biosciences, Aalst, Belgium) + 2 g bovine serum albumin (A9418, Sigma-Aldrich, Overijse, Belgium) + 1 g NaN
3 (1.06688.0100 Merck, Overijse, Belgium) in 100 mL H
2O). The samples were stained with 10 µL of PE mouse anti-human CD47 (556046, BD Biosciences) or with 10 µL PE Mouse IgG1, κ isotype control (555749, BD Biosciences) for 40 min at 4 °C. The cells were then washed with FACS buffer and 2 µL of 7AAD (420403, Biolegend, London, United Kingdom) was added to each sample for 15 min before being quantified with a flow cytometer (CytoFLEX flow cytometer, Beckman Coulter, Indianapolis, IN, USA). 10,000 events were collected and only viable cells (7AAD−) cells were analyzed for CD47 (
Figure S1). Data were analyzed and gated using the FlowJo software version 10 (FlowJo LLC, Ashland, OR, USA).
2.7. Immunofluorescence Analysis of CD47 on Spheroid and Tumor Sections
The spheroids were collected immediately and 24 h after treatment and fixed overnight in 4% paraformaldehyde at 4 °C. The fixed spheroids were transferred to microarray molds of 4% agarose as described before [
20]. The solidified agarose microarray supports were dehydrated and embedded in paraffin overnight. In ovo tumors derived from the CAM assay were also collected immediately or 24 h after treatment and fixed with 4% paraformaldehyde for 14 h at 37 °C prior to paraffin embedding. Both sample types were taken together for the remaining part of the immunofluorescence staining protocol. Slides of 5 µm were cut, deparaffinised and rehydrated. Heat-induced antigen retrieval was performed with sodium citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0) at 96 °C for 20 min. Slides were washed with washing buffer (TBS plus 0.025% Triton X-100) with gentle agitation (2 × 5 min) before blocking in 10% bovine serum albumin in TBS at room temperature for 2 h. Incubation with mouse monoclonal anti-CD47 primary antibody was done overnight at 4 °C (1/40 dilution; clone B6H12.2, MA5-11895, ThermoFisher Scientific, Merelbeke, Belgium). All slides were washed with washing buffer (2 × 5 min) and subsequently stained with Alexa Fluor 594-conjugated Donkey anti-Mouse IgG (H + L) highly cross-adsorbed secondary antibody for CD47 (1/500 dilution, Cat. No. A-21203, ThermoFisher Scientific). Incubation with secondary antibody at room temperature for 1 h was performed in the dark to avoid photobleaching. Lastly, all slides were rinsed with washing buffer (3 × 5 min), mounted with VECTASHIELD
® Antifade mounting medium containing counter staining DAPI (H-1200, VECTOR Laboratories, Peterborough, United Kingdom). Images were taken with an Olympus BX51 fluorescence microscope (Cat. No. WS-BX51-0169, Olympus Life Sciences, Calcutta, India).
All slides within the same experiment were imaged on the same day, and all images in this study were taken at fixed microscope and capture settings. Images were also batch processed in ImageJ to limit additional variation and potential bias. CD47 expression was quantified by averaging (arithmetic mean) the ratio of mean fluorescence intensities (MFI) of CD47 and DAPI (MFI
CD47/MFI
DAPI). For the spheroids, the entire spheroid was analyzed. For the in ovo tumor sections, three representative areas (100 µm × 100 µm) with only human cells were analyzed (
Figure S2). This ratio accounts for the influence of the number of cells under observation on CD47 intensity within that region. The final ratio was normalized to the mean of the untreated section.
For in vivo evaluation of CD47, the images were measured using by using DAPI to binary mask for the locations of the individual nuclei. This was accomplished by taking the raw fluorescent intensities for DAPI and removing background signal and luminance artifacts through the use of a one sided low pass filter formed by a Gaussian kernel of 25 pixels (10 µm) in standard deviation. A kernel size of 25 was used for 20× images and 12 for 10×. Resultant values where saturated to 95th percentile of the image intensities and binarized to 0.40 of the normalized dynamic range. Individual nuclei in the images were indexed using connected component analysis and objects smaller than 20 pixels2 (100 µm2) were removed. The masks of each of the nuclei were dilated using a circular structuring element with diameter of 51 pixels (20.4 µm2) to form a tissue mask. Each pixel of the resulting tissue mask was indexed to the nearest nuclei. To correct for background signal, images were taken with tissue and non-tissue areas, and fluorescence intensity of the CD47 channel was preprocessed by saturating on the lower side to 5th percentile of the raw image intensity. Finally, the mean fluorescence intensity for each cell of the CD47 channel was taken for each nuclei, by measuring the overlap with the nuclei mask, overlap with the tissue mask or both and the mean across all nuclei for each of these metrics was reported for per tissue.
2.8. Statistical Analysis
Statistical differences for the experimental studies were analyzed using the linear mixed model with JMP Pro 13 (SAS Software, Tervuren, Belgium). NTP treatment was set as the fixed effect and the random effects tested include the date the experiment was performed or when the slides were imaged. The interactions between treatment and the date were also tested. The random slope model was only used when the interaction was significant (p ≤ 0.05). The fixed effect test was used to determine if there was a statistical difference between treatment (p ≤ 0.05), and the Dunnett’s test for statistical significance was performed post-hoc to calculate the adjusted p value compared to untreated controls. A p value less than or equal to 0.05 was considered statistically significant. Data are represented as mean ± SEM and all individual values are reported.
2.9. Computational Details
We performed MD simulations in order to understand the interaction mechanisms of the native and oxidized CD47 with SIRPα and B6H12.2 antibodies at the molecular level. The GROMACS 5.1 software was employed for the simulations, applying the GROMOS 45a3 force field [
21]. As model systems we used the CD47-SIRPα and CD47-B6H12.2 complex structures obtained from the Protein Data Bank (ID: 2JJT and 5TZU).
The preparation steps of the model systems are given in detail in the
supplementary materials. Briefly, we used two model structures, i.e., the native CD47–SIRPα (or CD47-B6H12.2) and oxidized CD47
OX–SIRPα (or CD47
OX–B6H12.2) protein complexes. These systems were placed in simulation boxes filled with water molecules including 0.1 M Na
+ and Cl
− ions. Prior to the equilibration, the systems were energy minimized using the steepest descent algorithm. Subsequently, a 50 ps equilibration run was carried out applying the NVT ensemble (i.e., a system with constant number of particles N, volume V, and temperature T) and position restraints on the heavy atoms of the proteins. Further, the model systems were equilibrated for another 50 ps applying weak coupling thermo- and barostats and employing NPT ensemble (i.e., a system with constant number of particles N, pressure p, and temperature T). Finally, 350 ns production runs were performed using again the NPT ensemble, but employing the Nose-Hoover thermostat and the isotropic Parrinello-Rahman barostat, in this case removing the applied position restraints. All simulations were carried out at 310 K and 1 bar, and 1.4 nm cut-off distance was used for the Coulomb and van der Waals interactions. The trajectory of the production runs was used for data collection, i.e., for calculation of the root mean square deviation (RMSD) and distance between salt bridges of the CD47/CD47
OX and SIRPα/B6H12.2 complexes. The PyMOL molecular graphics tool was used to prepare the images presented in this paper.
From the last 50 ns trajectory of each 350 ns production run, we extracted three systems (at 300, 325 and 350 ns) for both native and oxidized complexes (i.e., 3 × 4 = 12 model systems in total). These systems were further used in our umbrella sampling (US) simulations. In the US simulations, we used 30 windows separated by 0.1 nm, which were extracted from the pulling simulations of CD47/CD47
OX. Note that SIRPα (or B6H12.2) was restrained and used as a reference in the pulling simulations, and the pulling of CD47/CD47
OX was performed along the
z-axis. Moreover, the movement of CD47/CD47
OX in the xy-plane was also restrained by using the so-called flat-bottomed position restraint, with a radius of 0.1 nm and a force constant of 500 kJ/(mol nm
2). A 1000 kJ/(mol nm
2) spring constant together with 0.001 nm/ps pulling rate were applied in the pulling simulations, which led to the disintegration of CD47/CD47
OX from SIRPα (or B6H12.2). Using each individual umbrella window, we further performed US simulations for 25 ns and the last 23 ns trajectory was used for data collection, i.e., the initial 2 ns was used to relax the system. Finally, the weighted histogram analysis method (WHAM) [
22] was employed to calculate the potential of mean forces or free energy profiles (FEPs) of the dissociation of CD47/CD47
OX from the SIRPα (or B6H12.2) protein complex. The final FEP for each complex was averaged from three FEPs. The errors associated with the FEPs were estimated by means of the bootstrapping method. In total 30 × 3 × 4 = 360 US simulations were performed to obtain the final FEPs.
4. Discussion
In this paper, we investigated the effect of NTP on the innate immunosuppressive checkpoint ligand, CD47, and used in silico techniques to gain insight into the mechanisms of action and immunologic consequence. We provided new fundamental insight into the chemical interactions of NTP and cancer cells, though the clinical effectiveness of these observations are a part of our ongoing investigation.
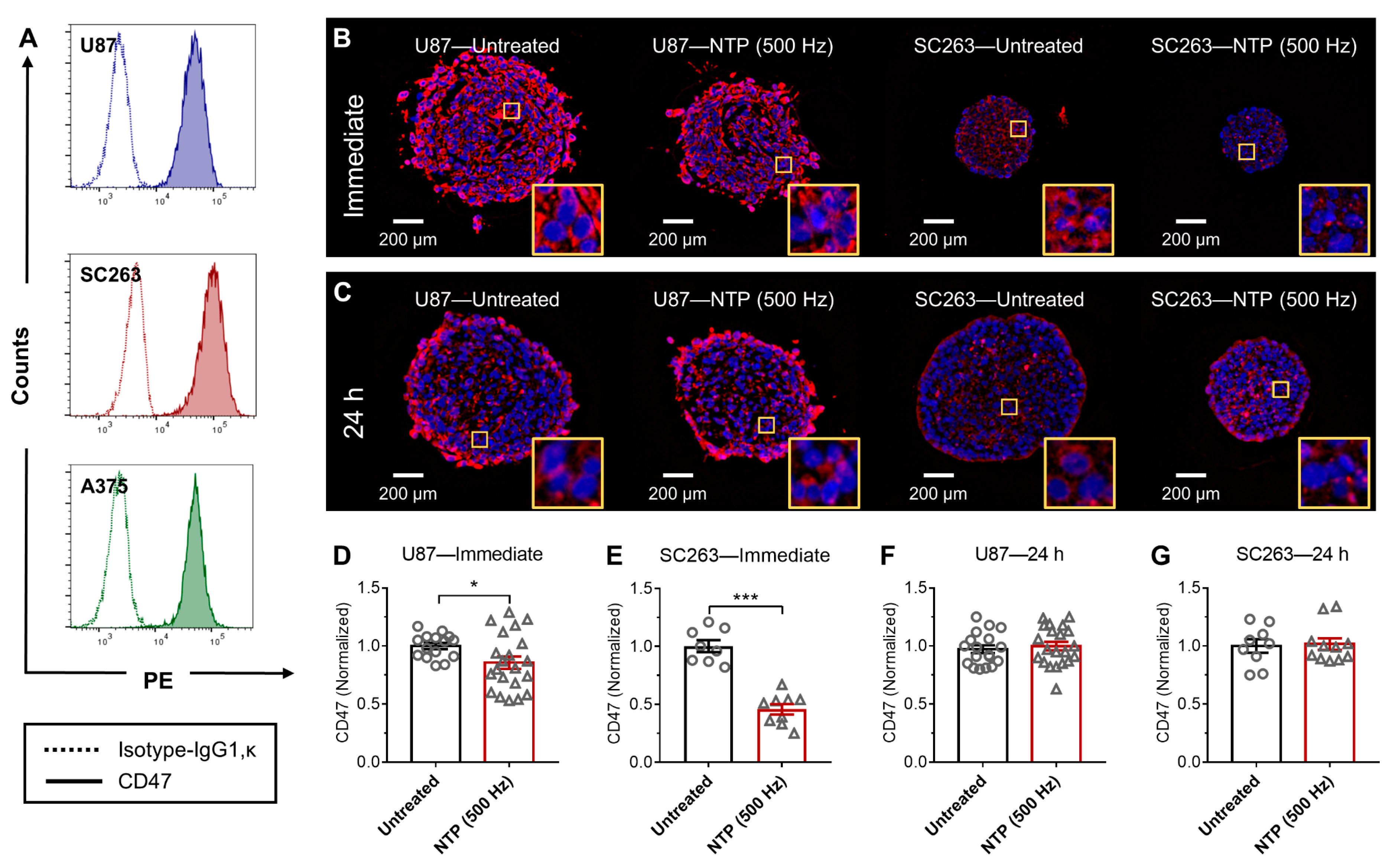
When NTP is generated, a unique mixture of highly reactive RONS is produced. After exposure to NTP, the CD47 expression on the surface of cancer cells was immediately reduced in 3D in vitro tumor models (
Figure 1 and
Figure 2). Furthermore, in vivo treatment of melanoma tumors not only reduced tumor burden but also modulated CD47 expression (
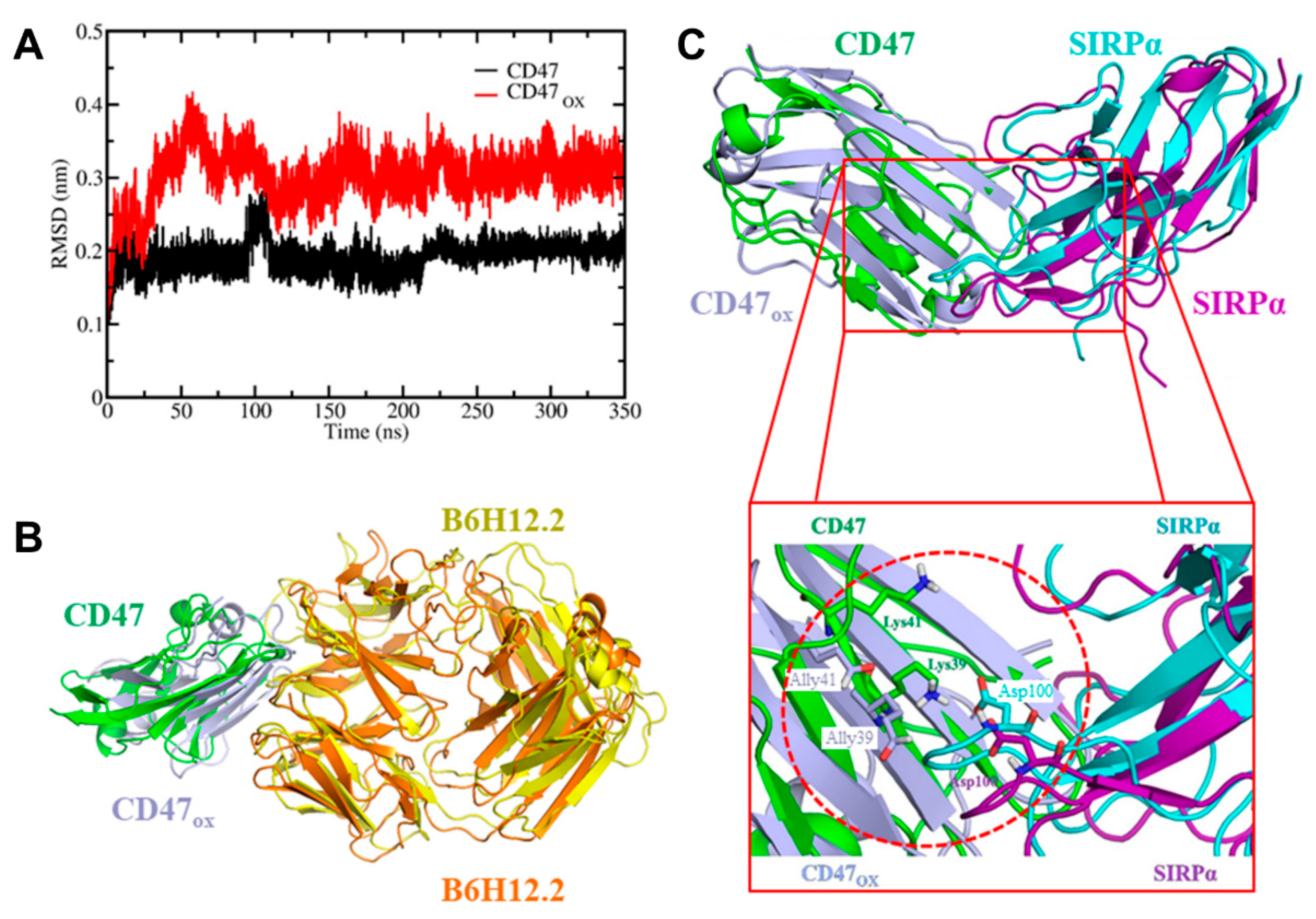
Figure 3). Due to the timing of the analysis and the rapid reduction of CD47 expression, it is likely that the RONS generated by NTP were oxidizing the protein and affecting its binding affinity. This was confirmed in silico, via RMSD analysis (
Figure 4A) and by calculating the FEP of CD47/CD47
OX with the monoclonal antibody used for fluorescence staining, B6H12.2 (
Figure S4C). CD47 is only one of many immune checkpoints overexpressed on the surface of cancer cells; others include Galectin-9 and programmed cell death ligand 1 (PD-L1) [
30]. To gain deeper insight into this, mass spectrometry analysis of these proteins before and after NTP treatment could be employed. NTP-generated RONS could consequently also oxidize these surface markers and affect binding to their immune receptors. Further investigation into the degree of oxidation and the duration before the protein is restored is still required for clinical application.
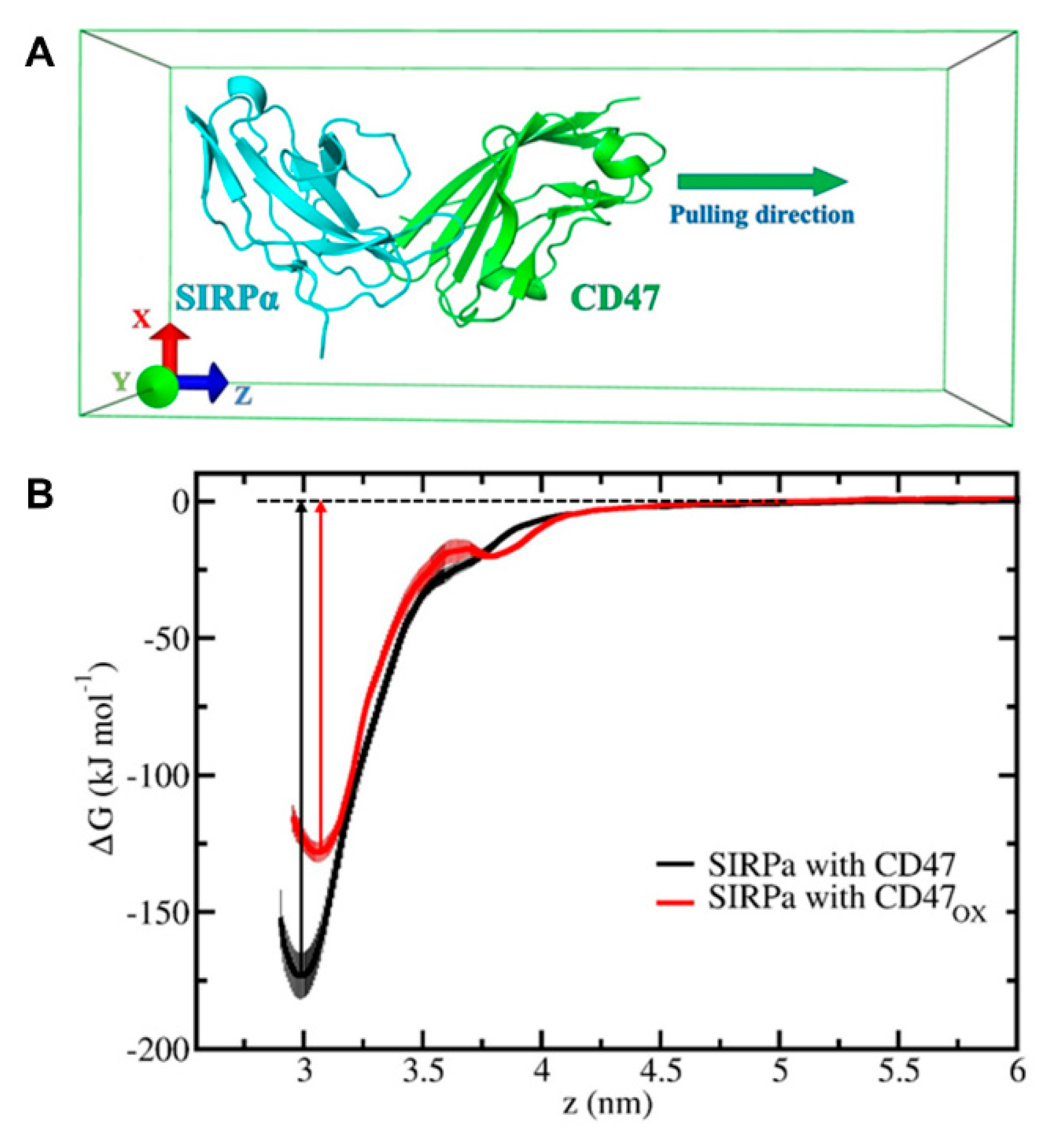
To investigate the immunologic outcome of NTP-oxidized CD47, we performed MD/US simulations to understand the nanoscale mechanisms of NTP action on the CD47 protein and the subsequent effect on binding affinity with its immune receptor, SIRPα (
Figure 4). Computer simulations have played a vital role in studying protein-protein interactions in many different fields [
31]. Particularly, simulations help to understand the nature of these interactions at atomic level precision, which is beyond the resolution of an experimental technique. To date, the interaction between cancer cell and immune cell proteins (e.g., CD47–SIRPα) under oxidative stress has not yet been investigated by computer simulations. Our simulation results revealed in detail the disruption mechanism of the binding of CD47
OX to SIRPα and show that the free energy of dissociation decreased by ~45 kJ/mol after oxidation (
Figure 5B). This suggests that NTP treatment of the tumor can reduce the ability of CD47 to bind to SIRPα on innate immune cells. This would consequently help increase tumor immunogenicity and support the patient’s anti-cancer immune response. Such computer simulations, despite their computational cost, are highly valuable for gaining insights in cancer immunotherapy, without the high financial and labor costs of experimental studies (e.g., autologous/allogenic co-culture assays, in vivo experiments, gene knockout studies).
The results presented here not only add to fundamental knowledge of NTP-cell interactions, but also complement the current understanding that NTP can increase tumor immunogenicity via induction of immunogenic cancer cell death [
8,
10,
11]. Our lab has previously identified an ICD-inducing regime of NTP and reported the increase of surface CRT, a pro-phagocytic signal [
8]. Furthermore, we demonstrated that the short-lived RONS produced during treatment, were the major effectors in that regime [
8]. In the present study, we showed that NTP operated at the same ICD-inducing regime can also increase tumor immunogenicity by reducing the immunosuppressive signal, CD47 (
Figure 1 and
Figure 2). Interestingly, while CRT was increased at 24 h, we observed here that CD47 was reduced immediately and returned to baseline 24 h later. This suggests that while RONS were required to stimulate cellular pathways to increased CRT expression, CD47 destruction was inflicted via direct oxidation. As mentioned above, the oxidation of CD47 with NTP shown here, provokes intrigue into how NTP can affect other highly expressed immunosuppressive markers and checkpoints. This would open up a new paradigm in ‘plasma oncology’, where NTP could play a role in reducing the strategies used by cancer cells to evade and suppress the immune system.
Taken together, our results suggest a dual role of NTP therapy to simultaneously increase immunogenic signals and reduce immunosuppressive ones. Due to the short lifetimes and reactivity of this unique mixture of RONS, a highly localized treatment can be performed. This could be an advantage over current ICD inducers which are normally systemic or deeply penetrating (e.g., anthracycline chemotherapeutics, low dose radiation), at least for tumors that are accessible with the NTP device, such as melanoma and head and neck cancer. It remains open to be discovered whether combining NTP with currently available anti-CD47 therapies (e.g., Hu5F9-G4, a humanized IgG4 isotype immune checkpoint inhibitor of CD47) [
32,
33] will be beneficial or if combination strategies with other checkpoint inhibitors that target a separate axis of anti-cancer immunity are more favorable. Research into NTP for cancer therapy, having only just started in the past decade, has already gained significant momentum, and clinical pilot studies have also been initiated. Metelmann, et al., used NTP to treat patients with advanced head and neck cancer and infected ulcerations [
34,
35] and Friedman, et al., used NTP to treat actinic keratosis, a common pre-cancerous skin disease [
36]. Both studies demonstrated that treatment led to positive responses without short- or long-term adverse effects. It is possible that clinical application of NTP could also be affecting immune responses, although this has not yet been observed or analyzed directly in patients. Studies into both roles of NTP therapy, as we propose, would be of great interest for fundamental understanding and clinical translation.