Increased Expression of AKT3 in Neuroendocrine Differentiated Prostate Cancer Cells Alters the Response Towards Anti-Androgen Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
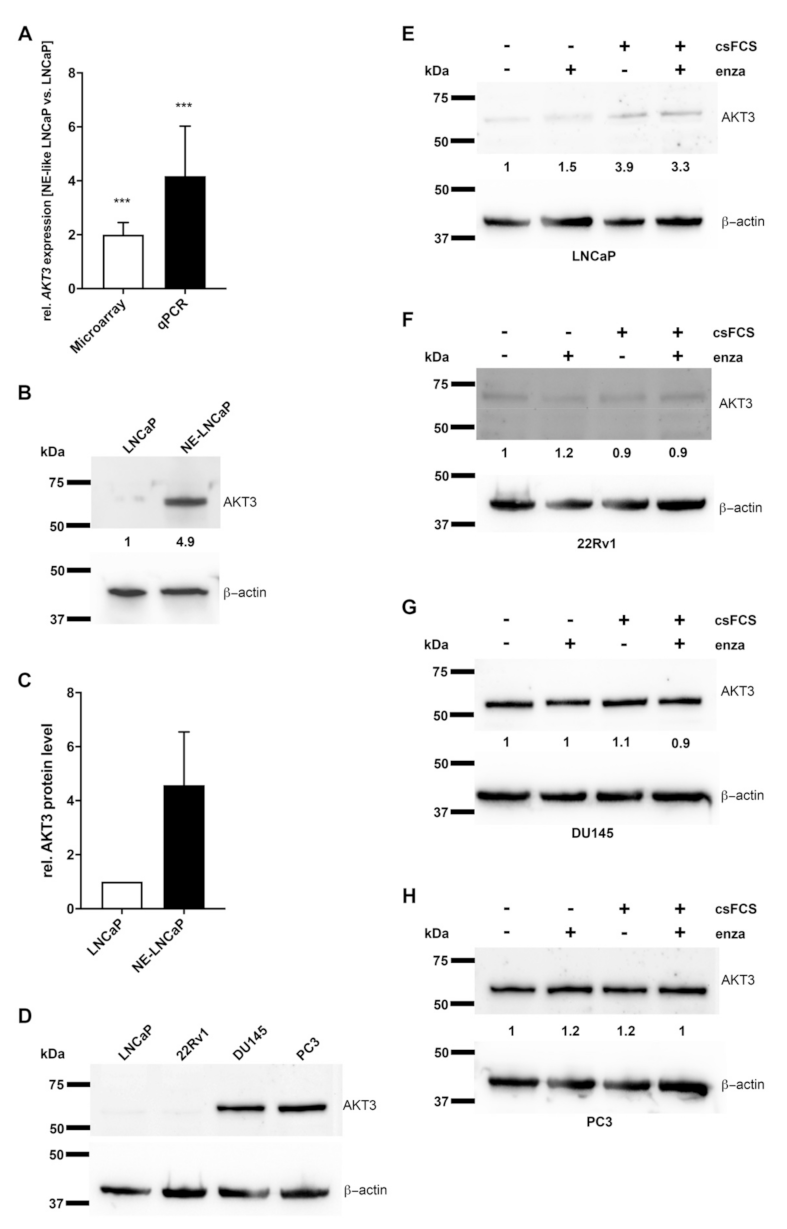
2.1. AKT3 Expression Is Increased in NE-Like LNCaP Cells
2.2. AKT3 Protein Expression Negatively Correlates with AR Signaling
2.3. Neuroendocrine Differentiated PCa Cells Express AKT3
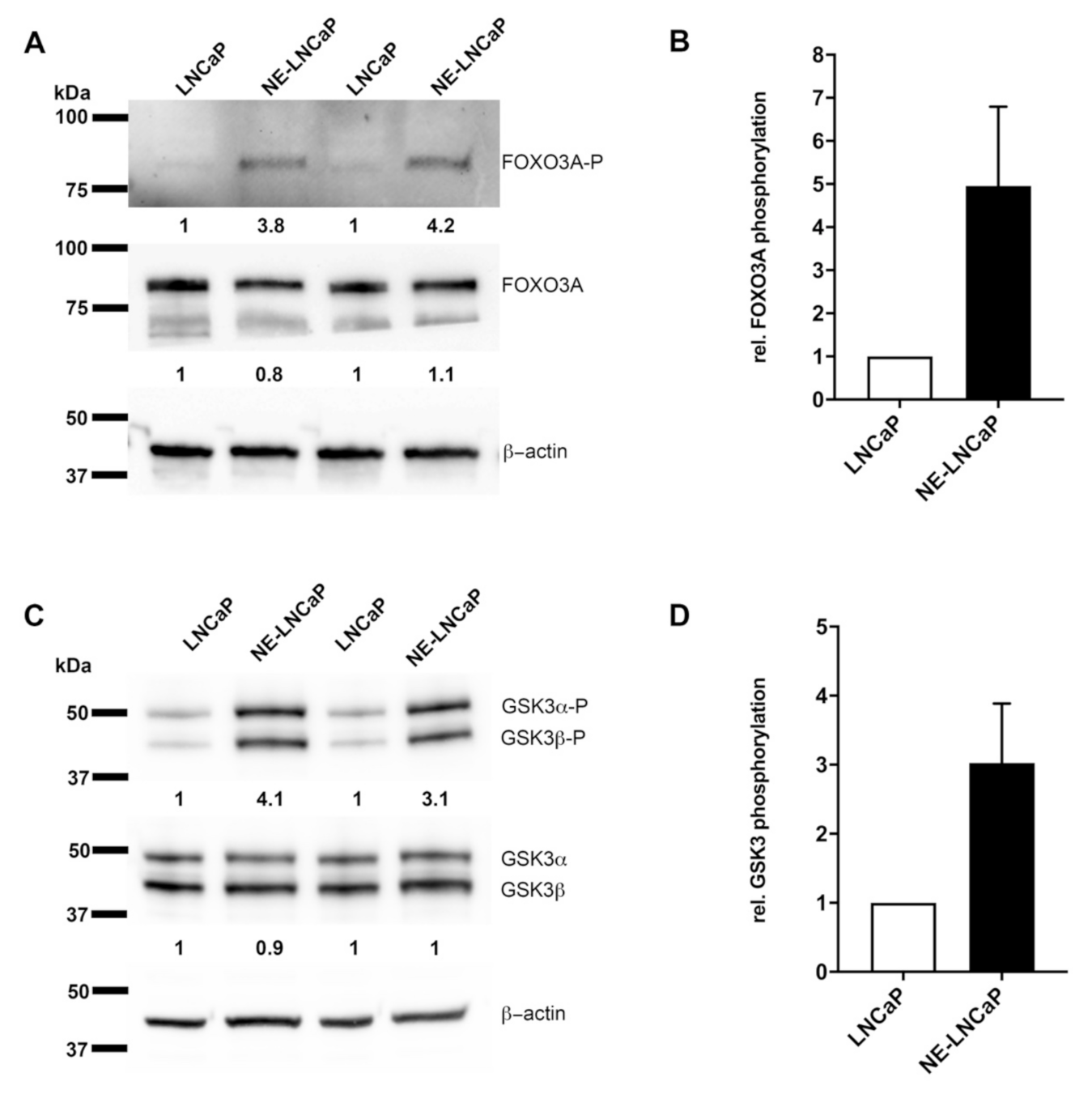
2.4. AKT3 Signaling Pathway Is Activated in NE-Like LNCaP Cells
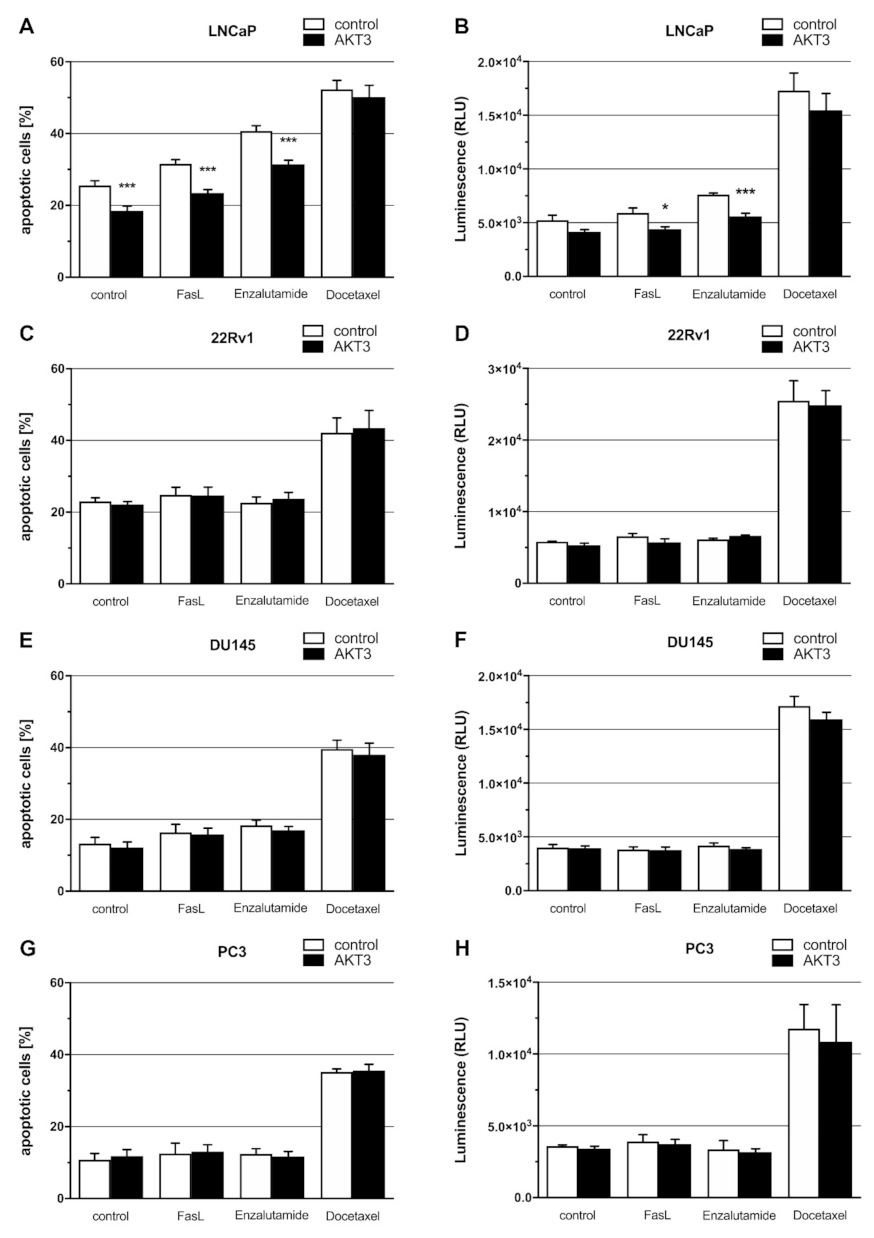
2.5. Expression of AKT3 Has an Anti-Apoptotic Effect on AR-Positive LNCaP Cells
2.6. Members of miR-17 Family Post-Transcriptionally Regulate AKT3 Biosynthesis
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. RNA Extraction
4.3. Quantitative Real-Time PCR
4.4. SDS-PAGE and Western Blotting
4.5. Human Prostate Specimens
4.6. Immunohistochemistry
4.7. Double Immunofluorescence
4.8. Plasmids
4.9. Transfection
4.10. Cell Proliferation Assay
4.11. Colony Formation Assay
4.12. Flow Cytometric Determination of Apoptosis
4.13. Caspase 3/7 Activity Assay
4.14. Target Gene Prediction
4.15. Dual-Luciferase Assay
4.16. Data Analysis and Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.N.; Patel, N.; Bershadskiy, A.; Sokoloff, A.; Singer, E.A. Androgen synthesis inhibitors in the treatment of castration-resistant prostate cancer. Asian J. Androl. 2014, 16, 387. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S. Enzalutamide: The emperor of all anti-androgens. Transl. Androl. Urol. 2013, 2, 119. [Google Scholar] [PubMed]
- Balk, S.P. Androgen receptor as a target in androgen-independent prostate cancer. Urology 2002, 60, 132–138. [Google Scholar] [CrossRef]
- Perrot, V. Neuroendocrine differentiation in the progression of prostate cancer: An update on recent developments. Open J. Urol. 2012, 2, 173. [Google Scholar] [CrossRef][Green Version]
- Abrahamsson, P. Neuroendocrine cells in tumour growth of the prostate. Endocr. Relat. Cancer 1999, 6, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Shariff, A.H.; Ather, M.H. Neuroendocrine differentiation in prostate cancer. Urology 2006, 68, 2–8. [Google Scholar] [CrossRef]
- Vashchenko, N.; Abrahamsson, P.-A. Neuroendocrine differentiation in prostate cancer: Implications for new treatment modalities. Eur. Urol. 2005, 47, 147–155. [Google Scholar] [CrossRef]
- Uchida, K.; Masumori, N.; Takahashi, A.; Itoh, N.; Kato, K.; Matusik, R.J.; Tsukamoto, T. Murine androgen-independent neuroendocrine carcinoma promotes metastasis of human prostate cancer cell line LNCaP. Prostate 2006, 66, 536–545. [Google Scholar] [CrossRef]
- Berruti, A.; Mosca, A.; Tucci, M.; Terrone, C.; Torta, M.; Tarabuzzi, R.; Russo, L.; Cracco, C.; Bollito, E.; Scarpa, R.M. Independent prognostic role of circulating chromogranin A in prostate cancer patients with hormone-refractory disease. Endocr. Relat. Cancer 2005, 12, 109–117. [Google Scholar] [CrossRef]
- Wu, C.; Huang, J. Phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin pathway is essential for neuroendocrine differentiation of prostate cancer. J. Biol. Chem. 2007, 282, 3571–3583. [Google Scholar] [CrossRef] [PubMed]
- Wee, P.; Wang, Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers 2017, 9, 52. [Google Scholar]
- Altomare, D.A.; Testa, J.R. Perturbations of the AKT signaling pathway in human cancer. Oncogene 2005, 24, 7455–7464. [Google Scholar] [PubMed]
- Cheng, J.Q.; Lindsley, C.W.; Cheng, G.Z.; Yang, H.; Nicosia, S.V. The Akt/PKB pathway: Molecular target for cancer drug discovery. Oncogene 2005, 24, 7482–7492. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Cantley, L. PI3K pathway alterations in cancer: Variations on a theme. Oncogene 2008, 27, 5497–5510. [Google Scholar] [CrossRef]
- Stahl, J.M.; Sharma, A.; Cheung, M.; Zimmerman, M.; Cheng, J.Q.; Bosenberg, M.W.; Kester, M.; Sandirasegarane, L.; Robertson, G.P. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004, 64, 7002–7010. [Google Scholar] [CrossRef]
- Lin, F.-M.; Yost, S.E.; Wen, W.; Frankel, P.H.; Schmolze, D.; Chu, P.-G.; Yuan, Y.-C.; Liu, Z.; Yim, J.; Chen, Z. Differential gene expression and AKT targeting in triple negative breast cancer. Oncotarget 2019, 10, 4356. [Google Scholar] [CrossRef]
- Li, Y.; Dong, W.; Yang, H.; Xiao, G. Propofol suppresses proliferation and metastasis of colorectal cancer cells by regulating miR-124-3p. 1/AKT3. Biotechnol. Lett. 2020, 42, 493–504. [Google Scholar] [CrossRef]
- Zinda, M.J.; Johnson, M.A.; Paul, J.D.; Horn, C.; Konicek, B.W.; Lu, Z.H.; Sandusky, G.; Thomas, J.E.; Neubauer, B.L.; Lai, M.T. AKT-1,-2, and-3 are expressed in both normal and tumor tissues of the lung, breast, prostate, and colon. Clin. Cancer Res. 2001, 7, 2475–2479. [Google Scholar]
- Nakatani, K.; Thompson, D.A.; Barthel, A.; Sakaue, H.; Liu, W.; Weigel, R.J.; Roth, R.A. Up-regulation of Akt3 in estrogen receptor-deficient breast cancers and androgen-independent prostate cancer lines. J. Biol. Chem. 1999, 274, 21528–21532. [Google Scholar] [CrossRef]
- Le Page, C.; Koumakpayi, I.; Alam-Fahmy, M.; Mes-Masson, A.; Saad, F. Expression and localisation of Akt-1, Akt-2 and Akt-3 correlate with clinical outcome of prostate cancer patients. Br. J. Cancer 2006, 94, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Alwhaibi, A.; Kolhe, R.; Gao, F.; Cobran, E.K.; Somanath, P.R. Genome atlas analysis based profiling of Akt pathway genes in the early and advanced human prostate cancer. Oncoscience 2019, 6, 317. [Google Scholar] [CrossRef] [PubMed]
- Dankert, J.T.; Wiesehöfer, M.; Czyrnik, E.D.; Singer, B.B.; von Ostau, N.; Wennemuth, G. The deregulation of miR-17/CCND1 axis during neuroendocrine transdifferentiation of LNCaP prostate cancer cells. PLoS ONE 2018, 13, e0200472. [Google Scholar] [CrossRef] [PubMed]
- Coppola, V.; De Maria, R.; Bonci, D. MicroRNAs and prostate cancer. Endocr. Relat. Cancer 2010, 17, F1. [Google Scholar] [CrossRef] [PubMed]
- Szczyrba, J.; Löprich, E.; Wach, S.; Jung, V.; Unteregger, G.; Barth, S.; Grobholz, R.; Wieland, W.; Stöhr, R.; Hartmann, A. The microRNA profile of prostate carcinoma obtained by deep sequencing. Mol. Cancer Res. 2010, 8, 529–538. [Google Scholar] [CrossRef]
- Wach, S.; Nolte, E.; Szczyrba, J.; Stöhr, R.; Hartmann, A.; Ørntoft, T.; Dyrskjøt, L.; Eltze, E.; Wieland, W.; Keck, B. MicroRNA profiles of prostate carcinoma detected by multiplatform microRNA screening. Int. J. Cancer 2012, 130, 611–621. [Google Scholar] [CrossRef]
- Chlenski, A.; Nakashiro, K.i.; Ketels, K.V.; Korovaitseva, G.I.; Oyasu, R. Androgen receptor expression in androgen-independent prostate cancer cell lines. Prostate 2001, 47, 66–75. [Google Scholar]
- Smith, R.; Liu, M.; Liby, T.; Bayani, N.; Bucher, E.; Chiotti, K.; Derrick, D.; Chauchereau, A.; Heiser, L.; Alumkal, J. Enzalutamide response in a panel of prostate cancer cell lines reveals a role for glucocorticoid receptor in enzalutamide resistant disease. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Khurana, N.; Kim, H.; Chandra, P.K.; Talwar, S.; Sharma, P.; Abdel-Mageed, A.B.; Sikka, S.C.; Mondal, D. Multimodal actions of the phytochemical sulforaphane suppress both AR and AR-V7 in 22Rv1 cells: Advocating a potent pharmaceutical combination against castration-resistant prostate cancer. Oncol. Rep. 2017, 38, 2774–2786. [Google Scholar] [CrossRef]
- Zhang, T.; Karsh, L.I.; Nissenblatt, M.J.; Canfield, S.E. Androgen receptor splice variant, AR-V7, as a biomarker of resistance to androgen axis-targeted therapies in advanced prostate cancer. Clin. Genitourin. Cancer 2020, 18, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Chan, S.C.; Brand, L.J.; Hwang, T.H.; Silverstein, K.A.; Dehm, S.M. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013, 73, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Di Sant’Agnese, P.A.; de Mesy Jensen, K.L. Endocrine-paracrine cells of the prostate and prostatic urethra: An ultrastructural study. Human Pathol. 1984, 15, 1034–1041. [Google Scholar] [CrossRef]
- Di Sant’Agnese, P.A. Neuroendocrine cells of the prostate and neuroendocrine differentiation in prostatic carcinoma: A review of morphologic aspects. Urology 1998, 51, 121–124. [Google Scholar] [CrossRef]
- Szczyrba, J.; Niesen, A.; Wagner, M.; Wandernoth, P.M.; Aumüller, G.; Wennemuth, G. Neuroendocrine cells of the prostate derive from the neural crest. J. Biol. Chem. 2017, 292, 2021–2031. [Google Scholar] [CrossRef]
- Tai, S.; Sun, Y.; Squires, J.M.; Zhang, H.; Oh, W.K.; Liang, C.Z.; Huang, J. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 2011, 71, 1668–1679. [Google Scholar] [CrossRef] [PubMed]
- Ather, M.H.; Abbas, F.; Faruqui, N.; Israr, M.; Pervez, S. Correlation of three immunohistochemically detected markers of neuroendocrine differentiation with clinical predictors of disease progression in prostate cancer. BMC Urol. 2008, 8, 21. [Google Scholar] [CrossRef]
- Kasprzak, A.; Zabel, M.; Biczysko, W.a. Selected markers (chromogranin A, neuron-specific enolase, synaptophysin, protein gene product 9.5) in diagnosis and prognosis of neuroendocrine pulmonary tumours. Pol. J. Pathol. 2007, 58, 23–33. [Google Scholar]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef]
- Das, T.; Suman, S.; Alatassi, H.; Ankem, M.; Damodaran, C. Inhibition of AKT promotes FOXO3a-dependent apoptosis in prostate cancer. Cell Death Dis. 2016, 7, e2111. [Google Scholar] [CrossRef]
- Cross, D.A.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995, 378, 785–789. [Google Scholar] [CrossRef]
- Pap, M.; Cooper, G.M. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J. Biol. Chem. 1998, 273, 19929–19932. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, D.; Dedhar, S.; Wu, H.; Nelson, C. PTEN and GSK3 β: Key regulators of progression to androgen-independent prostate cancer. Oncogene 2006, 25, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yan, X.; Wu, D.; Yang, Y.; Li, M.; Su, Y.; Yang, W.; Shan, Z.; Gao, Y.; Jin, Z. High expression of Ras-related protein 1A promotes an aggressive phenotype in colorectal cancer via PTEN/FOXO3/CCND1 pathway. J. Exp. Clin. Cancer Res. 2018, 37, 178. [Google Scholar] [CrossRef] [PubMed]
- Ai, B.; Kong, X.; Wang, X.; Zhang, K.; Yang, X.; Zhai, J.; Gao, R.; Qi, Y.; Wang, J.; Wang, Z. LINC01355 suppresses breast cancer growth through FOXO3-mediated transcriptional repression of CCND1. Cell Death Dis. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Diehl, J.A.; Zindy, F.; Sherr, C.J. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 1997, 11, 957–972. [Google Scholar] [CrossRef]
- Diehl, J.A.; Cheng, M.; Roussel, M.F.; Sherr, C.J. Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998, 12, 3499–3511. [Google Scholar]
- Chen, Y.; Martinez, L.A.; LaCava, M.; Coghlan, L.; Conti, C.J. Increased cell growth and tumorigenicity in human prostate LNCaP cells by overexpression to cyclin D1. Oncogene 1998, 16, 1913–1920. [Google Scholar] [CrossRef][Green Version]
- Drobnjak, M.; Osman, I.; Scher, H.I.; Fazzari, M.; Cordon-Cardo, C. Overexpression of cyclin D1 is associated with metastatic prostate cancer to bone. Clin. Cancer Res. 2000, 6, 1891–1895. [Google Scholar]
- Lin, H.-P.; Lin, C.-Y.; Huo, C.; Jan, Y.-J.; Tseng, J.-C.; Jiang, S.S.; Kuo, Y.-Y.; Chen, S.-C.; Wang, C.-T.; Chan, T.-M. AKT3 promotes prostate cancer proliferation cells through regulation of Akt, B-Raf & TSC1/TSC2. Oncotarget 2015, 6, 27097. [Google Scholar]
- Shao, Y.; Aplin, A.E. Akt3-mediated resistance to apoptosis in B-RAF–targeted melanoma cells. Cancer Res. 2010, 70, 6670–6681. [Google Scholar] [CrossRef]
- Xie, S.; Lin, H.K.; Ni, J.; Yang, L.; Wang, L.; di Sant’Agnese, P.A.; Chang, C. Regulation of interleukin-6-mediated PI3K activation and neuroendocrine differentiation by androgen signaling in prostate cancer LNCaP cells. Prostate 2004, 60, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Martín-Orozco, R.M.; Almaraz-Pro, C.; Rodríguez-Ubreva, F.J.; Cortés, M.A.; Ropero, S.; Colomer, R.; López-Ruiz, P.; Colás, B. EGF Prevents the Neuroendocrine Differentiation of LNCaP Cells Induced By Serum Deprivation: The Modulator Role of P13K/Akt. Neoplasia 2007, 9, 614–624. [Google Scholar] [CrossRef] [PubMed][Green Version]
- ChunJiao, S.; Huan, C.; ChaoYang, X.; GuoMei, R. Uncovering the roles of miRNAs and their relationship with androgen receptor in prostate cancer. IUBMB Life 2014, 66, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Mei, Y.; Li, K.; Huang, X.; Yang, H. Downregulation of miR-17-92a cluster promotes autophagy induction in response to celastrol treatment in prostate cancer cells. Biochem. Biophys. Res. Commun. 2016, 478, 804–810. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Singh, H.; Rajapakshe, K.; Tachibana, K.; Ganesan, N.; Pan, Y.; Gunaratne, P.H.; Coarfa, C.; Bedrosian, I. Regulation of miRNA-29c and its downstream pathways in preneoplastic progression of triple-negative breast cancer. Oncotarget 2017, 8, 19645. [Google Scholar] [CrossRef]
- De Bono, J.S.; De Giorgi, U.; Rodrigues, D.N.; Massard, C.; Bracarda, S.; Font, A.; Arija, J.A.A.; Shih, K.C.; Radavoi, G.D.; Xu, N. Randomized phase II study evaluating Akt blockade with ipatasertib, in combination with abiraterone, in patients with metastatic prostate cancer with and without PTEN loss. Clin. Cancer Res. 2019, 25, 928–936. [Google Scholar]
- Ipatasertib Plus Abiraterone Plus Prednisone/Prednisolone, Relative to Placebo Plus Abiraterone Plus Prednisone/Prednisolone in Adult Male Patients with Metastatic Castrate-Resistant Prostate Cancer. Available online: https://ClinicalTrials.gov/show/NCT03072238 (accessed on 12 October 2020).
- Spahn, M.; Kneitz, S.; Scholz, C.J.; Stenger, N.; Rüdiger, T.; Ströbel, P.; Riedmiller, H.; Kneitz, B. Expression of microRNA-221 is progressively reduced in aggressive prostate cancer and metastasis and predicts clinical recurrence. Int. J. Cancer 2010, 127, 394–403. [Google Scholar]
- Kneitz, B.; Krebs, M.; Kalogirou, C.; Schubert, M.; Joniau, S.; van Poppel, H.; Lerut, E.; Kneitz, S.; Scholz, C.J.; Ströbel, P. Survival in patients with high-risk prostate cancer is predicted by miR-221, which regulates proliferation, apoptosis, and invasion of prostate cancer cells by inhibiting IRF2 and SOCS3. Cancer Res. 2014, 74, 2591–2603. [Google Scholar] [CrossRef]
- D’Amico, A.V.; Whittington, R.; Malkowicz, S.B.; Schultz, D.; Blank, K.; Broderick, G.A.; Tomaszewski, J.E.; Renshaw, A.A.; Kaplan, I.; Beard, C.J. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998, 280, 969–974. [Google Scholar] [CrossRef]



| Patient | Age (Years) | Serum PSA (ng/mL) | pT-Stage | Gleason | GG | pN+ | Adj. ADT |
|---|---|---|---|---|---|---|---|
| 1 | 69 | 31.00 | pT3a | 3 + 4 | 2 | yes | yes |
| 2 | 72 | 32.00 | pT3b | 4 + 3 | 3 | no | yes |
| 3 | 65 | 83.70 | pT4 | 3 + 4 | 2 | no | yes |
| 4 | 73 | 50.10 | pT4 | 4 + 5 | 5 | no | yes |
| 5 | 62 | 23.40 | pT3b | 3 + 3 | 1 | no | yes |
| 6 | 72 | 23.92 | pT3a | 3 + 4 | 2 | no | yes |
| 7 | 63 | 22.61 | pT4 | 4 + 3 | 3 | yes | yes |
| 8 | 73 | 32.80 | pT3b | 4 + 4 | 4 | yes | yes |
| 9 | 77 | 58.00 | pT3b | 3 + 4 | 2 | yes | no |
| 10 | 76 | 33.50 | pT3b | 4 + 3 | 3 | no | yes |
| 11 | 63 | 25.41 | pT3b | 3 + 4 | 2 | yes | yes |
| 12 | 70 | 48.35 | pT3a | 3 + 3 | 1 | yes | yes |
| 13 | 67 | 153.00 | pT3a | 3 + 4 | 2 | yes | yes |
| 14 | 56 | 42.70 | pT3a | 3 + 4 | 2 | no | yes |
| 15 | 77 | 110.00 | pT3b | 5 + 4 | 5 | no | yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiesehöfer, M.; Czyrnik, E.D.; Spahn, M.; Ting, S.; Reis, H.; Dankert, J.T.; Wennemuth, G. Increased Expression of AKT3 in Neuroendocrine Differentiated Prostate Cancer Cells Alters the Response Towards Anti-Androgen Treatment. Cancers 2021, 13, 578. https://doi.org/10.3390/cancers13030578
Wiesehöfer M, Czyrnik ED, Spahn M, Ting S, Reis H, Dankert JT, Wennemuth G. Increased Expression of AKT3 in Neuroendocrine Differentiated Prostate Cancer Cells Alters the Response Towards Anti-Androgen Treatment. Cancers. 2021; 13(3):578. https://doi.org/10.3390/cancers13030578
Chicago/Turabian StyleWiesehöfer, Marc, Elena Dilara Czyrnik, Martin Spahn, Saskia Ting, Henning Reis, Jaroslaw Thomas Dankert, and Gunther Wennemuth. 2021. "Increased Expression of AKT3 in Neuroendocrine Differentiated Prostate Cancer Cells Alters the Response Towards Anti-Androgen Treatment" Cancers 13, no. 3: 578. https://doi.org/10.3390/cancers13030578
APA StyleWiesehöfer, M., Czyrnik, E. D., Spahn, M., Ting, S., Reis, H., Dankert, J. T., & Wennemuth, G. (2021). Increased Expression of AKT3 in Neuroendocrine Differentiated Prostate Cancer Cells Alters the Response Towards Anti-Androgen Treatment. Cancers, 13(3), 578. https://doi.org/10.3390/cancers13030578









