Value and Unmet Needs in Non-Invasive Human Papillomavirus (HPV) Testing for Oropharyngeal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
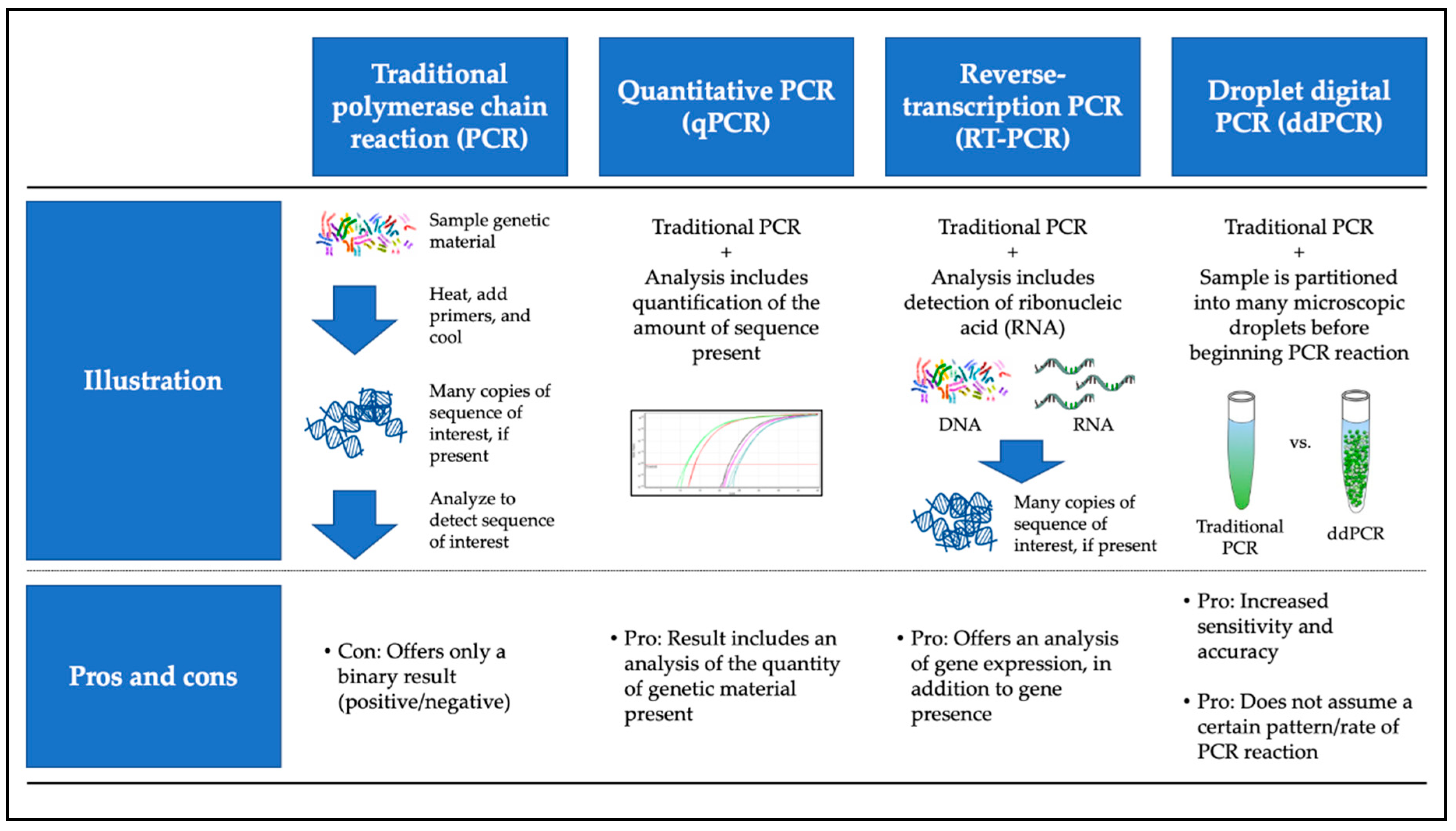
2. HPV Detection Methods
2.1. Mouth and Throat Samples
2.1.1. Collection Methods
2.1.2. Testing Methods
2.2. Serum Samples
3. Utility of Detection: Public Health and Clinical Scenarios
3.1. Non-Invasive Testing to Study Epidemiology of Oral/Oropharyngeal HPV
3.2. Immunogenicity
3.3. Screening
3.4. Monitoring Treatment Effect
3.5. Surveillance for Recurrence
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Senkomago, V.; Henley, S.J.; Thomas, C.C.; Mix, J.M.; Markowitz, L.E.; Saraiya, M. Human Papillomavirus-Attributable Cancers—United States, 2012–2016. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Anderson, W.F.; Gillison, M.L. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J. Clin. Oncol. 2008, 26, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Mahal, B.A.; Catalano, P.J.; Haddad, R.I.; Hanna, G.J.; Kass, J.I.; Schoenfeld, J.D.; Tishler, R.B.; Margalit, D.N. Incidence and Demographic Burden of HPV-Associated Oropharyngeal Head and Neck Cancers in the United States. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Mazul, A.L.; Farquhar, D.; Brennan, P.; Anantharaman, D.; Abedi-Ardekani, B.; Weissler, M.C.; Hayes, D.N.; Olshan, A.F.; Zevallos, J.P. Long-term Survival in Head and Neck Cancer: Impact of Site, Stage, Smoking, and Human Papillomavirus Status. Laryngoscope 2019, 129, 2506–2513. [Google Scholar] [CrossRef] [PubMed]
- Farmer, E.; Cheng, M.A.; Hung, C.F.; Wu, T.C. Vaccination Strategies for the Control and Treatment of HPV Infection and HPV-Associated Cancer. Recent Results Cancer Res. 2021, 217, 157–195. [Google Scholar] [CrossRef]
- Combes, J.D.; Dalstein, V.; Gheit, T.; Clifford, G.M.; Tommasino, M.; Clavel, C.; Lacau St Guily, J.; Franceschi, S.; SPLIT Study Group. Prevalence of human papillomavirus in tonsil brushings and gargles in cancer-free patients: The SPLIT study. Oral Oncol. 2017, 66, 52–57. [Google Scholar] [CrossRef]
- Chikandiwa, A.; Pisa, P.T.; Chersich, M.F.; Muller, E.E.; Mayaud, P.; Delany-Moretlwe, S. Oropharyngeal HPV infection: Prevalence and sampling methods among HIV-infected men in South Africa. Int. J. STD AIDS 2018, 29, 776–780. [Google Scholar] [CrossRef]
- Dona, M.G.; Pichi, B.; Rollo, F.; Benevolo, M.; Latini, A.; Laquintana, V.; Pellini, R.; Colafigli, M.; Frasca, M.; Giuliani, M.; et al. Human papillomavirus detection in matched oral rinses, oropharyngeal and oral brushings of cancer-free high-risk individuals. Oral Oncol. 2019, 91, 1–6. [Google Scholar] [CrossRef]
- Benevolo, M.; Rollo, F.; Giuliani, M.; Pichi, B.; Latini, A.; Pellini, R.; Vescio, M.F.; Morrone, A.; Cristaudo, A.; Dona, M.G. Abnormal cytology in oropharyngeal brushings and in oral rinses is not associated with HPV infection: The OHMAR study. Cancer Cytopathol. 2020, 128, 648–655. [Google Scholar] [CrossRef]
- Laprise, C.; Madathil, S.A.; Schlecht, N.F.; Castonguay, G.; Soulieres, D.; Nguyen-Tan, P.F.; Allison, P.; Coutlee, F.; Hier, M.; Rousseau, M.C.; et al. Human papillomavirus genotypes and risk of head and neck cancers: Results from the HeNCe Life case-control study. Oral Oncol. 2017, 69, 56–61. [Google Scholar] [CrossRef]
- De Souza, M.M.A.; Hartel, G.; Whiteman, D.C.; Antonsson, A. Detection of oral HPV infection—Comparison of two different specimen collection methods and two HPV detection methods. Diagn. Microbiol. Infect. Dis. 2018, 90, 267–271. [Google Scholar] [CrossRef]
- Stankiewicz Karita, H.C.; Magaret, A.; Huang, M.L.; Jerome, K.R.; Feng, Q.; Wald, A. Quantitative Oral HPV16 and HPV18 Detection in Persons Attending Dental Clinics. Sex. Transm. Dis. 2020, 47, 100–104. [Google Scholar] [CrossRef]
- Tsikis, S.; Hoefer, L.; Bethimoutis, G.; Nicolaidou, E.; Paparizos, V.; Antoniou, C.; Chardalias, L.; Stavropoulos, G.E.; Sharma, S.; Long, B.C.; et al. Risk factors, prevalence, and site concordance of human papillomavirus in high-risk Greek men. Eur. J. Cancer Prev. 2018, 27, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.H.; Kemp, T.J.; Isaacs-Soriano, K.; Abrahamsen, M.; Pan, Y.; Lazcano-Ponce, E.; Salmeron, J.; Pinto, L.A.; Giuliano, A.R. HPV-specific antibodies at the oral cavity up to 30months after the start of vaccination with the quadrivalent HPV vaccine among mid-adult aged men. Vaccine 2019, 37, 2864–2869. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; McNeel, T.S.; Fakhry, C. Understanding personal risk of oropharyngeal cancer: Risk-groups for oncogenic oral HPV infection and oropharyngeal cancer. Ann. Oncol. 2017, 28, 3065–3069. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; Clemens, G.; Troy, T.; Castillo, R.G.; Struijk, L.; Waterboer, T.; Bender, N.; Pierorazio, P.M.; Best, S.R.; Strickler, H.; et al. Evaluating the Utility and Prevalence of HPV Biomarkers in Oral Rinses and Serology for HPV-related Oropharyngeal Cancer. Cancer Prev. Res. 2019, 12, 689–700. [Google Scholar] [CrossRef]
- Hanna, G.J.; Lau, C.J.; Mahmood, U.; Supplee, J.G.; Mogili, A.R.; Haddad, R.I.; Janne, P.A.; Paweletz, C.P. Salivary HPV DNA informs locoregional disease status in advanced HPV-associated oropharyngeal cancer. Oral Oncol. 2019, 95, 120–126. [Google Scholar] [CrossRef]
- Fakhry, C.; Blackford, A.L.; Neuner, G.; Xiao, W.; Jiang, B.; Agrawal, A.; Gillison, M.L. Association of Oral Human Papillomavirus DNA Persistence With Cancer Progression After Primary Treatment for Oral Cavity and Oropharyngeal Squamous Cell Carcinoma. JAMA Oncol. 2019, 5, 985–992. [Google Scholar] [CrossRef]
- Kofler, B.; Borena, W.; Dudas, J.; Innerhofer, V.; Dejaco, D.; Steinbichler, T.B.; Widmann, G.; von Laer, D.; Riechelmann, H. Post-Treatment HPV Surface Brushings and Risk of Relapse in Oropharyngeal Carcinoma. Cancers 2020, 12, 1069. [Google Scholar] [CrossRef]
- Tanaka, H.; Takemoto, N.; Horie, M.; Takai, E.; Fukusumi, T.; Suzuki, M.; Eguchi, H.; Komukai, S.; Tatsumi, M.; Isohashi, F.; et al. Circulating Tumor HPV DNA Complements PET-CT in Guiding Management after Radiotherapy in HPV-Related Squamous Cell Carcinoma of the Head and Neck. Int. J. Cancer 2020. [Google Scholar] [CrossRef]
- Reder, H.; Taferner, V.F.; Wittekindt, C.; Brauninger, A.; Speel, E.M.; Gattenlohner, S.; Wolf, G.; Klussmann, J.P.; Wuerdemann, N.; Wagner, S. Plasma cell-free human papillomavirus oncogene E6- and E7-DNA predicts outcome in oropharyngeal squamous cell carcinoma. J. Mol. Diagn. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chera, B.S.; Kumar, S.; Shen, C.; Amdur, R.; Dagan, R.; Green, R.; Goldman, E.; Weiss, J.; Grilley-Olson, J.; Patel, S.; et al. Plasma Circulating Tumor HPV DNA for the Surveillance of Cancer Recurrence in HPV-Associated Oropharyngeal Cancer. J. Clin. Oncol. 2020, 38, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Chera, B.S.; Kumar, S.; Beaty, B.T.; Marron, D.; Jefferys, S.; Green, R.; Goldman, E.C.; Amdur, R.; Sheets, N.; Dagan, R.; et al. Rapid Clearance Profile of Plasma Circulating Tumor HPV Type 16 DNA during Chemoradiotherapy Correlates with Disease Control in HPV-Associated Oropharyngeal Cancer. Clin. Cancer Res. 2019, 25, 4682–4690. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, J.C.; Oton-Gonzalez, L.; Mazziotta, C.; Lanzillotti, C.; Iaquinta, M.R.; Tognon, M.; Martini, F. Simultaneous Detection and Viral DNA Load Quantification of Different Human Papillomavirus Types in Clinical Specimens by the High Analytical Droplet Digital PCR Method. Front. Microbiol. 2020, 11, 591452. [Google Scholar] [CrossRef]
- Bettampadi, D.; Sirak, B.A.; Abrahamsen, M.E.; Reich, R.R.; Villa, L.L.; Ponce, E.L.; Giuliano, A.R. Factors associated with persistence and clearance of high-risk oral HPV among participants in the HPV Infection in Men (HIM) study. Clin. Infect. Dis. 2020, ciaa1701. [Google Scholar] [CrossRef]
- Handisurya, A.; Schellenbacher, C.; Haitel, A.; Senger, T.; Kirnbauer, R. Human papillomavirus vaccination induces neutralising antibodies in oral mucosal fluids. Br. J. Cancer 2016, 114, 409–416. [Google Scholar] [CrossRef]
- Loimaranta, V.; Sievi, K.; Werner, J.; Pawlita, M.; Waterboer, T.; Butt, J.; Syrjanen, S. Comparison of multiplex-serology and ELISA based methods in detecting HPV16 L1 antibody responses in paired saliva and serum samples of healthy men. J. Virol. Methods 2019, 270, 26–33. [Google Scholar] [CrossRef]
- Ahn, S.M.; Chan, J.Y.; Zhang, Z.; Wang, H.; Khan, Z.; Bishop, J.A.; Westra, W.; Koch, W.M.; Califano, J.A. Saliva and plasma quantitative polymerase chain reaction-based detection and surveillance of human papillomavirus-related head and neck cancer. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 846–854. [Google Scholar] [CrossRef]
- Smith, E.M.; Hoffman, H.T.; Summersgill, K.S.; Kirchner, H.L.; Turek, L.P.; Haugen, T.H. Human papillomavirus and risk of oral cancer. Laryngoscope 1998, 108, 1098–1103. [Google Scholar] [CrossRef]
- Schwartz, S.M.; Daling, J.R.; Doody, D.R.; Wipf, G.C.; Carter, J.J.; Madeleine, M.M.; Mao, E.J.; Fitzgibbons, E.D.; Huang, S.; Beckmann, A.M.; et al. Oral cancer risk in relation to sexual history and evidence of human papillomavirus infection. J. Natl. Cancer Inst. 1998, 90, 1626–1636. [Google Scholar] [CrossRef]
- Pickard, R.K.; Xiao, W.; Broutian, T.R.; He, X.; Gillison, M.L. The prevalence and incidence of oral human papillomavirus infection among young men and women, aged 18-30 years. Sex. Transm. Dis. 2012, 39, 559–566. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.; Agrawal, Y.; Halpern, J.; Bodison, S.; Gillison, M.L. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J. Infect. Dis. 2009, 199, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Broutian, T.; Pickard, R.K.; Tong, Z.Y.; Xiao, W.; Kahle, L.; Graubard, B.I.; Chaturvedi, A.K. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA 2012, 307, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Bergman, H.; Buckley, B.S.; Villanueva, G.; Petkovic, J.; Garritty, C.; Lutje, V.; Riveros-Balta, A.X.; Low, N.; Henschke, N. Comparison of different human papillomavirus (HPV) vaccine types and dose schedules for prevention of HPV-related disease in females and males. Cochrane Database Syst. Rev. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Wilkin, T.J.; Chen, H.; Cespedes, M.S.; Leon-Cruz, J.T.; Godfrey, C.; Chiao, E.Y.; Bastow, B.; Webster-Cyriaque, J.; Feng, Q.; Dragavon, J.; et al. A Randomized, Placebo-Controlled Trial of the Quadrivalent Human Papillomavirus Vaccine in Human Immunodeficiency Virus-Infected Adults Aged 27 Years or Older: AIDS Clinical Trials Group Protocol A5298. Clin. Infect. Dis. 2018, 67, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Kacew, A.J.; Grimes, A.C.; Roth, M.; Teoh, D.; Landier, W.; Strohbehn, G.W.; Paskett, E.D. The case for catch-up human papillomavirus vaccination in at-risk populations: Rural communities and survivors of pediatric and young adult cancers. CA Cancer J. Clin. 2020, 70, 518–519. [Google Scholar] [CrossRef] [PubMed]
- Enerly, E.; Berger, S.; Kjaer, S.K.; Sundstrom, K.; Campbell, S.; Tryggvadottir, L.; Munk, C.; Hortlund, M.; Group, T.; Joshi, A.; et al. Use of real-world data for HPV vaccine trial follow-up in the Nordic region. Contemp. Clin. Trials 2020, 92, 105996. [Google Scholar] [CrossRef]
- Fakhry, C.; Rosenthal, B.T.; Clark, D.P.; Gillison, M.L. Associations between oral HPV16 infection and cytopathology: Evaluation of an oropharyngeal “pap-test equivalent“ in high-risk populations. Cancer Prev. Res. 2011, 4, 1378–1384. [Google Scholar] [CrossRef]
- D’Souza, G.; Kreimer, A.R.; Viscidi, R.; Pawlita, M.; Fakhry, C.; Koch, W.M.; Westra, W.H.; Gillison, M.L. Case-control study of human papillomavirus and oropharyngeal cancer. N. Engl. J. Med. 2007, 356, 1944–1956. [Google Scholar] [CrossRef]
- Franceschi, S.; Combes, J.D.; Dalstein, V.; Caudroy, S.; Clifford, G.; Gheit, T.; Tommasino, M.; Clavel, C.; Lacau St Guily, J.; Birembaut, P.; et al. Deep brush-based cytology in tonsils resected for benign diseases. Int. J. Cancer 2015, 137, 2994–2999. [Google Scholar] [CrossRef]
- Zhang, Y.; Waterboer, T.; Haddad, R.I.; Miles, B.A.; Wentz, A.; Gross, N.D.; Fakhry, C.; Quon, H.; Lorch, J.H.; Gourin, C.G.; et al. Human papillomavirus (HPV) 16 antibodies at diagnosis of HPV-related oropharyngeal cancer and antibody trajectories after treatment. Oral Oncol. 2017, 67, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.J.; Supplee, J.G.; Kuang, Y.; Mahmood, U.; Lau, C.J.; Haddad, R.I.; Janne, P.A.; Paweletz, C.P. Plasma HPV cell-free DNA monitoring in advanced HPV-associated oropharyngeal cancer. Ann. Oncol. 2018, 29, 1980–1986. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.J.; Sridharan, V.; Margalit, D.N.; La Follette, S.K.; Chau, N.G.; Rabinowits, G.; Lorch, J.H.; Haddad, R.I.; Tishler, R.B.; Anderson, K.S.; et al. Salivary and serum HPV antibody levels before and after definitive treatment in patients with oropharyngeal squamous cell carcinoma. Cancer Biomark. 2017, 19, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, S.C.; Muzaffar, S.J.; Ahmed, I.; Dhanda, J.; Paleri, V.; Mehanna, H. Efficacy, outcomes, and complication rates of different surgical and nonsurgical treatment modalities for recurrent/residual oropharyngeal carcinoma: A systematic review and meta-analysis. Head Neck 2016, 38, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Wuerdemann, N.; Jain, R.; Adams, A.; Speel, E.M.; Wagner, S.; Joosse, S.A.; Klussmann, J.P. Cell-Free HPV-DNA as a Biomarker for Oropharyngeal Squamous Cell Carcinoma-A Step Towards Personalized Medicine? Cancers 2020, 12, 2997. [Google Scholar] [CrossRef] [PubMed]
| First Author | Year | N | Setting | Collection Method | Testing Method | Genotypes Included | Findings |
|---|---|---|---|---|---|---|---|
| Mouth/Throat Sample Collection | |||||||
| Stankiewicz Karita [12] | 2020 | 15,313 | Epidemiology, Seattle, Washington, United States | Oral rinse | RT-qPCR | 16, 18 | 1% overall prevalence, OR 3.2 for men vs. women |
| Kofler [19] | 2020 | 62 | Surveillance for recurrence | Endoscopic oropharynx brushing | RT-PCR | Broad (40 types) | Clearance of oropharyngeal HPV DNA predicts lower chance of recurrence |
| Benevolo [9] | 2020 | 310 | Screening for HPV-related malignancy | Oral rinse vs. brushing | PCR | Broad (37 types) | HPV genetic material does not correlate with cytologic abnormalities |
| Dona [8] | 2019 | 163 | Epidemiology, Rome, Italy, high-risk individuals | Oral rinse vs. oropharyngeal brushing vs. oral brushing | PCR | Broad (37 types) | 51.2% agreement for oral rinse vs. oropharyngeal brushings, 74.1% for high-risk genotypes |
| D’Souza [16] | 2019 | 694 | Screening for HPV-related malignancy | Oral rinse, serum | PCR, ELISA | Broad | Low sensitivity (43–88%) and high specificity (≥98%) in the screening setting; serum Ab testing performs better than oral rinse |
| Hanna [17] | 2019 | 21 | Risk stratification after OPC diagnosis, monitoring treatment | Oral rinse, serum | ddPCR | 16, 18, 31, 33, 45 | Baseline plasma ctHPVDNA levels associated with poor outcomes; trends in salivary DNA predicts outcomes |
| Fakhry [18] | 2019 | 396 | Surveillance for recurrence | Oral rinse | PCR | Broad (37 types) | Detection of oral HPV after therapy portends worse RFS and OS |
| Chikandiwa [7] | 2018 | 181 | Epidemiology, Johannesburg, South Africa, HIV-infected men | Paired oral rinse vs. oral swab | PCR | Broad (37 types) | 1.8% prevalence in oral rinse vs. 0.6% in oral swab |
| Tsikis [13] | 2018 | 294 | Epidemiology, Athens, Greece, high-risk men | Oral rinse vs. anal swab vs. penile swab | Next-generation sequencing | Broad | 49% prevalence at any site: 33% anal, 23% penile, 4% oral; Low concordance (≤2%) between oral and anogenital site |
| De Souza [11] | 2018 | 96 | Epidemiology, Brisbane, Australia | Oral rinse vs. spit (commercial saliva kit) | PCR (single primer vs. nested) | Broad | Oral rinse: 11.5% (nested PCR), 10.4% (single primer PCR) Spit: 16.7% (nested PCR), 3.1% (single primer PCR) |
| Combes [6] | 2017 | 692 | Epidemiology, France | Oral rinse vs. brushing from tonsillectomy specimen | PCR (bead-based multiplex assay) | Broad (21 types) | 13.1% prevalence in rinse vs. 3.6% in tonsil brushings |
| D’Souza [15] | 2017 | 13,089 | Epidemiology, screening for HPV-related malignancy | Oral rinse | PCR | Broad (37 types) | 3.5% prevalence of HPV infection, 37 per 10,000 annual OPC incidence |
| Laprise [10] | 2017 | 918 | Screening for HPV-related malignancy | Oral rinse vs. brushing | PCR | Broad (37 types) | HPV infection associated with OR 10.8 for OPC, 47.2 with HPV16 infection |
| Serum-Based Sample Collection | |||||||
| Tanaka [20] | 2020 | 35 | Surveillance for recurrence | Serum (ctDNA) | ddPCR | 16 | ctHPV16DNA, when combined with PET-CT, predicts recurrence |
| Reder [21] | 2020 | 50 | Surveillance for recurrence | Serum (ctDNA) | RT-qPCR | 16 | Lower post-therapy ctHPVDNA corresponds with reduced chance of recurrence |
| Chera [22] | 2020 | 115 | Surveillance for recurrence | Serum (ctDNA) | ddPCR | 16, 18, 31, 33, 35 | Undetectable ctHPVDNA at all post-treatment timepoints has 100% NPV for recurrence; two consecutive positive ctHPVDNA tests after treatment has 94% PPV for recurrence |
| Chera [23] | 2019 | 103 | Monitoring treatment | Serum (ctDNA) | ddPCR | 16, 18, 31, 33, 35 | Poor ctHPVDNA clearance associated with treatment failure; ctHPVDNA copy number associated with tumor burden and HPV genome integration |
| Includes both Mouth/Throat and Serum-Based Samples | |||||||
| Parker [14] | 2019 | 150 | Immunogenicity, international, adult males receiving quadrivalent vaccine | Matched oral rinse, serum at multiple timepoints | ELISA | 16, 18 | Oral anti-HPV Abs present in majority at month 7, minority at month 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kacew, A.J.; Hanna, G.J. Value and Unmet Needs in Non-Invasive Human Papillomavirus (HPV) Testing for Oropharyngeal Cancer. Cancers 2021, 13, 562. https://doi.org/10.3390/cancers13030562
Kacew AJ, Hanna GJ. Value and Unmet Needs in Non-Invasive Human Papillomavirus (HPV) Testing for Oropharyngeal Cancer. Cancers. 2021; 13(3):562. https://doi.org/10.3390/cancers13030562
Chicago/Turabian StyleKacew, Alec J., and Glenn J. Hanna. 2021. "Value and Unmet Needs in Non-Invasive Human Papillomavirus (HPV) Testing for Oropharyngeal Cancer" Cancers 13, no. 3: 562. https://doi.org/10.3390/cancers13030562
APA StyleKacew, A. J., & Hanna, G. J. (2021). Value and Unmet Needs in Non-Invasive Human Papillomavirus (HPV) Testing for Oropharyngeal Cancer. Cancers, 13(3), 562. https://doi.org/10.3390/cancers13030562






