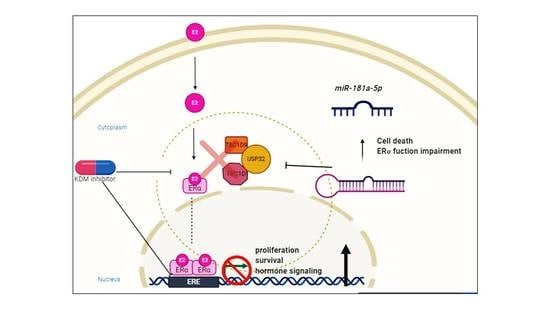
Regulatory Interplay between miR-181a-5p and Estrogen Receptor Signaling Cascade in Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
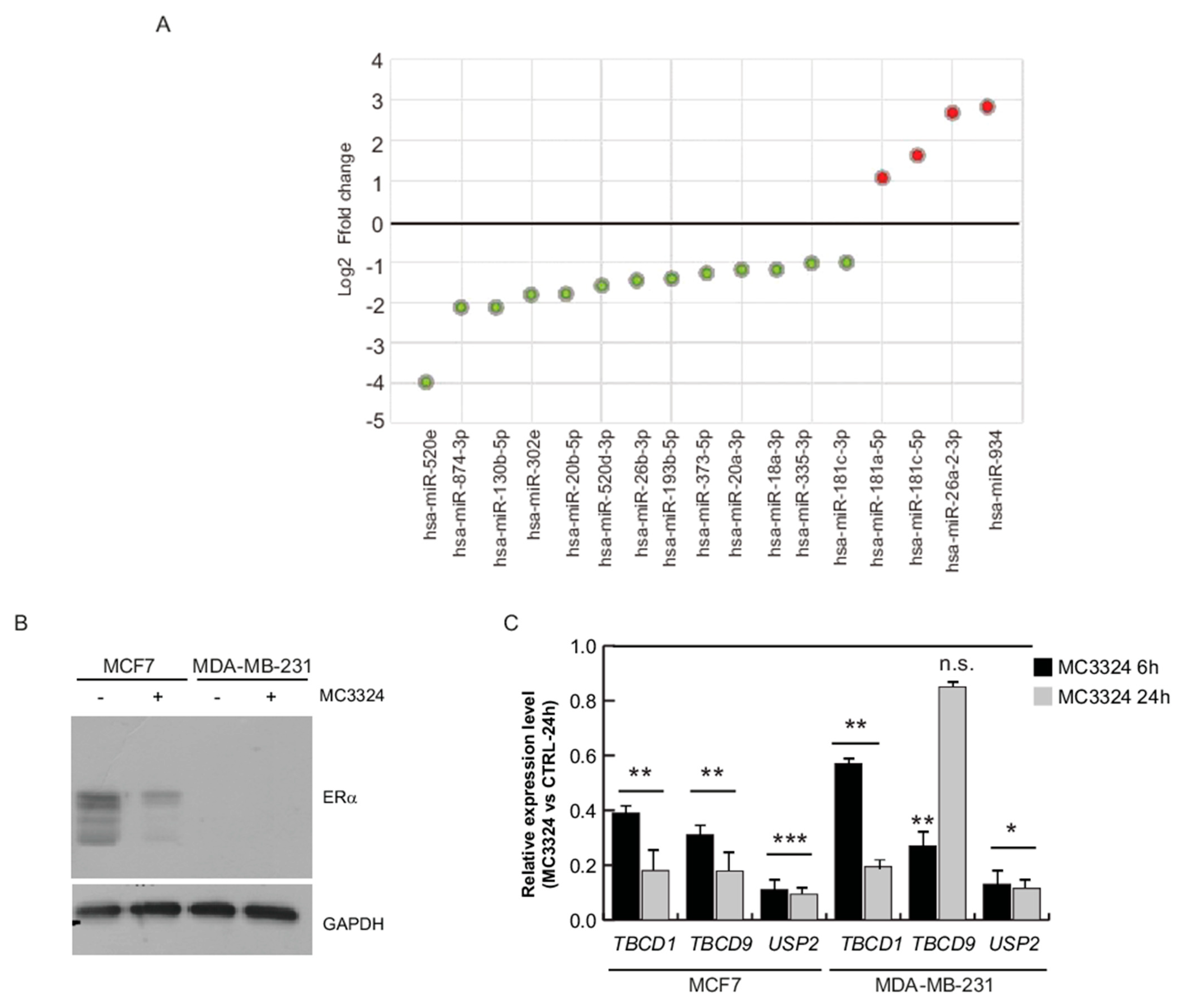
2.1. Inhibition of LSD1 and UTX Affects miRNA Profile
2.2. Integrative Analysis of Multi-Omics Data: miRNome, mRNA Transcriptome, and Interactome
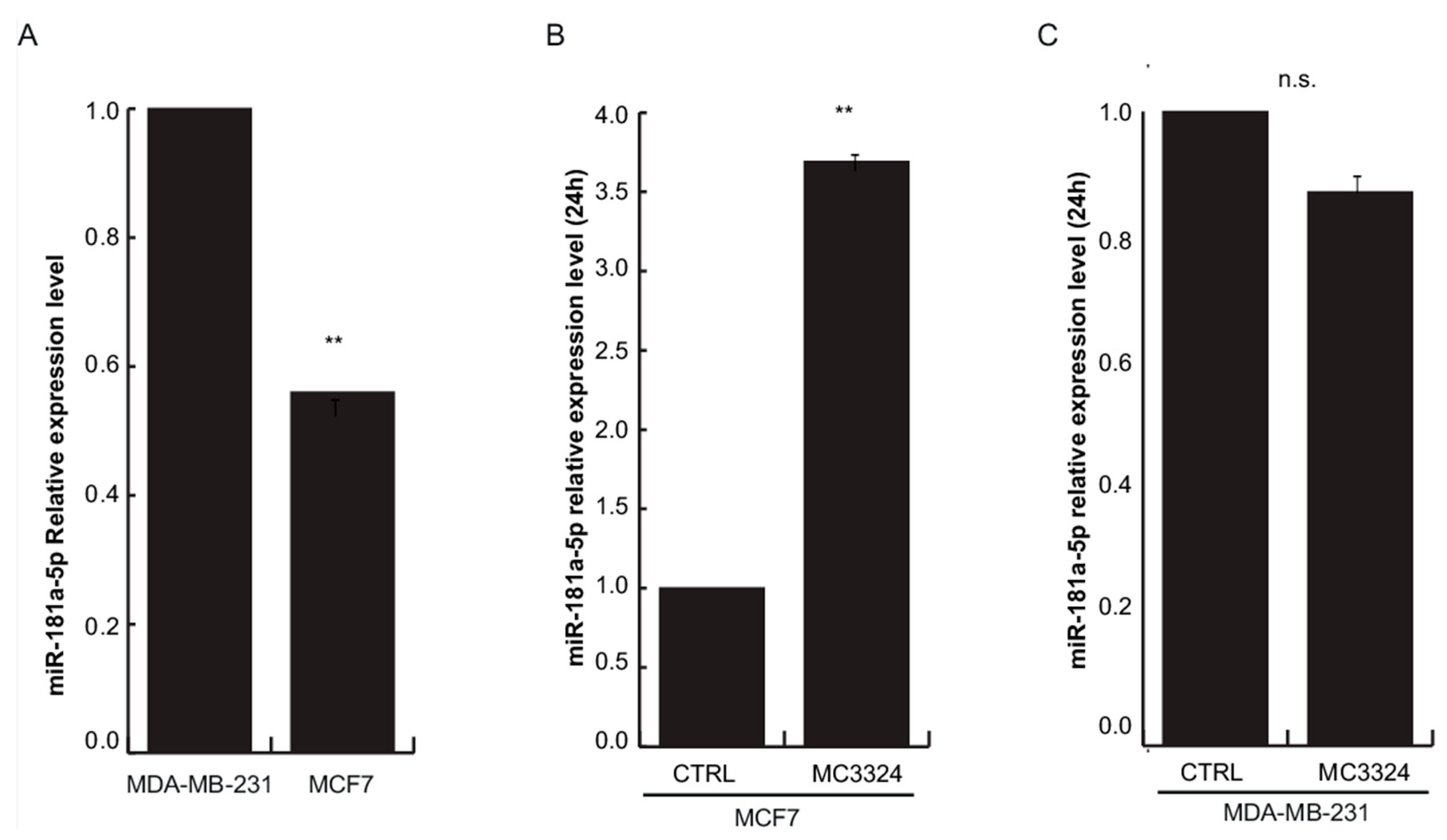
2.3. ERα-Mediated miRNA Signature
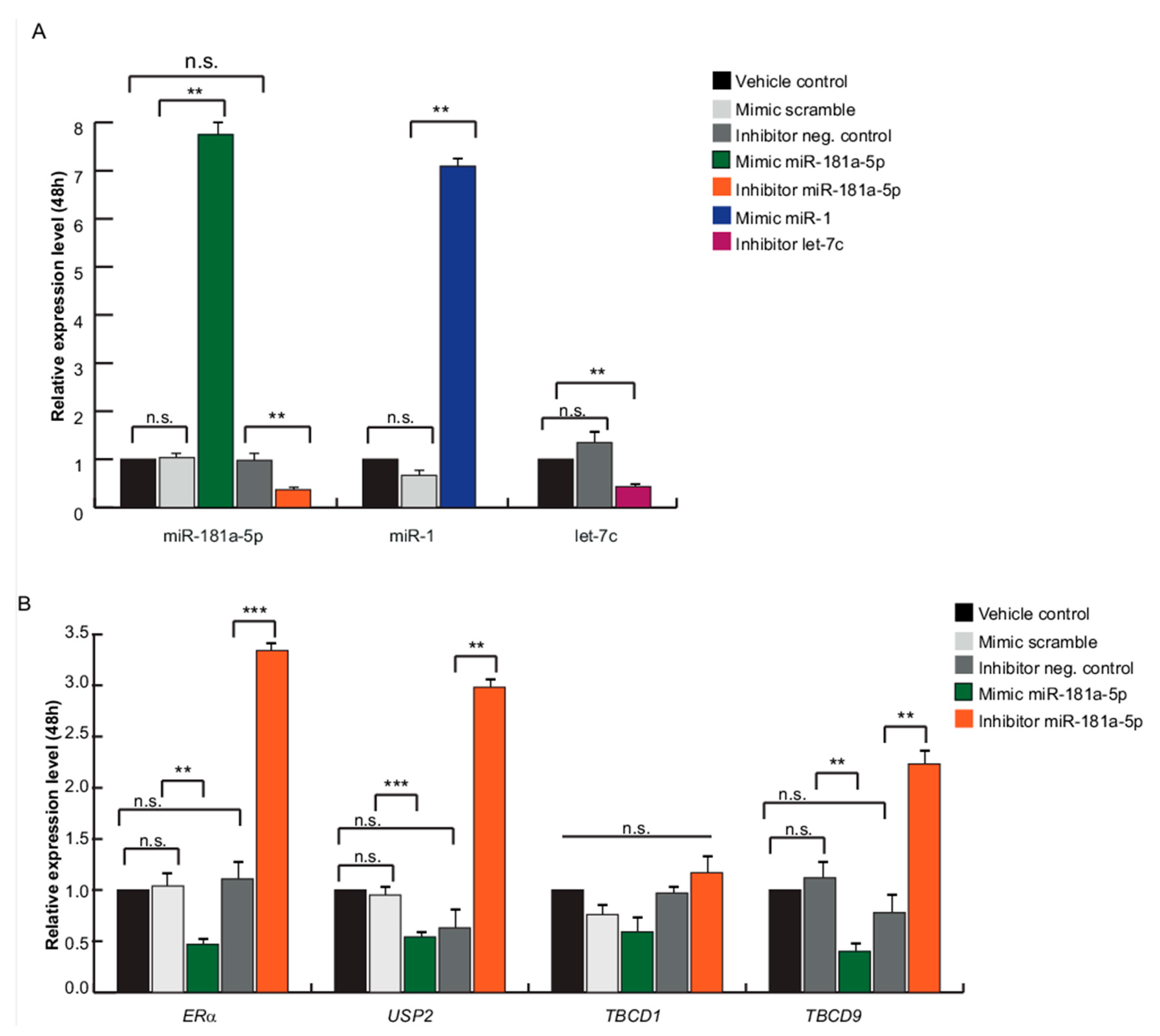
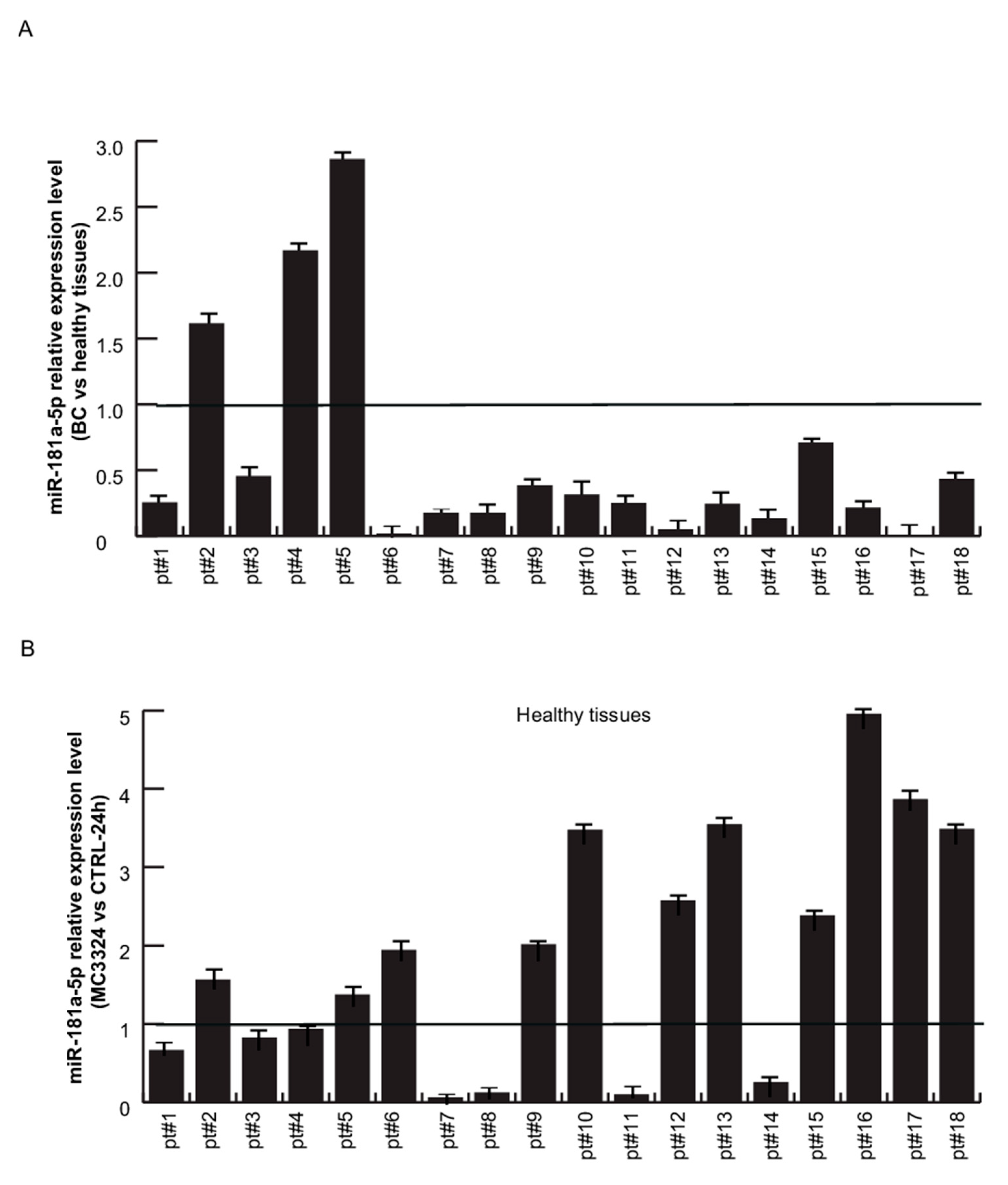
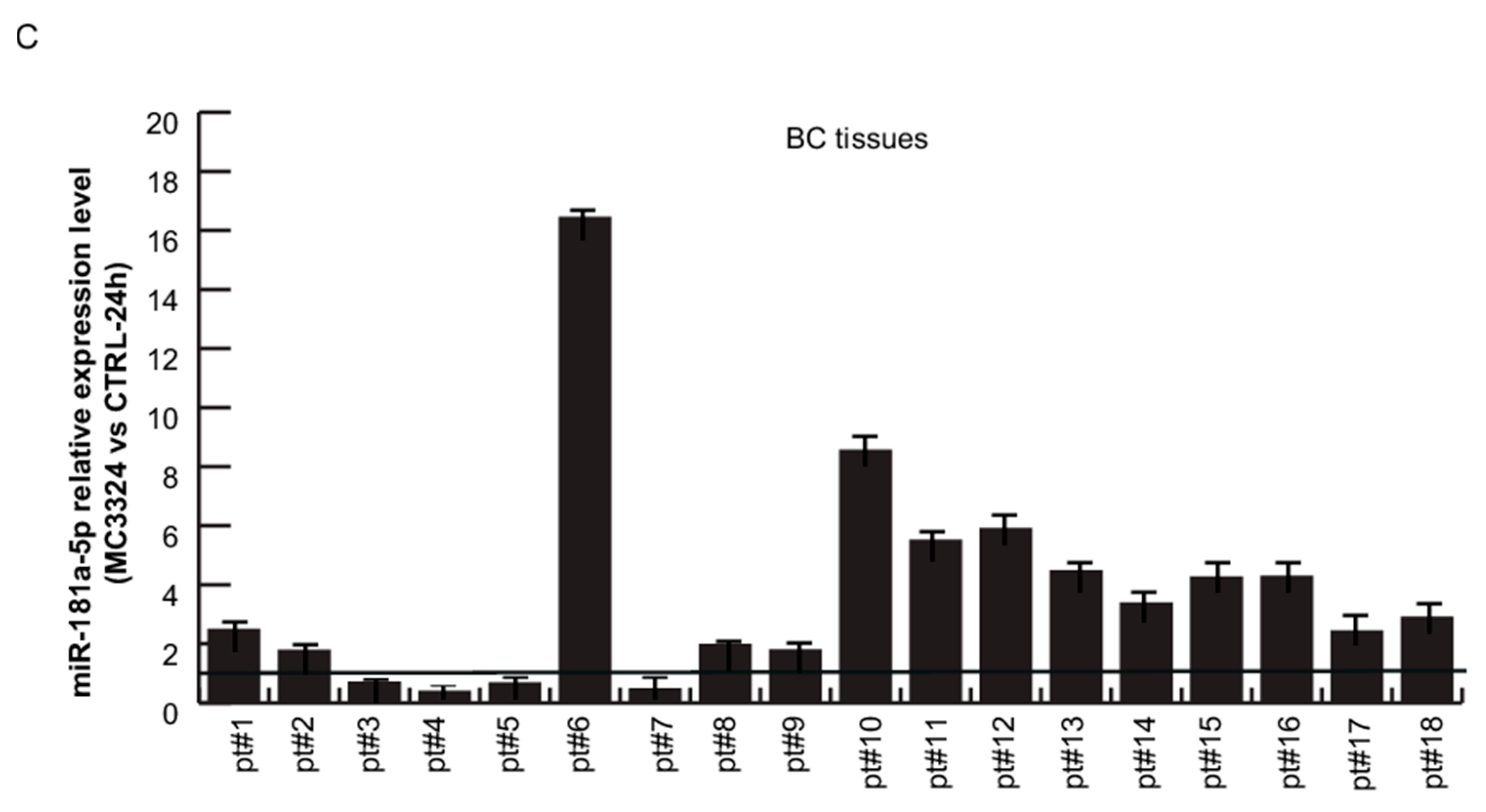
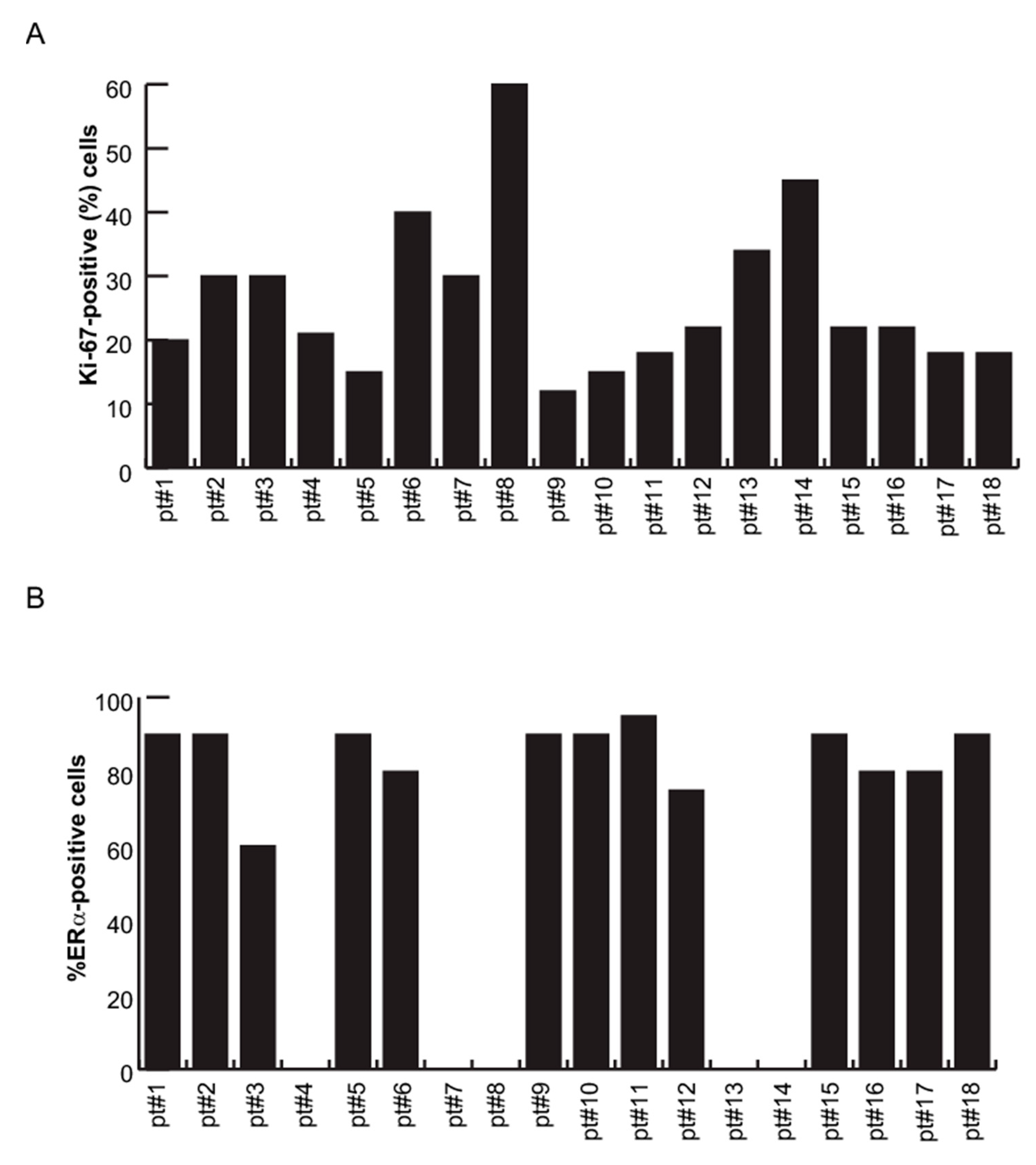
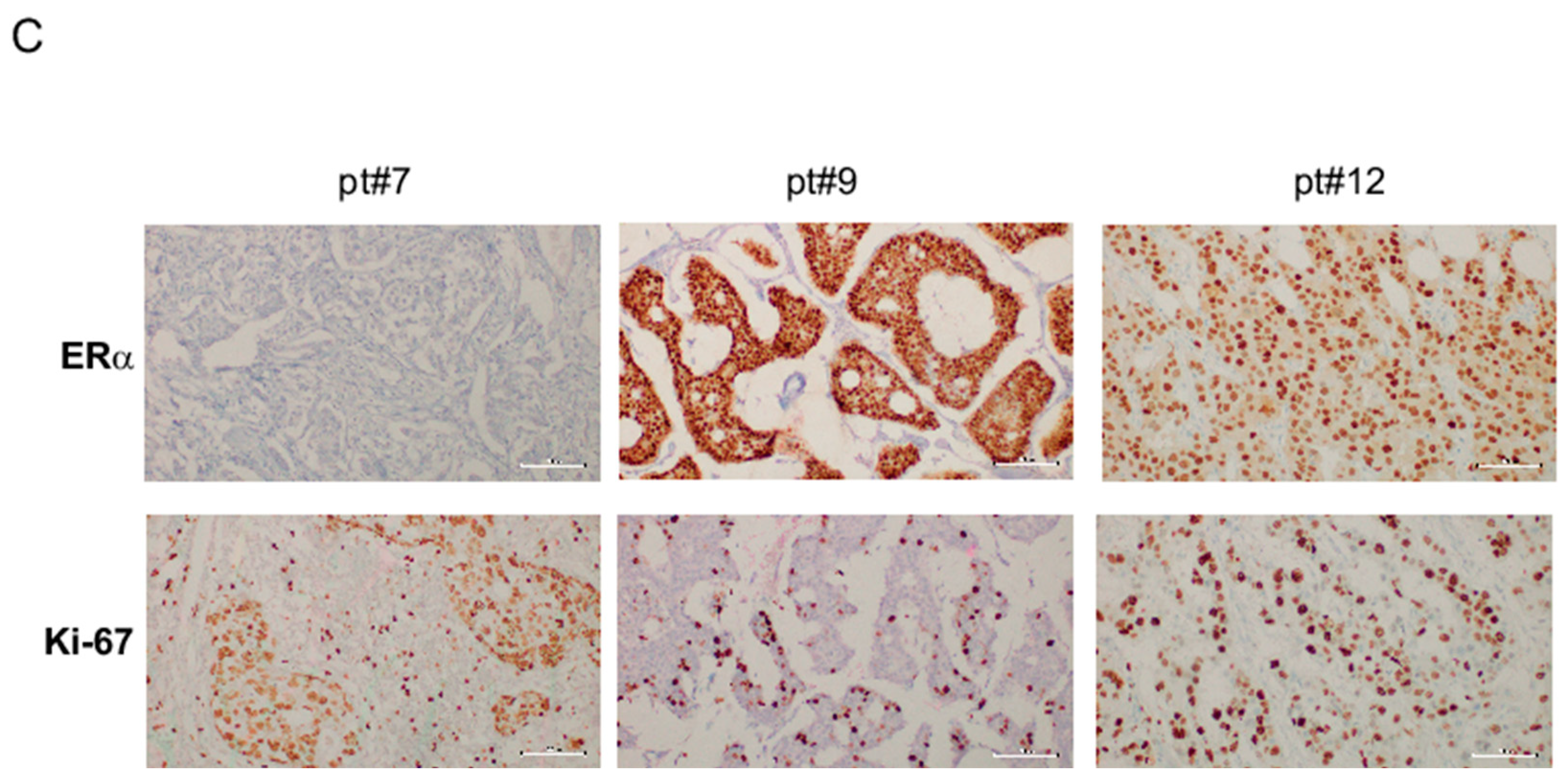
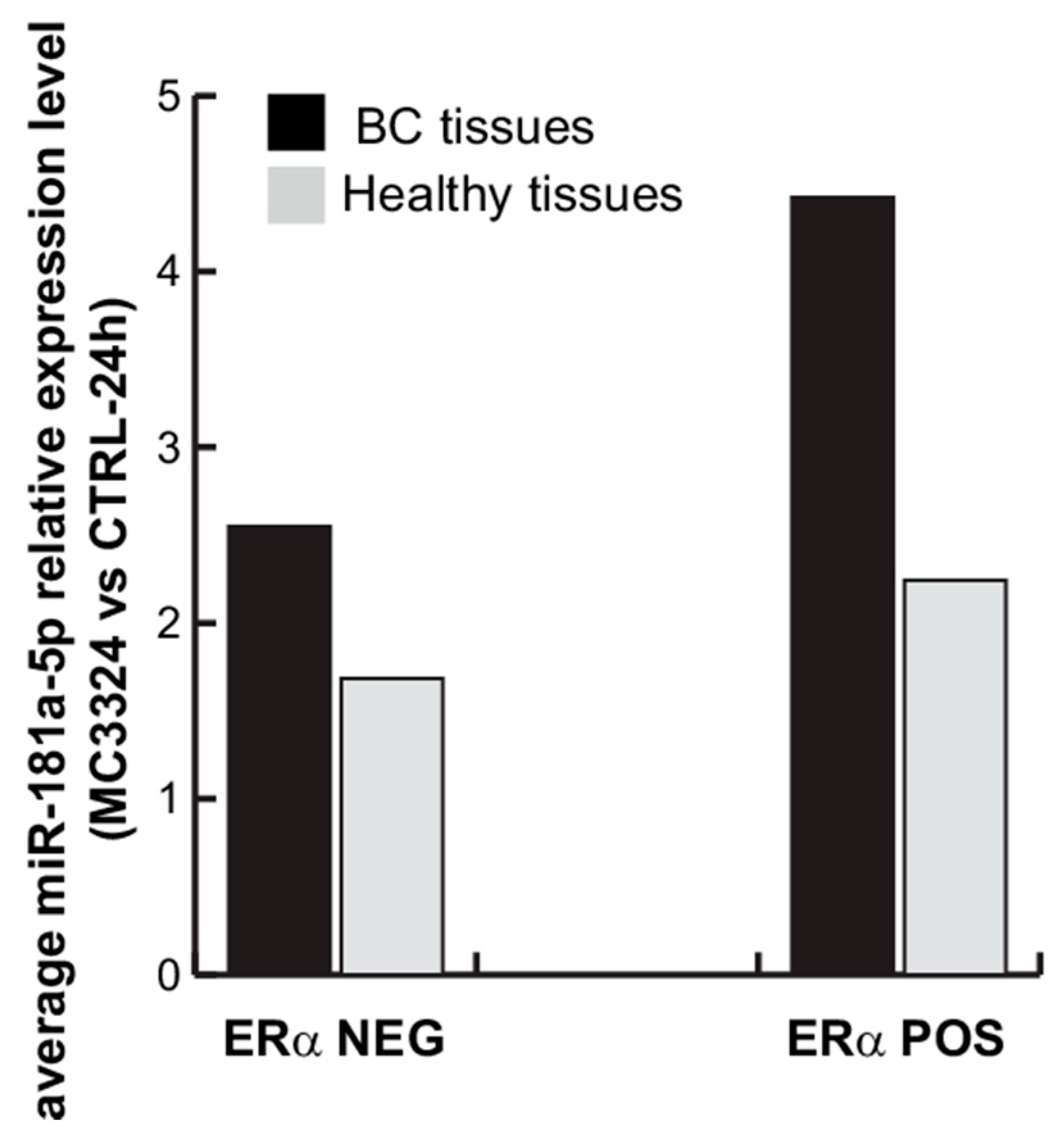
2.4. miR-181a-5p as a Potential Hallmark of BC
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. miRNome Profiling
4.3. RNA Isolation and miRNA Expression Validation
4.4. Gene Expression Analysis by qPCR Quantitative Real-Time PCR
4.5. Pre-miR Precursor and Inhibitor of miR-181a-5p Reverse Transfection
4.6. Computational Prediction and Gene Enrichment Analysis of miRNA Target Genes
4.7. Data Collection and Sampling of Primary BC Tissue
4.8. Western Blot Analysis
4.9. IHC Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The, L. Globocan 2018: Counting the toll of cancer. Lancet 2018, 392, 985. [Google Scholar] [CrossRef]
- American Institute for Cancer Research. Available online: https://www.aicr.org/ (accessed on 18 November 2020).
- American Cancer Society. Breast Cancer Facts &Figures 2017–2018; American Cancer Society, Inc.: Atlanta, GA, USA, 2017. [Google Scholar]
- Rani, A.; Stebbing, J.; Giamas, G.; Murphy, J. Endocrine Resistance in Hormone Receptor Positive Breast Cancer-From Mechanism to Therapy. Front. Endocrinol. (Lausanne) 2019, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Eguchi, H.; Nakachi, K.; Tanimoto, K.; Higashi, Y.; Suemasu, K.; Iino, Y.; Morishita, Y.; Hayashi, S. Distinct mechanisms of loss of estrogen receptor alpha gene expression in human breast cancer: Methylation of the gene and alteration of trans-acting factors. Carcinogenesis 2000, 21, 2193–2201. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.J.; Cheung, K.L. Endocrine Therapy for Breast Cancer: A Model of Hormonal Manipulation. Oncol. Ther. 2018, 6, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Chang, J.; Fu, P. Endocrine therapy resistance in breast cancer: Current status, possible mechanisms and overcoming strategies. Future Med. Chem. 2015, 7, 1511–1519. [Google Scholar] [CrossRef]
- Decker, J.T.; Hall, M.S.; Blaisdell, R.B.; Schwark, K.; Jeruss, J.S.; Shea, L.D. Dynamic microRNA activity identifies therapeutic targets in trastuzumab-resistant HER2(+) breast cancer. Biotechnol. Bioeng. 2018, 115, 2613–2623. [Google Scholar] [CrossRef]
- Muluhngwi, P.; Klinge, C.M. Roles for miRNAs in endocrine resistance in breast cancer. Endocr. Relat. Cancer 2015, 22, R279–R300. [Google Scholar] [CrossRef]
- Howard, E.W.; Yang, X. microRNA Regulation in Estrogen Receptor-Positive Breast Cancer and Endocrine Therapy. Biol. Proced. Online 2018, 20, 17. [Google Scholar] [CrossRef]
- Loh, H.Y.; Norman, B.P.; Lai, K.S.; Rahman, N.; Alitheen, N.B.M.; Osman, M.A. The Regulatory Role of MicroRNAs in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 4940. [Google Scholar] [CrossRef]
- Si, W.; Shen, J.; Zheng, H.; Fan, W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin. Epigenetics 2019, 11, 25. [Google Scholar] [CrossRef]
- Lindholm, E.M.; Ragle Aure, M.; Haugen, M.H.; Kleivi Sahlberg, K.; Kristensen, V.N.; Nebdal, D.; Borresen-Dale, A.L.; Lingjaerde, O.C.; Engebraaten, O. miRNA expression changes during the course of neoadjuvant bevacizumab and chemotherapy treatment in breast cancer. Mol. Oncol. 2019, 13, 2278–2296. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, E.; Lamberti, J.; Saggese, P.; Pecoraro, G.; Memoli, D.; Cappa, V.M.; Ravo, M.; Iorio, R.; Tarallo, R.; Rizzo, F.; et al. Small Non-Coding RNA Profiling Identifies miR-181a-5p as a Mediator of Estrogen Receptor Beta-Induced Inhibition of Cholesterol Biosynthesis in Triple-Negative Breast Cancer. Cells 2020, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, M.; Roske, A.; Laufer, T.; Fehlmann, T.; Backes, C.; Kern, F.; Kohlhaas, J.; Schrors, H.; Saiz, A.; Zabler, C.; et al. MicroRNA in diagnosis and therapy monitoring of early-stage triple-negative breast cancer. Sci. Rep. 2018, 8, 11584. [Google Scholar] [CrossRef] [PubMed]
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef]
- Klinge, C.M. miRNAs regulated by estrogens, tamoxifen, and endocrine disruptors and their downstream gene targets. Mol. Cell. Endocrinol. 2015, 418 Pt 3, 273–297. [Google Scholar] [CrossRef]
- Castellano, L.; Giamas, G.; Jacob, J.; Coombes, R.C.; Lucchesi, W.; Thiruchelvam, P.; Barton, G.; Jiao, L.R.; Wait, R.; Waxman, J.; et al. The estrogen receptor-α-induced microRNA signature regulates itself and its transcriptional response. PNAS 2009, 106, 15732–15737. [Google Scholar] [CrossRef]
- Jiang, C.F.; Li, D.M.; Shi, Z.M.; Wang, L.; Liu, M.M.; Ge, X.; Liu, X.; Qian, Y.C.; Wen, Y.Y.; Zhen, L.L.; et al. Estrogen regulates miRNA expression: Implication of estrogen receptor and miR-124/AKT2 in tumor growth and angiogenesis. Oncotarget 2016, 7, 36940–36955. [Google Scholar] [CrossRef]
- Bertoli, G.; Cava, C.; Castiglioni, I. MicroRNAs: New Biomarkers for Diagnosis, Prognosis, Therapy Prediction and Therapeutic Tools for Breast Cancer. Theranostics 2015, 5, 1122–1143. [Google Scholar] [CrossRef]
- Benedetti, R.; Dell’Aversana, C.; De Marchi, T.; Rotili, D.; Liu, N.Q.; Novakovic, B.; Boccella, S.; Di Maro, S.; Cosconati, S.; Baldi, A.; et al. Inhibition of Histone Demethylases LSD1 and UTX Regulates ERalpha Signaling in Breast Cancer. Cancers (Basel) 2019, 11, 2027. [Google Scholar] [CrossRef]
- Rotili, D.; Tomassi, S.; Conte, M.; Benedetti, R.; Tortorici, M.; Ciossani, G.; Valente, S.; Marrocco, B.; Labella, D.; Novellino, E.; et al. Pan-histone demethylase inhibitors simultaneously targeting Jumonji C and lysine-specific demethylases display high anticancer activities. J. Med. Chem. 2014, 57, 42–55. [Google Scholar] [CrossRef]
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkouni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-miRPath v3.0: Deciphering microRNA function with experimental support. Nucleic Acids Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef] [PubMed]
- John, B.; Enright, A.J.; Aravin, A.; Tuschl, T.; Sander, C.; Marks, D.S. Human MicroRNA targets. PLoS Biol. 2004, 2, e363. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.S.; Ebert, M.S.; van Oudenaarden, A. Genome-wide dissection of microRNA functions and cotargeting networks using gene set signatures. Mol. Cell 2010, 38, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Krek, A.; Grun, D.; Poy, M.N.; Wolf, R.; Rosenberg, L.; Epstein, E.J.; MacMenamin, P.; da Piedade, I.; Gunsalus, K.C.; Stoffel, M.; et al. Combinatorial microRNA target predictions. Nat. Genet. 2005, 37, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, M.; Iovino, N.; Unnerstall, U.; Gaul, U.; Segal, E. The role of site accessibility in microRNA target recognition. Nat. Genet. 2007, 39, 1278–1284. [Google Scholar] [CrossRef]
- Miranda, K.C.; Huynh, T.; Tay, Y.; Ang, Y.S.; Tam, W.L.; Thomson, A.M.; Lim, B.; Rigoutsos, I. A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell 2006, 126, 1203–1217. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife 2015, 4. [Google Scholar] [CrossRef]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Yang, C.; Tabatabaei, S.N.; Ruan, X.; Hardy, P. The Dual Regulatory Role of MiR-181a in Breast Cancer. Cell. Physiol. Biochem. 2017, 44, 843–856. [Google Scholar] [CrossRef]
- Jiao, X.; Zhao, L.; Ma, M.; Bai, X.; He, M.; Yan, Y.; Wang, Y.; Chen, Q.; Zhao, X.; Zhou, M.; et al. MiR-181a enhances drug sensitivity in mitoxantone-resistant breast cancer cells by targeting breast cancer resistance protein (BCRP/ABCG2). Breast Cancer Res. Treat. 2013, 139, 717–730. [Google Scholar] [CrossRef]
- Kastrati, I.; Canestrari, E.; Frasor, J. PHLDA1 expression is controlled by an estrogen receptor-NFkappaB-miR-181 regulatory loop and is essential for formation of ER+ mammospheres. Oncogene 2015, 34, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, J.; Li, S.; Ma, R.; Cao, H.; Ji, M.; Jing, C.; Tang, J. The function role of miR-181a in chemosensitivity to adriamycin by targeting Bcl-2 in low-invasive breast cancer cells. Cell. Physiol. Biochem. 2013, 32, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kuscu, C.; Banach, A.; Zhang, Q.; Pulkoski-Gross, A.; Kim, D.; Liu, J.; Roth, E.; Li, E.; Shroyer, K.R.; et al. miR-181a-5p Inhibits Cancer Cell Migration and Angiogenesis via Downregulation of Matrix Metalloproteinase-14. Mol. Cell. Pathobiol. 2015, 75, 2674–2685. [Google Scholar] [CrossRef] [PubMed]
- Grober, O.M.; Mutarelli, M.; Giurato, G.; Ravo, M.; Cicatiello, L.; De Filippo, M.R.; Ferraro, L.; Nassa, G.; Papa, M.F.; Paris, O.; et al. Global analysis of estrogen receptor beta binding to breast cancer cell genome reveals an extensive interplay with estrogen receptor alpha for target gene regulation. BMC Genom. 2011, 12, 36. [Google Scholar] [CrossRef]
- Andres, S.A.; Wittliff, J.L. Co-expression of genes with estrogen receptor-alpha and progesterone receptor in human breast carcinoma tissue. Horm. Mol. Biol. Clin. Investig. 2012, 12, 377–390. [Google Scholar] [CrossRef]
- Andres, S.A.; Smolenkova, I.A.; Wittliff, J.L. Gender-associated expression of tumor markers and a small gene set in breast carcinoma. Breast 2014, 23, 226–233. [Google Scholar] [CrossRef]
- Qin, S.; Ma, F.; Chen, L. Gene regulatory networks by transcription factors and microRNAs in breast cancer. Bioinformatics 2015, 31, 76–83. [Google Scholar] [CrossRef]
- Akhavantabasi, S.; Akman, H.B.; Sapmaz, A.; Keller, J.; Petty, E.M.; Erson, A.E. USP32 is an active, membrane-bound ubiquitin protease overexpressed in breast cancers. Mamm. Genome 2010, 21, 388–397. [Google Scholar] [CrossRef]
- Zhang, Y.; Martens, J.W.; Yu, J.X.; Jiang, J.; Sieuwerts, A.M.; Smid, M.; Klijn, J.G.; Wang, Y.; Foekens, J.A. Copy number alterations that predict metastatic capability of human breast cancer. Cancer Res. 2009, 69, 3795–3801. [Google Scholar] [CrossRef]
- Indrieri, A.; Carrella, S.; Carotenuto, P.; Banfi, S.; Franco, B. The Pervasive Role of the miR-181 Family in Development, Neurodegeneration, and Cancer. Int. J. Mol. Sci. 2020, 21, 2092. [Google Scholar] [CrossRef]
- Berber, U.; Yilmaz, I.; Narli, G.; Haholu, A.; Kucukodaci, Z.; Demirel, D. miR-205 and miR-200c: Predictive Micro RNAs for Lymph Node Metastasis in Triple Negative Breast Cancer. J. Breast Cancer 2014, 17, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Ota, D.; Mimori, K.; Yokobori, T.; Iwatsuki, M.; Kataoka, A.; Masuda, N.; Ishii, H.; Ohno, S.; Mori, M. Identification of recurrence-related microRNAs in the bone marrow of breast cancer patients. Int. J. Oncol. 2011, 38, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.; Dell’Aversana, C.; Sgueglia, G.; Carissimo, A.; Altucci, L. HDAC2-dependent miRNA signature in acute myeloid leukemia. FEBS Lett. 2019, 593, 2574–2584. [Google Scholar] [CrossRef] [PubMed]


| Gene Target | miRNA | ||||
|---|---|---|---|---|---|
| ER_3324 DOWN PROTEIN INTERACTORS | ESR1 (ERα) | miR-181c-5p | miR-185-3p | ||
| ENAH | miR-181a-5p | miR-181c-5p | |||
| STRBP | miR-181a-5p | ||||
| TBC1D9 | miR-181a-5p | miR-181c-5p | miR-451 | ||
| USP32 | let-7f-5p | ||||
| PKP4 | miR-193a-3p | ||||
| OSBPL8 | miR-181a-5p | miR-181c-5p | |||
| TBL1XR1 | miR-181a-5p | miR-181c-5p | miR-193a-3p | ||
| LSD1_3324 DOWN PROTEIN INTERACTORS | ANKRD50 | miR-181a-5p | miR-181c-5p | ||
| CCDC6 | miR-181a-5p | miR-181c-5p | |||
| DCLK1 | miR-181a-5p | miR-181c-5p | |||
| TANC2 | miR-146a-5p | miR-181a-5p | miR-181c-5 | ||
| TBC1D1 | miR-181a-5p | miR-181c-5p | |||
| USP32 | let-7f-5p | ||||
| ER_3324 UP PROTEIN INTERACTORS | SCD | let-7d-5p | |||
| RSBN1 | miR-20b-5p | miR-31-5p | miR-520d-3p | miR-520e | |
| ARHGAP17 | miR-101-3p | ||||
| LSD1_3324 UP PROTEIN INTERACTORS | MAP2K7 | miR-142-5p | |||
| MYH10 | miR-300 | ||||
| NAP1L1 | let-7d-5p | miR-101-3p | miR-148a-3p | ||
| TFRC | miR-144-3p | miR-148a-3p | miR-31-5p | miR-758-3p | |
| Cytological Classification | IHC Classification | Grade | Staging | PR (%) | HER2 | |
|---|---|---|---|---|---|---|
| PT#1 | C5 | Invasive carcinoma of no special type (NST) | G2 | pT1N0 | 70 | 3+ |
| PT#2 | Invasive carcinoma of no special type (NST) | G2 | pT1N0 | 70 | 0 | |
| PT#3 | Invasive carcinoma of no special type (NST) | G3 | pT2N2 | 60 | 1+ | |
| PT#4 | Invasive carcinoma of no special type (NST) | G3 | pT4bN0 | 0 | 3+ | |
| PT#5 | Invasive carcinoma of no special type (NST) | G2 | pT1N1a | 0.8 | 1+ | |
| PT#6 | Invasive carcinoma of no special type (NST) | G3 | pT4bN2a | 0.65 | 1+ | |
| PT#7 | Invasive carcinoma of no special type (NST) | G2 | pT2N1a | 0 | 0 | |
| PT#8 | Invasive apocrine carcinoma (IAC) | G3 | pT2N1a | 0 | 3+ | |
| PT#9 | Invasive mucinous carcinoma (IMC) | G2 | pT1cN0 | 0.7 | 0 | |
| PT#10 | Invasive Ductal Carcinoma (IDC) | G1 | pT2N0 | 0.03 | 0 | |
| PT#11 | Invasive carcinoma of no special type (NST) | G2 | pT2N1 | 0.9 | 1+ | |
| PT#12 | Invasive Ductal Carcinoma (IDC) | G3 | pT2N0 | 0.7 | 1+ | |
| PT#13 | Invasive Ductal Carcinoma (IDC) | G2 | pT1cN0 | 0 | 0 | |
| PT#14 | Invasive Ductal Carcinoma (IDC) | G3 | pT1bN1a | 0 | 2+ | |
| PT#15 | Invasive Ductal Carcinoma (IDC) | G2 | pT2N2 | <5 | 1+ | |
| PT#16 | Invasive Ductal Carcinoma (IDC) | G2 | pT1cN0 | 0.8 | 1+ | |
| PT#17 | Invasive Ductal Carcinoma (IDC) | G2 | pT1cN0 | 0.6 | 0 | |
| PT#18 | Invasive Ductal Carcinoma (IDC) | G2 | pT2N1M1 (bone) | 0.7 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedetti, R.; Papulino, C.; Sgueglia, G.; Chianese, U.; De Marchi, T.; Iovino, F.; Rotili, D.; Mai, A.; Niméus, E.; Dell’ Aversana, C.; et al. Regulatory Interplay between miR-181a-5p and Estrogen Receptor Signaling Cascade in Breast Cancer. Cancers 2021, 13, 543. https://doi.org/10.3390/cancers13030543
Benedetti R, Papulino C, Sgueglia G, Chianese U, De Marchi T, Iovino F, Rotili D, Mai A, Niméus E, Dell’ Aversana C, et al. Regulatory Interplay between miR-181a-5p and Estrogen Receptor Signaling Cascade in Breast Cancer. Cancers. 2021; 13(3):543. https://doi.org/10.3390/cancers13030543
Chicago/Turabian StyleBenedetti, Rosaria, Chiara Papulino, Giulia Sgueglia, Ugo Chianese, Tommaso De Marchi, Francesco Iovino, Dante Rotili, Antonello Mai, Emma Niméus, Carmela Dell’ Aversana, and et al. 2021. "Regulatory Interplay between miR-181a-5p and Estrogen Receptor Signaling Cascade in Breast Cancer" Cancers 13, no. 3: 543. https://doi.org/10.3390/cancers13030543
APA StyleBenedetti, R., Papulino, C., Sgueglia, G., Chianese, U., De Marchi, T., Iovino, F., Rotili, D., Mai, A., Niméus, E., Dell’ Aversana, C., & Altucci, L. (2021). Regulatory Interplay between miR-181a-5p and Estrogen Receptor Signaling Cascade in Breast Cancer. Cancers, 13(3), 543. https://doi.org/10.3390/cancers13030543