Hepatitis B Virus Covalently Closed Circular DNA Predicts Postoperative Liver Cancer Metastasis Independent of Virological Suppression
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Quantification of cccDNA
2.3. Assays for HBsAg, Anti-HCV, and Anti-Hepatitis D
2.4. Diagnosis Criteria for HCC and Postoperative Follow-Ups
2.5. Western Blot Analysis for Twist1 and Slug
2.6. Statistical Analysis
2.7. Tissue HBV-DNA Extraction and Quantitative Assay
3. Results
3.1. Development of a Novel Method to Quantify cccDNA by the Use of PNA-Clamping
3.2. Comparison between the PNA-Clamping Method with the Exonucleases-Based Methods to the cccDNA Assay
3.3. Baseline Clinicopathological and Virological Data for HCC Patients Receiving Surgical Resection
3.4. Clinicopathological and Virological Factors Associated with cccDNA Amounts
3.5. Clinicopathological and Virological Factors Associated with Postoperative Prognosis
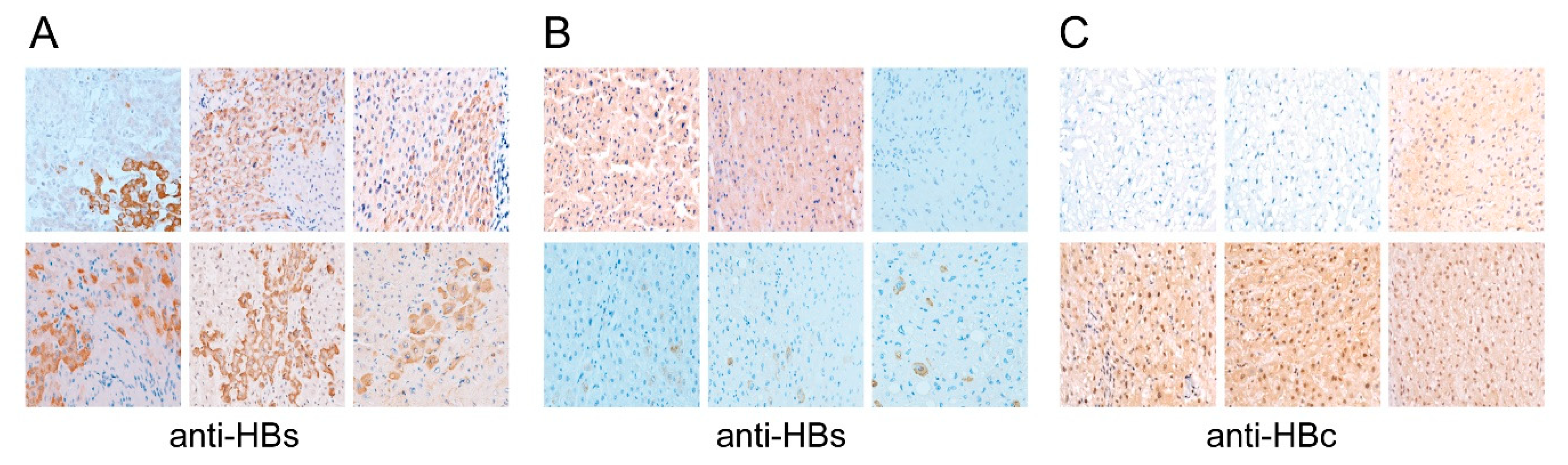
3.6. Tissue Distribution Patterns of HBsAg and cccDNA Levels Predicted Postoperative Outcomes in Postoperative Serum HBV-DNA Negative Subgroup
3.7. The cccDNA Level Was Associated with Tissue Twist1 Expression Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liaw, Y.F.; Chu, C.M. Hepatitis B virus infection. Lancet 2009, 373, 582–592. [Google Scholar] [CrossRef]
- Tseng, T.C.; Liu, C.J.; Chen, C.L.; Yang, H.C.; Su, T.H.; Wang, C.C.; Yang, W.T.; Kuo, S.F.; Liu, C.H.; Chen, P.J.; et al. Risk stratification of hepatocellular carcinoma in hepatitis B virus e antigen-negative carriers by combining viral biomarkers. J. Infect. Dis 2013, 208, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.T.; Lai, M.W. Eliminating hepatitis B virus through neonatal vaccination: Can we make it? J. Hepatol. 2012, 57, 484–485. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sung, J.J.; Tsoi, K.K.; Wong, V.W.; Li, K.C.; Chan, H.L. Meta-analysis: Treatment of hepatitis B infection reduces risk of hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2008, 28, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, T.; Suzuki, F.; Kobayashi, M.; Seko, Y.; Kawamura, Y.; Sezaki, H.; Akuta, N.; Suzuki, Y.; Saitoh, S.; Arase, Y.; et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology 2013, 58, 98–107. [Google Scholar] [CrossRef]
- Wu, C.Y.; Chen, Y.J.; Ho, H.J.; Hsu, Y.C.; Kuo, K.N.; Wu, M.S.; Lin, J.T. Association Between Nucleoside Analogues and Risk of Hepatitis B Virus–Related Hepatocellular Carcinoma Recurrence Following Liver Resection. JAMA 2012, 308, 1906–1914. [Google Scholar] [CrossRef]
- Liang, K.H.; Hsu, C.W.; Chang, M.L.; Chen, Y.C.; Lai, M.W.; Yeh, C.T. Peginterferon Is Superior to Nucleos(t)ide Analogues for Prevention of Hepatocellular Carcinoma in Chronic Hepatitis B. J. Infect. Dis. 2016, 213, 966–974. [Google Scholar] [CrossRef]
- Lucifora, J.; Xia, Y.; Reisinger, F.; Zhang, K.; Stadler, D.; Cheng, X.; Sprinzl, M.F.; Koppensteiner, H.; Makowska, Z.; Volz, T.; et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus. Science 2014, 343, 1221–1228. [Google Scholar] [CrossRef]
- Marcellin, P.; Lau, G.K.; Bonino, F.; Farci, P.; Hadziyannis, S.; Jin, R.; Lu, Z.M.; Piratvisuth, T.; Germanidis, G.; Yurdaydin, C.; et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N. Engl. J. Med. 2004, 351, 1206–1217. [Google Scholar] [CrossRef]
- Chang, T.T.; Gish, R.G.; de Man, R.; Gadano, A.; Sollano, J.; Chao, Y.C.; Lok, A.S.; Han, K.H.; Goodman, Z.; Zhu, J.; et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N. Engl. J. Med. 2006, 354, 1001–1010. [Google Scholar] [CrossRef]
- Lai, C.L.; Shouval, D.; Lok, A.S.; Chang, T.T.; Cheinquer, H.; Goodman, Z.; DeHertogh, D.; Wiber, R.; Zink, R.C.; Cross, A.; et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N. Engl. J. Med. 2006, 354, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J. Hepatol. 2012, 57, 167–185. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Xu, H.; Cao, L.; Ye, M. HBeAg Seroconversion in HBeAg-Positive Chronic Hepatitis B Patients Receiving Long-Term Nucleos(t)ide Analog Treatment: A Systematic Review and Network Meta-Analysis. PLoS ONE 2017, 12, e0169444. [Google Scholar] [CrossRef] [PubMed]
- Kranidiotia, H.; Manolakopoulosb, S.; Khakoo, S.I. Outcome after discontinuation of nucleot(s)ide analogues in chronic hepatitis B: Relapse rate and associated factors. Ann. Gastroenterol. 2015, 28, 173–181. [Google Scholar]
- Lok, A.S.; Zoulim, F.; Dusheiko, G.; Ghany, M.G. Hepatitis B cure: From discovery to regulatory approval. J. Hepatol. 2017, 67, 847–861. [Google Scholar] [CrossRef]
- Lai, M.W.; Hsu, C.W.; Lin, C.L.; Chien, R.N.; Lin, W.R.; Chang, C.S.; Liang, K.H.; Yeh, C.T. Multiple doses of hepatitis B recombinant vaccine for chronic hepatitis B patients with low surface antigen levels: A pilot study. Hepatol. Int. 2018, 12, 456–464. [Google Scholar] [CrossRef]
- Chan, H.L.; Wong, V.W.; Tse, A.M.; Tse, C.H.; Chim, A.M.; Chan, H.Y.; Wong, G.L.; Sung, J.J. Serum hepatitis B surface antigen quantitation can reflect hepatitis B virus in the liver and predict treatment response. Clin. Gastroenterol. Hepatol. 2007, 5, 1462–1468. [Google Scholar] [CrossRef]
- Thompson, A.J.; Nguyen, T.; Iser, D.; Ayres, A.; Jackson, K.; Littlejohn, M.; Slavin, J.; Bowden, S.; Gane, E.J.; Abbott, W.; et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: Disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology 2010, 51, 1933–1944. [Google Scholar] [CrossRef]
- van Zonneveld, M.; Honkoop, P.; Hansen, B.E.; Niesters, H.G.; Darwish Murad, S.; de Man, R.A.; Schalm, S.W.; Janssen, H.L. Long-term follow-up of alpha-interferon treatment of patients with chronic hepatitis B. Hepatology 2004, 39, 804–810. [Google Scholar] [CrossRef]
- Flink, H.J.; van Zonneveld, M.; Hansen, B.E.; de Man, R.A.; Schalm, S.W.; Janssen, H.L. Treatment with Peg-interferon α-2b for HBeAg-positive chronic hepatitis B: HBsAg loss is associated with HBV genotype. Am. J. Gastroenterol. 2006, 101, 297–303. [Google Scholar] [CrossRef]
- Lai, C.L.; Gane, E.; Liaw, Y.F.; Hsu, C.W.; Thongsawat, S.; Wang, Y.; Chen, Y.; Heathcote, E.J.; Rasenack, J.; Bzowei, N.; et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N. Engl. J. Med. 2007, 357, 2576–2588. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, P.; Heathcote, E.J.; Buti, M.; Gane, E.; de Man, R.A.; Krastev, Z.; Germanidis, G.; Lee, S.S.; Flisiak, R.; Kaita, K.; et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N. Engl. J. Med. 2008, 359, 2442–2455. [Google Scholar] [CrossRef] [PubMed]
- Heathcote, E.J.; Marcellin, P.; Buti, M.; Gane, E.; De Man, R.A.; Krastev, Z.; Germanidis, G.; Lee, S.S.; Flisiak, R.; Kaita, K.; et al. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology 2011, 140, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, P.; Ahn, S.H.; Ma, X.; Caruntu, F.A.; Tak, W.Y.; Elkashab, M.; Chuang, W.L.; Lim, S.G.; Tabak, F.; Mehta, R.; et al. Combination of Tenofovir Disoproxil Fumarate and Peginterferon alpha-2a Increases Loss of Hepatitis B Surface Antigen in Patients With Chronic Hepatitis B. Gastroenterology 2016, 150, 134–144 e110. [Google Scholar] [CrossRef]
- Werle-Lapostolle, B.; Bowden, S.; Locarnini, S.; Wursthorn, K.; Petersen, J.; Lau, G.; Trepo, C.; Marcellin, P.; Goodman, Z.; Delaney, W.E., 4th; et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology 2004, 126, 1750–1758. [Google Scholar] [CrossRef]
- Laras, A.; Koskinas, J.; Dimou, E.; Kostamena, A.; Hadziyannis, S.J. Intrahepatic levels and replicative activity of covalently closed circular hepatitis B virus DNA in chronically infected patients. Hepatology 2006, 44, 694–702. [Google Scholar] [CrossRef]
- Xia, Y.; Stadler, D.; Ko, C.; Protzer, U. Analyses of HBV cccDNA Quantification and Modification. Methods Mol. Biol. 2017, 1540, 59–72. [Google Scholar]
- Tu, T.; Zehnder, B.; Qu, B.; Ni, Y.; Main, N.; Allweiss, L.; Dandri, M.; Shackel, N.; George, J.; Urban, S. A novel method to precisely quantify hepatitis B virus covalently closed circular (ccc)DNA formation and maintenance. Antiviral Res. 2020, 181, 104865. [Google Scholar] [CrossRef]
- Chuaypen, N.; Sriprapun, M.; Praianantathavorn, K.; Payungporn, S.; Wisedopas, N.; Poovorawan, Y.; Tangkijvanich, P. Kinetics of serum HBsAg and intrahepatic cccDNA during pegylated interferon therapy in patients with HBeAg-positive and HBeAg-negative chronic hepatitis B. J. Med. Virol. 2017, 89, 130–138. [Google Scholar] [CrossRef]
- Nielsen, P.E.; Egholm, M.; Berg, R.H.; Buchardt, O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science 1991, 254, 1497–1500. [Google Scholar] [CrossRef]
- Nagai, Y.; Miyazawa, H.; Huqun; Tanaka, T.; Udagawa, K.; Kato, M.; Fukuyama, S.; Yokote, A.; Kobayashi, K.; Kanazawa, M.; et al. Genetic heterogeneity of the epidermal growth factor receptor in non-small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid-locked nucleic acid PCR clamp. Cancer Res. 2005, 65, 7276–7282. [Google Scholar] [CrossRef] [PubMed]
- Soh, J.; Toyooka, S.; Aoe, K.; Asano, H.; Ichihara, S.; Katayama, H.; Hiraki, A.; Kiura, K.; Aor, M.; Sano, Y.; et al. Usefulness of EGFR mutation screening in pleural fluid to predict the clinical outcome of gefitinib treated patients with lung cancer. Int. J. Cancer 2006, 119, 2353–2358. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, H.; Tanaka, T.; Nagai, Y.; Matsuoka, M.; Huqun; Sutani, A.; Udagawa, K.; Zhang, J.; Hirama, T.; Murayama, Y.; et al. Peptide nucleic acid-locked nucleic acid polymerase chain reaction clamp-based detection test for gefitinib-refractory T790M epidermal growth factor receptor mutation. Cancer Sci. 2008, 99, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.T.; Chien, R.N.; Chu, C.M.; Liaw, Y.F. Clearance of the original hepatitis B virus YMDD-motif mutants with emergence of distinct lamivudine-resistant mutants during prolonged lamivudine therapy. Hepatology 2000, 31, 1318–1326. [Google Scholar] [CrossRef]
- Chu, Y.D.; Lai, H.Y.; Pai, L.M.; Huang, Y.H.; Lin, Y.H.; Liang, K.H.; Yeh, C.T. The methionine salvage pathway-involving ADI1 inhibits hepatoma growth by epigenetically altering genes expression via elevating S-adenosylmethionine. Cell Death Dis. 2019, 10, 240. [Google Scholar] [CrossRef]
- Mazumdar, M.; Glassman, J.R. Categorizing a prognostic variable: Review of methods, code for easy implementation and applications to decision-making about cancer treatments. Stat. Med. 2000, 19, 113–132. [Google Scholar] [CrossRef]
- Yeh, C.T.; So, M.; Ng, J.; Yang, H.W.; Chang, M.L.; Lai, M.W.; Chen, T.C.; Lin, C.Y.; Yeh, T.S.; Lee, W.C. Hepatitis B virus-DNA level and basal core promoter A1762T/G1764A mutation in liver tissue independently predict postoperative survival in hepatocellular carcinoma. Hepatology 2010, 52, 1922–1933. [Google Scholar] [CrossRef]
- Jiang, P.X.; Mao, R.C.; Dong, M.H.; Yu, X.P.; Xun, Q.; Wang, J.Y.; Jing, L.; Qiang, D.; Zhang, J.M. Exonuclease I and III improve the detection efficacy of hepatitis B virus covalently closed circular DNA. Hepatobiliary Pancreat Dis. Int. 2019, 18, 458–463. [Google Scholar] [CrossRef]
- Qu, B.; Ni, Y.; Lempp, F.A.; Vondran, F.W.R.; Urban, S. T5 Exonuclease Hydrolysis of Hepatitis B Virus Replicative Intermediates Allows Reliable Quantification and Fast Drug Efficacy Testing of Covalently Closed Circular DNA by PCR. J. Virol. 2018, 92, e01117-18. [Google Scholar] [CrossRef]
- Heerboth, S.; Housman, G.; Leary, M.; Longacre, M.; Byler, S.; Lapinska, K.; Willbanks, A.; Sarkar, S. EMT and tumor metastasis. Clin. Transl. Med. 2015, 4, 6. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, L.; Yoo, S.; Wang, W.; Blank, S.; Fiel, M.I.; Kadri, H.; Luan, W.; Warren, L.; Zhu, J.; et al. Impact of non-neoplastic vs intratumoural hepatitis B viral DNA and replication on hepatocellular carcinoma recurrence. Br. J. Cancer 2016, 115, 841–847. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Q.; Fiel, M.I.; Luan, W.; Blank, S.; Kadri, H.; Kim, K.W.; Hiotis, S.P. Impact of intrahepatic hepatitis B DNA and covalently closed circular DNA on survival after hepatectomy in HBV-associated hepatocellular carcinoma patients. Ann. Surg. Oncol. 2013, 20, 3761–3770. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.K.; Yuen, M.F.; Poon, R.T.; Yuen, J.C.; Fung, J.; Lai, C.L. Quantification of hepatitis B virus covalently closed circular DNA in patients with hepatocellular carcinoma. J. Hepatol. 2006, 45, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Wang, X.; Xu, Z.; Tang, N. HBx-dependent activation of Twist mediates STAT3 control of epithelium-mesenchymal transition of liver cells. J. Cell Biochem. 2013, 114, 1097–1104. [Google Scholar] [CrossRef]




| Variable | Value |
|---|---|
| Clinical characteristics | |
| Sex, male/female, n (%) | 282 (80.6%)/68 (19.4%) |
| Age, years, mean ± SD | 53.5 ± 13.4 |
| Anti-HCV positive a, n (%) | 36 (10.3%) |
| Antiviral therapy used after operation, n (%) | 56 (16.0%) |
| Cirrhosis, n (%) | 209 (59.7%) |
| Alcoholism, n (%) | 108 (30.9%) |
| Ascites, n (%) | 26 (7.4%) |
| Serology | |
| HBeAg, n (%) | 18 (5.1%) |
| Serum HBV DNA, ×106 copies/mL b, median (range) | 0.758 (0, 149.6) |
| AFP, ng/mL, median (range) | 25.0 (0.9, 685353) |
| Albumin, g/L, mean ± SD | 4.0 ± 0.6 |
| Bilirubin, mg/dL, mean ± SD | 1.1 ± 1.3 |
| Prothrombin time, s, mean ± SD | 12.2 ± 1.5 |
| Creatinine, mg/dL, mean ± SD | 1.1 ± 1.1 |
| AST, U/L, mean ± SD | 66.7 ± 87.0 |
| ALT, U/L, mean ± SD | 71.2 ± 94.7 |
| Child-Pugh functional class | |
| A/B, n (%) | 323 (92.3%)/27 (7.7%) |
| Tumor characteristics | |
| Maximum tumor size, cm, mean ± SD | 6.2 ± 5.6 |
| Tumor number ≤ 3, n (%) | 330 (94.3%) |
| Microvascular invasion, n (%) | 119 (34.0%) |
| Macrovascular invasion, n (%) | 50 (14.3%) |
| Histology grade, mean ± SD | 2.7 ± 0.7 |
| Tumor capsule, n (%) | 255 (72.9%) |
| BCLC tumor stage | |
| 0-A/B, n (%) | 153 (43.7%)/197 (56.3%) |
| Virological characteristics in para-neoplastic tissues | |
| Positive tissue HBsAg, n (%) | 331 (94.6%) |
| Clustered distribution of HBsAg, n (%) | 106 (30.3%) f |
| Diffuse distribution of HBsAg, n (%) | 170 (48.6%) f |
| Scattered distribution of HBsAg, n (%) | 109 (31.1%) f |
| Positive tissue HBcAg, n (%) | 219 (62.6%) |
| Predominantly cytoplasmic HBcAg c, n (%) | 126 (36.0%) |
| Predominantly nuclear HBcAg d, n (%) | 93 (26.6%) |
| Tissue HBV DNA, ×106 copies/g, median (range) | 12.0 (0, 22455.5) e |
| Tissue cccDNA, ×106 copies/g, median (range) | 0.0 (0, 123.0) e |
| Variable | Beta (95% Confidence Interval) | p |
|---|---|---|
| Clinical characteristics | ||
| Sex, male = 1 | 0.474 (−2.152, 3.101) | 0.723 |
| Age, years | 0.058 (−0.020, 0.135) | 0.144 |
| Anti-HCV positive a = 1 | −2.530 (−5.941, 0.881) | 0.145 |
| Cirrhosis, positive = 1 | 2.360 (0.256, 4.465) | 0.028 |
| Alcoholism, positive = 1 | −1.217 (−3.463, 1.030) | 0.288 |
| Ascites, positive = 1 | −0.464 (−4.427, 3.499) | 0.818 |
| Serology | ||
| HBeAg, positive = 1 | 12.193 (7.666, 16.719) | <0.001 |
| Serum HBV-DNA, ×106 copies/mL b | 0.158 (0.049, 0.267) | 0.005 |
| AFP, ×100 ng/mL | 0.000 (−0.003, 0.002) | 0.690 |
| Albumin, g/L | 0.397 (−1.427, 2.221) | 0.669 |
| Bilirubin, mg/dL | −0.462 (−1.261, 0.336) | 0.256 |
| Prothrombin time, s | −0.426 (−1.139, 0.286) | 0.240 |
| Creatinine, mg/dL | −0.309 (−1.272, 0.654) | 0.529 |
| AST, U/L | −0.001 (−0.013, 0.011) | 0.916 |
| ALT, U/L | 0.003 (−0.008, 0.014) | 0.600 |
| Child-Pugh functional class, class B = 1 | −1.107 (−5.001, 2.787) | 0.576 |
| Tumor characteristics | ||
| Maximum tumor size, cm | −0.114 (−0.299, 0.071) | 0.227 |
| Tumor number | −0.894 (−1.896, 0.107) | 0.080 |
| Microvascular invasion, positive = 1 | −0.173 (−2.367, 2.021) | 0.877 |
| Macrovascular invasion, positive = 1 | 1.370 (−1.596, 4.337) | 0.364 |
| Histology grade | −0.837 (−2.372, 0.698) | 0.284 |
| Tumor capsule, positive = 1 | 0.003 (−2.334, 2.340) | 0.998 |
| BCLC tumor stage, stage B = 1 | −2.337 (−4.417, −0.256) | 0.028 |
| Virological characteristics in para-neoplastic tissues | ||
| Positive tissue HBsAg, positive = 1 | 2.328 (−1.882, 7.275) | 0.247 |
| Clustered distribution of HBsAg, positive = 1 | 1.046 (−1.213, 3.305) | 0.363 |
| Diffuse distribution of HBsAg, positive = 1 | 1.056 (−1.082, 3.072) | 0.347 |
| Scattered distribution of HBsAg, positive = 1 | 1.141 (−2.834, 1.653) | 0.605 |
| Positive tissue HBcAg, positive = 1 | 1.078 (1.072, 5.315) | 0.003 |
| Predominantly cytoplasmic HBcAg c, positive = 1 | −0.024 (−2.197, 2.150) | 0.983 |
| Predominantly nuclear HBcAg d, positive = 1 | 3.887 (1.570, 6.204) | 0.001 |
| Tissue HBV DNA, ×106 copies/g | 0.001 (0.000, 0.001) | 0.051 |
| Multivariate linear regression analysis | ||
| Cirrhosis, positive = 1 | 1.813 (−0.244, 3.870) | 0.084 |
| HBeAg, positive = 1 | 10.913 (6.260, 15.565) | <0.001 |
| Serum HBV-DNA, ×106 copies/mL b | 0.135 (0.029, 0.241) | 0.012 |
| BCLC tumor stage, stage B = 1 | −1.984 (−4.008, 0.039) | 0.055 |
| Positive tissue HBcAg, positive = 1 | 1.330 (−0.990, 3.651) | 0.260 |
| Predominantly nuclear HBcAg d, positive = 1 | 1.033 (−1.571, 3.637) | 0.436 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p |
| Clinical characteristics | ||||
| Sex, male = 1 | 1.145 (0.531, 2.472) | 0.730 | ||
| Age, per year increase | 1.004 (0.982, 1.027) | 0.714 | ||
| Anti-HCV positive a = 1 | 0.567 (0.175, 1.835) | 0.344 | ||
| Antiviral therapy used after operation, Yes = 1 | 0.451 (0.177, 1.153) | 0.096 | ||
| Postoperative undetectable HBV DNA b, Yes = 1 | 0.348 (0.136, 0.890) | 0.027 | 0.453 (0.173, 1.185) | 0.107 |
| Cirrhosis, positive = 1 | 0.840 (0.459, 1.535) | 0.570 | ||
| Alcoholism, positive = 1 | 1.391 (0.748, 2.587) | 0.297 | ||
| Ascites, positive = 1 | 2.361 (0.994, 5.611) | 0.052 | ||
| Serology | ||||
| HBeAg, positive = 1 | 1.469 (0.453, 4.763) | 0.521 | ||
| Preoperative serum HBV DNA, ×106 copies/mL | 1.031 (1.016, 1.047) | <0.001 | 1.013 (0.981, 1.046) | 0.433 |
| AFP, per 100 ng/mL increase | 1.000 (1.000, 1.001) | 0.376 | ||
| Albumin, per g/L increase | 0.345 (0.217, 0.549) | <0.001 | 0.392 (0.212, 0.725) | 0.003 |
| Bilirubin, per mg/dL increase | 1.063 (0.899, 1.258) | 0.476 | ||
| Prothrombin time, per s increase | 1.143 (0.969, 1.348) | 0.113 | ||
| Creatinine, per mg/dL increase | 0.304 (0.069, 1.338) | 0.115 | ||
| AST, per U/L increase | 1.004 (1.002, 1.006) | <0.001 | 1.000 (0.996, 1.004) | 0.859 |
| ALT, per U/L increase | 1.000 (0.997, 1.003) | 0.999 | ||
| Child-Pugh functional class, class B = 1 | 2.576 (1.085, 6.116) | 0.032 | 0.905 (0.301, 2.722) | 0.859 |
| Tumor characteristics | ||||
| Maximum tumor size, per cm increase | 1.024 (0.998, 1.051) | 0.066 | ||
| Tumor number, per number increase | 1.307 (1.016, 1.683) | 0.038 | 1.161 (0.874, 1.542) | 0.303 |
| Microvascular invasion, positive = 1 | 2.036 (1.101, 3.763) | 0.023 | 1.987 (0.999, 3.954) | 0.050 |
| Macrovascular invasion, positive = 1 | 2.605 (1.279, 5.307) | 0.008 | 1.371 (0.592, 3.174) | 0.461 |
| Histology grade, per grade increase | 1.487 (0.961, 2.302) | 0.075 | ||
| Tumor capsule, positive = 1 | 0.743 (0.392, 1.407) | 0.361 | ||
| BCLC tumor stage, stage B = 1 | 2.488 (1.294, 4.785) | 0.006 | 1.933 (0.900, 4.155) | 0.091 |
| Virological characteristics in non-tumor parts | ||||
| Positive tissue HBsAg, positive = 1 | 2.806 (0.384, 20.484) | 0.309 | ||
| Clustered distribution of tissue HBsAg, positive = 1 | 1.336 (0.710, 2.515) | 0.370 | ||
| Diffuse distribution of HBsAg, positive = 1 | 0.447 (0.232, 0.860) | 0.016 | 0.385 (0.191, 0.777) | 0.008 |
| Scattered distribution of HBsAg, positive = 1 | 1.411 (0.754, 2.641) | 0.282 | ||
| Positive tissue HBcAg, positive = 1 | 1.685 (0.858, 3.306) | 0.129 | ||
| Predominant cytoplasmic HBcAg c, positive = 1 | 1.069 (0.563, 2.030) | 0.838 | ||
| Predominant nuclear HBcAg d, positive = 1 | 1.578 (0.854, 2.918) | 0.145 | ||
| Tissue HBV DNA, per 108 copies/g | 1.009 (1.001, 1.017) | 0.021 | 1.000 (1.000, 1.000) | 0.670 |
| Tissue cccDNA, per 106 copies/g | 1.017 (1.002, 1.032) | 0.025 | 1.029 (1.010, 1.049) | 0.003 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p |
| Clinical characteristics | ||||
| Sex, male = 1 | 1.142 (0.779, 1.674) | 0.497 | ||
| Age, per year increase | 1.002 (0.991, 1.013) | 0.712 | ||
| Anti-HCV positive a = 1 | 0.962 (0.598, 1.550) | 0.875 | ||
| Antiviral therapy used after operation, Yes = 1 | 0.687 (0.458, 1.031) | 0.070 | ||
| Postoperative undetectable HBV DNA b, Yes = 1 | 0.570 (0.386, 0.842) | 0.005 | 0.690 (0.460, 1.034) | 0.072 |
| Cirrhosis, positive = 1 | 1.173 (0.866, 1.588) | 0.302 | ||
| Alcoholism, positive = 1 | 1.194 (0.875, 1.629) | 0.264 | ||
| Ascites, positive = 1 | 1.784 (1.081, 2.946) | 0.024 | 1.436 (0.841, 2.453) | 0.185 |
| Serology | ||||
| HBeAg, positive = 1 | 1.573 (0.853, 2.900) | 0.147 | ||
| Preoperative serum HBV-DNA, ×106 copies/mL | 1.012 (0.993, 1.030) | 0.214 | ||
| AFP, per 100 ng/mL increase | 1.000 (1.000, 1.001) | 0.380 | ||
| Albumin, per g/L increase | 0.599 (0.461, 0.779) | <0.001 | 0.763 (0.563, 1.034) | 0.082 |
| Bilirubin, per mg/dL increase | 0.896 (0.742, 1.032) | 0.255 | ||
| Prothrombin time, per s increase | 1.100 (1.011, 1.198) | 0.027 | 1.041 (0.944, 1.149) | 0.419 |
| Creatinine, per mg/dL increase | 0.932 (0.781, 1.111) | 0.430 | ||
| AST, per U/L increase | 1.003 (1.002, 1.005) | <0.001 | 1.002 (1.000, 1.004) | 0.116 |
| ALT, per U/L increase | 1.001 (1.000, 1.003) | 0.029 | 1.000 (0.998, 1.002) | 0.924 |
| Child-Pugh functional class, class B = 1 | 0.913 (0.482, 1.728) | 0.780 | ||
| Tumor characteristics | ||||
| Maximum tumor size, per cm increase | 1.011 (0.995, 1.038) | 0.176 | ||
| Tumor number, per number increase | 1.100 (0.948, 1.275) | 0.209 | ||
| Microvascular invasion, positive = 1 | 2.414 (1.780, 3.275) | <0.001 | 2.136 (1.543, 2.956) | <0.001 |
| Macrovascular invasion, positive = 1 | 1.274 (0.827, 1.962) | 0.271 | ||
| Histology grade, per grade increase | 1.139 (0.919, 1.412) | 0.233 | ||
| Tumor capsule, positive = 1 | 1.030 (0.739, 1.435) | 0.862 | ||
| BCLC tumor stage, stage B = 1 | 1.655 (1.227, 2.231) | 0.001 | 1.429 (1.036, 1.969) | 0.029 |
| Virological characteristics in non-tumor parts | ||||
| Positive tissue HBsAg, positive = 1 | 1.102 (0.562, 2.161) | 0.777 | ||
| Clustered distribution of tissue HBsAg, positive = 1 | 2.309 (1.710, 3.118) | <0.001 | 2.093 (1.524, 2.874) | <0.001 |
| Diffuse distribution of HBsAg, positive = 1 | 0.699 (0.519, 0.940) | 0.018 | 0.749 (0.545, 1.030) | 0.075 |
| Scattered distribution of HBsAg, positive = 1 | 0.831 (0.596, 1.160) | 0.277 | ||
| Positive tissue HBcAg, positive = 1 | 1.679 (1.218, 2.315) | 0.002 | 1.372 (0.923, 2.038) | 0.118 |
| Predominantly cytoplasmic HBcAg c, positive = 1 | 1.602 (1.185, 2.166) | 0.002 | 1.338 (0.924, 1.938) | 0.123 |
| Predominantly nuclear HBcAg d, positive = 1 | 1.083 (0.782, 1.498) | 0.632 | ||
| Tissue HBV-DNA, per 108 copies/g | 0.997 (0.986, 1.008) | 0.572 | ||
| Tissue cccDNA, per 106 copies/g | 1.008 (0.997, 1.019) | 0.156 | ||
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p |
| Clinical characteristics | ||||
| Sex, male = 1 | 1.222 (0.639, 2.335) | 0.544 | ||
| Age, per year increase | 0.996 (0.978, 1.013) | 0.621 | ||
| Anti-HCV positive a = 1 | 0.757 (0.327, 1.752) | 0.515 | ||
| Antiviral therapy used after operation, Yes = 1 | 0.377 (0.162, 0.876) | 0.023 | 0.857 (0.099, 7.415) | 0.857 |
| Postoperative undetectable HBV-DNA b, Yes = 1 | 0.341 (0.155, 0.750) | 0.007 | 0.427 (0.058, 3.127) | 0.402 |
| Cirrhosis, positive = 1 | 0.775 (0.479, 1.254) | 0.299 | ||
| Alcoholism, positive = 1 | 1.097 (0.658, 1.828) | 0.722 | ||
| Ascites, positive = 1 | 0.660 (0.207, 2.104) | 0.482 | ||
| Serology | ||||
| HBeAg, positive = 1 | 3.281 (1.622, 6.638) | 0.001 | 1.547 (0.679, 3.525) | 0.299 |
| Preoperative serum HBV-DNA, ×106 copies/mL | 0.998 (0.954, 1.044) | 0.920 | ||
| AFP, per 100 ng/mL increase | 1.001 (1.000, 1.001) | <0.001 | 1.000 (1.000, 1.001) | 0.004 |
| Albumin, per g/L increase | 0.671 (0.433, 1.038) | 0.073 | ||
| Bilirubin, per mg/dL increase | 0.862 (0.593, 1.254) | 0.438 | ||
| Prothrombin time, per s increase | 1.108 (0.965, 1.273) | 0.146 | ||
| Creatinine, per mg/dL increase | 0.381 (0.125, 1.164) | 0.090 | ||
| AST, per U/L increase | 1.002 (1.000, 1.005) | 0.065 | ||
| ALT, per U/L increase | 0.998 (0.994, 1.002) | 0.268 | ||
| Child-Pugh functional class, class B = 1 | 0.997 (0.363, 2.742) | 0.996 | ||
| Tumor characteristics | ||||
| Maximum tumor size, per cm increase | 1.026 (1.006, 1.047) | 0.011 | 1.021 (0.952, 1.095) | 0.561 |
| Tumor number, per number increase | 1.313 (1.064, 1.620) | 0.011 | 1.161 (0.921, 1.465) | 0.207 |
| Microvascular invasion, positive = 1 | 3.943 (2.419, 6.426) | <0.001 | 2.767 (1.620, 4.727) | <0.001 |
| Macrovascular invasion, positive = 1 | 2.701 (1.534, 4.758) | 0.001 | 1.915 (1.023, 3.583) | 0.042 |
| Histology grade, per grade increase | 1.409 (0.990, 2.007) | 0.057 | ||
| Tumor capsule, positive = 1 | 0.794 (0.473, 1.331) | 0.381 | ||
| BCLC tumor stage, stage B = 1 | 2.719 (1.595, 4.637) | <0.001 | 1.536 (0.724, 3.258) | 0.263 |
| Virological characteristics in non-tumor parts | ||||
| Positive tissue HBsAg, positive = 1 | 2.156 (0.527, 8.826) | 0.285 | ||
| Clustered distribution of tissue HBsAg, positive = 1 | 1.251 (0.753, 2.079) | 0.387 | ||
| Diffuse distribution of HBsAg, positive = 1 | 1.136 (0.701, 1.838) | 0.605 | ||
| Scattered distribution of HBsAg, positive = 1 | 0.732 (0.416, 1.291) | 0.281 | ||
| Positive tissue HBcAg, positive = 1 | 2.591 (1.433, 4.686) | 0.002 | 1.867 (0.995, 3.502) | 0.052 |
| Predominantly cytoplasmic HBcAg c, positive = 1 | 1.532 (0.934, 2.511) | 0.091 | ||
| Predominantly nuclear HBcAg d, positive = 1 | 1.587 (0.971, 2.597) | 0.066 | ||
| Tissue HBV-DNA, per 108 copies/g | 0.996 (0.975, 1.018) | 0.734 | ||
| Tissue cccDNA, per 106 copies/g | 1.017 (1.005, 1.029) | 0.005 | 1.026 (1.011, 1.042) | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-W.; Chu, Y.-D.; Lai, M.-W.; Lin, C.-L.; Liang, K.-H.; Lin, Y.-H.; Yeh, C.-T. Hepatitis B Virus Covalently Closed Circular DNA Predicts Postoperative Liver Cancer Metastasis Independent of Virological Suppression. Cancers 2021, 13, 538. https://doi.org/10.3390/cancers13030538
Hsu C-W, Chu Y-D, Lai M-W, Lin C-L, Liang K-H, Lin Y-H, Yeh C-T. Hepatitis B Virus Covalently Closed Circular DNA Predicts Postoperative Liver Cancer Metastasis Independent of Virological Suppression. Cancers. 2021; 13(3):538. https://doi.org/10.3390/cancers13030538
Chicago/Turabian StyleHsu, Chao-Wei, Yu-De Chu, Ming-Wei Lai, Chih-Lang Lin, Kung-Hao Liang, Yang-Hsiang Lin, and Chau-Ting Yeh. 2021. "Hepatitis B Virus Covalently Closed Circular DNA Predicts Postoperative Liver Cancer Metastasis Independent of Virological Suppression" Cancers 13, no. 3: 538. https://doi.org/10.3390/cancers13030538
APA StyleHsu, C.-W., Chu, Y.-D., Lai, M.-W., Lin, C.-L., Liang, K.-H., Lin, Y.-H., & Yeh, C.-T. (2021). Hepatitis B Virus Covalently Closed Circular DNA Predicts Postoperative Liver Cancer Metastasis Independent of Virological Suppression. Cancers, 13(3), 538. https://doi.org/10.3390/cancers13030538





