The Current View of Nonalcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Prevalence of NAFLD-HCC
3. Characteristics and Pathogenesis of NAFLD-HCC
4. Risk Factors for NAFLD-HCC
5. Treatment of NAFLD-HCC
6. Prognosis of NAFLD-HCC
7. Prevention of NAFLD-HCC
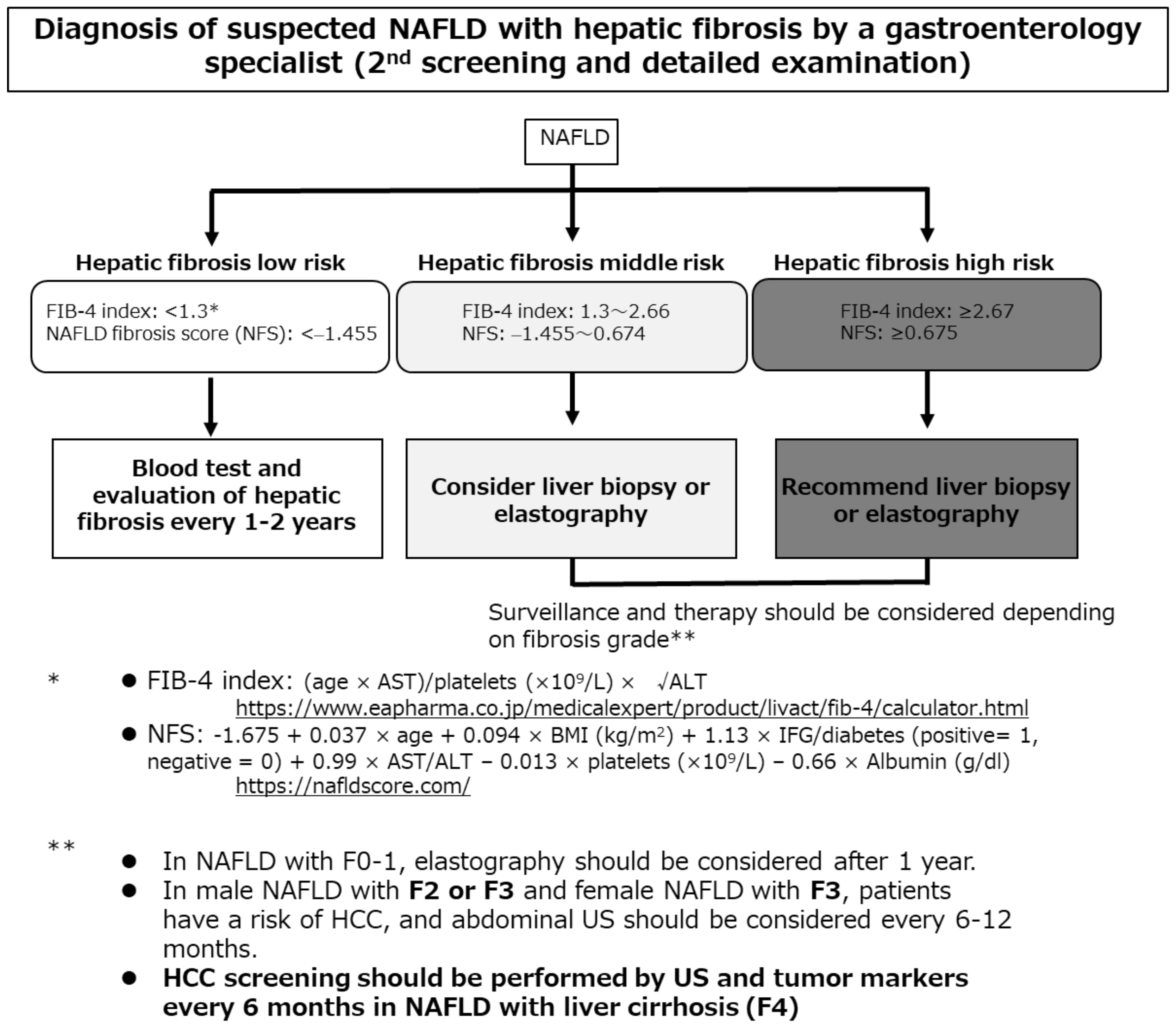
8. NAFLD-HCC Screening and Surveillance
9. Perspectives for Future Research
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eguchi, Y.; Hyogo, H.; Ono, M.; Mizuta, T.; Ono, N.; Fujimoto, K.; Chayama, K.; Saibara, T. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: A multicenter large retrospective study. J. Gastroenterol. 2012, 47, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, R.; Uchino, K.; Fujiwara, N.; Takehara, T.; Okanoue, T.; Seike, M.; Yoshiji, H.; Yatsuhashi, H.; Shimizu, M.; Torimura, T.; et al. A nationwide survey on non-B, non-C hepatocellular carcinoma in Japan: 2011–2015 update. J. Gastroenterol. 2019, 54, 367–376. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver; European Association for the Study of Diabetes. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Loomba, R.; Lim, J.K.; Patton, H.; El-Serag, H.B. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2020, 158, 1822–1830. [Google Scholar] [CrossRef]
- The Japanese Society of Gastroenterology; Japan Society of Hepatology. Evidence-Based Clinical Practice Guidelinefor Nonalcoholic Fatty liver Diseases/Nonalcoholic Steatohepatitis 2020 (Japanese), 2nd ed.; Nankodo Co., Ltd.: Tokyo, Japan, 2020. [Google Scholar]
- Margini, C.; Dufour, J.F. The story of HCC in NAFLD: From epidemiology, across pathogenesis, to prevention and treatment. Liver Int. 2016, 36, 317–324. [Google Scholar] [CrossRef]
- Yatsuji, S.; Hashimoto, E.; Tobari, M.; Taniai, M.; Tokushige, K.; Shiratori, K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J. Gastroenterol. Hepatol. 2009, 24, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ascha, M.S.; Hanouneh, I.A.; Lopez, R.; Tamimi, T.A.; Feldstein, A.F.; Zein, N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010, 51, 1972–1978. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Saitoh, S.; Koida, I.; Arase, Y.; Tsubota, A.; Chayama, K.; Kumada, H.; Kawanishi, M. A multivariate analysis of risk factors for hepatocellular carcinogenesis: A prospective observation of 795 patients with viral and alcoholic cirrhosis. Hepatology 1993, 18, 47–53. [Google Scholar] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Kawada, N.; Imanaka, K.; Kawaguchi, T.; Tamai, C.; Ishihara, R.; Matsunaga, T.; Gotoh, K.; Yamada, T.; Tomita, Y. Hepatocellular carcinoma arising from non-cirrhotic nonalcoholic steatohepatitis. J. Gastroenterol. 2009, 44, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, B.; Stål, P.; Wahlin, S.; Björkström, N.K.; Hagström, H. Characteristics and outcome of hepatocellular carcinoma in patients with NAFLD without cirrhosis. Liver Int. 2019, 39, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Piscaglia, F.; Svegliati-Baroni, G.; Barchetti, A.; Pecorelli, A.; Marinelli, S.; Tiribelli, C.; Bellentani, S.; HCC-NAFLD Italian Study Group. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology 2016, 63, 827–838. [Google Scholar] [CrossRef]
- Tokushige, K.; Hyogo, H.; Nakajima, T.; Ono, M.; Kawaguchi, T.; Honda, K.; Eguchi, Y.; Nozaki, Y.; Kawanaka, M.; Tanaka, S.; et al. Hepatocellular carcinoma in Japanese patients with nonalcoholic fatty liver disease and alcoholic liver disease: Multicenter survey. J. Gastroenterol. 2016, 51, 586–596. [Google Scholar] [CrossRef]
- Phipps, M.; Livanos, A.; Guo, A.; Pomenti, S.; Yeh, J.; Dakhoul, L.; Burney, H.; Kettler, C.; Liu, H.; Miller, E.; et al. Gender Matters: Characteristics of Hepatocellular Carcinoma in Women from a Large, Multicenter Study in the United States. Am. J. Gastroenterol. 2020, 115, 1486–1495. [Google Scholar]
- Bugianesi, E.; Leone, N.; Vanni, E.; Marchesini, G.; Brunello, F.; Carucci, P.; Musso, A.; De Paolis, P.; Capussotti, L.; Salizzoni, M.; et al. Expanding the natural history of nonalcoholic steatohepatitis: From cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002, 123, 134–140. [Google Scholar] [CrossRef]
- Marrero, J.A.; Fontana, R.J.; Su, G.L.; Conjeevaram, H.S.; Emick, D.M.; Lok, A.S. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology 2002, 36, 1349–1354. [Google Scholar] [CrossRef]
- Malik, S.M.; Gupte, P.A.; De Vera, M.E.; Ahmad, J. Liver Transplantation in Patients with Nonalcoholic Steatohepatitis-Related Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2009, 7, 800–806. [Google Scholar] [CrossRef]
- Shimada, M.; Hashimoto, E.; Taniai, M.; Hasegawa, K.; Okuda, H.; Hayashi, N.; Takasaki, K.; Ludwig, J. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J. Gastroenterol. 2009, 44, 89–95. [Google Scholar]
- Tokushige, K.; Hashimoto, E.; Horie, Y.; Taniai, M.; Higuchi, S. Hepatocellular carcinoma in Japanese patients with nonalcoholic fatty liver disease, alcoholic liver disease, and chronic liver disease of unknown etiology: Report of the nationwide survey. J. Gastroenterol. 2011, 46, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Kodama, K.; Tojjar, D.; Yamada, S.; Toda, K.; Patel, C.J.; Butte, A.J. Ethnic differences in the relationship between insulin sensitivity and insulin response: A systematic review and meta-analysis. Diabetes Care 2013, 36, 1789–1796. [Google Scholar] [PubMed]
- Wong, R.J.; Cheung, R.; Ahmed, A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014, 59, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, R.; Okanoue, T.; Fujiwara, N.; Okita, K.; Kiyosawa, K.; Omata, M.; Kumada, H.; Hayashi, N.; Koike, K. Clinical characteristics, treatment, and prognosis of non-B, non-C hepatocellular carcinoma: A large retrospective multicenter cohort study. J. Gastroenterol. 2015, 50, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Sada, Y.H.; El-Serag, H.B.; Kanwal, F.; Duan, Z.; Temple, S.; May, S.B.; Kramer, J.R.; Richardson, P.A.; Davila, J.A. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin. Gastroenterol. Hepatol. 2015, 13, 594–601.e591. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Otgonsuren, M.; Henry, L.; Venkatesan, C.; Mishra, A.; Erario, M.; Hunt, S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015, 62, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Kramer, J.R.; Mapakshi, S.; Natarajan, Y.; Chayanupatkul, M.; Richardson, P.A.; Li, L.; Desiderio, R.; Thrift, A.P.; Asch, S.M.; et al. Risk of Hepatocellular Cancer in Patients with Non-Alcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 1828–1837.e1822. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Bertot, L.C.; Wong, V.W.-S.; Castellanos, M.; La Fuente, R.A.-D.; Metwally, M.; Eslam, M.; Gonzalez-Fabian, L.; Sanz, M.A.-Q.; Conde-Martín, A.F.; et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients with Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 2018, 155, 443–457.e417. [Google Scholar] [CrossRef]
- Guzman, G.; Brunt, E.M.; Petrovic, L.M.; Chejfec, G.; Layden, T.J.; Cotler, S.J. Does nonalcoholic fatty liver disease predispose patients to hepatocellular carcinoma in the absence of cirrhosis? Arch. Pathol. Lab. Med. 2008, 132, 1761–1766. [Google Scholar]
- Paradis, V.; Zalinski, S.; Chelbi, E.; Guedj, N.; Degos, F.; Vilgrain, V.; Bedossa, P.; Belghiti, J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: A pathological analysis. Hepatology 2008, 49, 851–859. [Google Scholar] [CrossRef]
- Yasui, K.; Hashimoto, E.; Komorizono, Y.; Koike, K.; Arii, S.; Imai, Y.; Shima, T.; Kanbara, Y.; Saibara, T.; Mori, T.; et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 2011, 9, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Ertle, J.; Dechêne, A.; Sowa, J.P.; Penndorf, V.; Herzer, K.; Kaiser, G.; Schlaak, J.F.; Gerken, G.; Syn, W.-K.; Canbay, A. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int. J. Cancer 2011, 128, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Dyson, J.; Jaques, B.; Chattopadyhay, D.; Lochan, R.; Graham, J.; Das, D.; Aslam, T.; Patanwala, I.; Gaggar, S.; Cole, M.; et al. Hepatocellular cancer: The impact of obesity, type 2 diabetes and a multidisciplinary team. J. Hepatol. 2014, 60, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Perumpail, R.B.; Wong, R.J.; Ahmed, A.; Harrison, S.A. Hepatocellular Carcinoma in the Setting of Non-cirrhotic Nonalcoholic Fatty Liver Disease and the Metabolic Syndrome: US Experience. Dig. Dis. Sci. 2015, 60, 3142–3148. [Google Scholar] [CrossRef]
- Mittal, S.; El-Serag, H.B.; Sada, Y.H.; Kanwal, F.; Duan, Z.; Temple, S.; May, S.B.; Kramer, J.R.; Richardson, P.A.; Davila, J.A. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 124–131.e121. [Google Scholar] [CrossRef]
- Mohamad, B.; Shah, V.; Onyshchenko, M.; Elshamy, M.; Aucejo, F.; Lopez, R.; Hanouneh, I.A.; Alhaddad, R.; Alkhouri, N. Characterization of hepatocellular carcinoma (HCC) in non-alcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatol. Int. 2016, 10, 632–639. [Google Scholar] [CrossRef]
- Gawrieh, S.; Dakhoul, L.; Miller, E.; Scanga, A.; DeLemos, A.; Kettler, C.; Burney, H.; Liu, H.; Abu-Sbeih, H.; Chalasani, N.; et al. Characteristics, aetiologies and trends of hepatocellular carcinoma in patients without cirrhosis: A United States multicentre study. Aliment. Pharmacol. Ther. 2019, 50, 809–821. [Google Scholar] [CrossRef]
- Kodama, K.; Kawaguchi, T.; Hyogo, H.; Nakajima, T.; Ono, M.; Seike, M.; Takahashi, H.; Nozaki, Y.; Kawanaka, M.; Tanaka, S.; et al. Clinical features of hepatocellular carcinoma in nonalcoholic fatty liver disease patients without advanced fibrosis. J. Gastroenterol. Hepatol. 2019, 34, 1626–1632. [Google Scholar] [CrossRef]
- Tobari, M.; Hashimoto, E.; Taniai, M.; Kodama, K.; Kogiso, T.; Tokushige, K.; Yamamoto, M.; Takayoshi, N.; Satoshi, K.; Tatsuo, A. The characteristics and risk factors of hepatocellular carcinoma in nonalcoholic fatty liver disease without cirrhosis. J. Gastroenterol. Hepatol. 2020, 35, 862–869. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R.; Li, L.; Dai, J.; Natarajan, Y.; Yu, X.; Asch, S.M.; El-Serag, H.B. Effect of Metabolic Traits on the Risk of Cirrhosis and Hepatocellular Cancer in Nonalcoholic Fatty Liver Disease. Hepatology 2020, 71, 808–819. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Moschen, A.R. Multiple Parallel Hits Hypothesis in NAFLD—Revisited After a Decade. Hepatology 2020. [Google Scholar] [CrossRef]
- Ikejima, K.; Kon, K.; Yamashina, S. Nonalcoholic fatty liver disease and alcohol-related liver disease: From clinical aspects to pathophysiological insights. Clin. Mol. Hepatol. 2020, 26, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013, 499, 97–101. [Google Scholar] [PubMed]
- Martin, N.; Ziegler, D.V.; Parent, R.; Bernard, D. Hepatic Stellate Cell Senescence in Liver Tumorigenesis. Hepatology 2020. [Google Scholar] [CrossRef] [PubMed]
- Kucukoglu, O.; Sowa, J.P.; Mazzolini, G.D.; Syn, W.K.; Canbay, A. Hepatokines and adipokines in NASH-related hepatocellular carcinoma. J. Hepatol. 2020, 74, 442–457. [Google Scholar] [CrossRef]
- Enooku, K.; Nakagawa, H.; Fujiwara, N.; Kondo, M.; Minami, T.; Hoshida, Y.; Shibahara, J.; Tateishi, R.; Koike, K. Altered serum acylcarnitine profile is associated with the status of nonalcoholic fatty liver disease (NAFLD) and NAFLD-related hepatocellular carcinoma. Sci. Rep. 2019, 9, 10663. [Google Scholar] [CrossRef]
- Valenti, L.; Motta, B.M.; Soardo, G.; Iavarone, M.; Donati, B.; SanGiovanni, A.; Carnelutti, A.; Dongiovanni, P.; Rametta, R.; Bertelli, C.; et al. PNPLA3 I148M Polymorphism, Clinical Presentation, and Survival in Patients with Hepatocellular Carcinoma. PLoS ONE 2013, 8, e75982. [Google Scholar] [CrossRef]
- Su, W.; Wang, Y.; Jia, X.; Wu, W.; Li, L.; Tian, X.; Li, S.; Wang, C.; Xu, H.; Cao, J.; et al. Comparative proteomic study reveals 17β-HSD13 as a pathogenic protein in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA 2014, 111, 11437–11442. [Google Scholar] [CrossRef]
- Chen, J.; Zhuo, J.Y.; Yang, F.; Liu, Z.K.; Zhou, L.; Xie, H.Y.; Xu, X.; Zheng, S.S. 17-beta-hydroxysteroid dehydrogenase 13 inhibits the progression and recurrence of hepatocellular carcinoma. Hepatobiliary Pancreat. Dis. Int. 2018, 17, 220–226. [Google Scholar]
- Salomao, M.; Woojin, M.Y.; Brown, R.S., Jr.; Emond, J.C.; Lefkowitch, J.H. Steatohepatitic hepatocellular carcinoma (SH-HCC): A distinctive histological variant of HCC in hepatitis C virus-related cirrhosis with associated NAFLD/NASH. Am. J. Surg. Pathol. 2010, 34, 1630–1636. [Google Scholar]
- Taniai, M.; Hashimoto, E.; Tobari, M.; Kodama, K.; Tokushige, K.; Yamamoto, M.; Takayama, T.; Sugitani, M.; Sano, K.; Kondo, F.; et al. Clinicopathological investigation of steatohepatitic hepatocellular carcinoma: A multicenter study using immunohistochemical analysis of adenoma-related markers. Hepatol. Res. 2018, 48, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Muto, Y.; Sato, S.; Watanabe, A.; Moriwaki, H.; Suzuki, K.; Kato, A.; Kato, M.; Nakamura, T.; Higuchi, K.; Nishiguchi, S.; et al. Overweight and obesity increase the risk for liver cancer in patients with liver cirrhosis and long-term oral supplementation with branched-chain amino acid granules inhibits liver carcinogenesis in heavier patients with liver cirrhosis. Hepatol. Res. 2006, 35, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.; Arase, Y.; Ikeda, K.; Seko, Y.; Imai, N.; Hosaka, T.; Masahiro, K.; Satoshi, S.; Hitomi, S.; Norio, A.; et al. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am. J. Gastroenterol. 2012, 107, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Kogiso, T.; Sagawa, T.; Kodama, K.; Taniai, M.; Hashimoto, E.; Tokushige, K. Long-term outcomes of non-alcoholic fatty liver disease and the risk factors for mortality and hepatocellular carcinoma in a Japanese population. J. Gastroenterol. Hepatol. 2020, 35, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Tokushige, K.; Hashimoto, E.; Kodama, K. Hepatocarcinogenesis in non-alcoholic fatty liver disease in Japan. J. Gastroenterol. Hepatol. 2013, 28, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Seko, Y.; Sumida, Y.; Tanaka, S.; Mori, K.; Taketani, H.; Ishiba, H.; Hara, T.; Okajima, A.; Umemura, A.; Nishikawa, T.; et al. Development of hepatocellular carcinoma in Japanese patients with biopsy-proven non-alcoholic fatty liver disease: Association between PNPLA3 genotype and hepatocarcinogenesis/fibrosis progression. Hepatol. Res. 2017, 47, 1083–1092. [Google Scholar] [CrossRef]
- White, D.L.; Kanwal, F.; El-Serag, H.B. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin. Gastroenterol. Hepatol. 2012, 10, 1342–1359.e1342. [Google Scholar] [CrossRef]
- Kudo, M.; Matsui, O.; Izumi, N.; Iijima, H.; Kadoya, M.; Imai, Y.; Okusaka, T.; Miyayama, S.; Tsuchiya, K.; Ueshima, K.; et al. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer 2014, 3, 458–468. [Google Scholar] [CrossRef]
- Clinical Practice Guidelines for Liver Cancer 2017 Version, the Japan Society of Hepatology HCC Guidelines 2017; Kanehara & Co., LTD: Bunkyo-ku, Tokyo, 2017.
- Bruix, J.; Sherman, M. Diseases AAftSoL. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef]
- Adam, R.; Karam, V.; Cailliez, V.; Grady, J.G.O.; Mirza, D.; Cherqui, D.; Klempnauer, J.; Salizzoni, M.; Pratschke, J.; Jamieson, N.; et al. 2018 Annual Report of the European Liver Transplant Registry (ELTR)—50-year evolution of liver transplantation. Transpl. Int. 2018, 31, 1293–1317. [Google Scholar] [CrossRef]
- Cotter, T.G.; Charlton, M. Nonalcoholic Steatohepatitis after Liver Transplantation. Liver Transplant. 2020, 26, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Charlton, M.R.; Burns, J.M.; Pedersen, R.A.; Watt, K.D.; Heimbach, J.K.; Dierkhising, R.A. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011, 141, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, Y.; Wong, G.; Lee, I.H.; Akhtar, O.; Lopes, R.; Sumida, Y. Hepatocellular carcinoma and other complications of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in Japan: A structured literature review article. Hepatol. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.K.; Steel, J.L.; Chen, H.-W.; DeMateo, D.J.; Cardinal, J.S.; Behari, J.; Humar, A.; Marsh, J.W.; Geller, D.A.; Tsung, A. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology 2012, 55, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Benhammou, J.N.; Aby, E.S.; Shirvanian, G.; Manansala, K.; Hussain, S.K.; Tong, M.J. Improved survival after treatments of patients with nonalcoholic fatty liver disease associated hepatocellular carcinoma. Sci. Rep. 2020, 10, 9902. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Alejandro, R.; Croome, K.P.; Drage, M.; Sela, N.; Parfitt, J.; Chandok, N.; Marotta, P.; Dale, C.; Wall, W.; Quan, D. A comparison of survival and pathologic features of non-alcoholic steatohepatitis and hepatitis C virus patients with hepatocellular carcinoma. World J. Gastroenterol. 2012, 18, 4145–4149. [Google Scholar] [CrossRef]
- Cauchy, F.; Zalinski, S.; Dokmak, S.; Fuks, D.; Farges, O.; Castera, L.; Paradis, V.; Belghiti, J. Surgical treatment of hepatocellular carcinoma associated with the metabolic syndrome. Br. J. Surg. 2013, 100, 113–121. [Google Scholar] [CrossRef]
- Wakai, T.; Shirai, Y.; Sakata, J.; Korita, P.V.; Ajioka, Y.; Hatakeyama, K. Surgical Outcomes for Hepatocellular Carcinoma in Nonalcoholic Fatty Liver Disease. J. Gastrointest. Surg. 2011, 15, 1450–1458. [Google Scholar] [CrossRef]
- Takuma, Y.; Nouso, K.; Makino, Y.; Gotoh, T.; Toshikuni, N.; Morimoto, Y.; Shimomura, H.; Yamamoto, H. Outcomes after curative treatment for cryptogenic cirrhosis-associated hepatocellular carcinoma satisfying the Milan criteria. J. Gastroenterol. Hepatol. 2011, 26, 1417–1424. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zheng, Z.J.; Shi, R.; Su, Q.; Jiang, Q.; Kip, K.E. Metformin for liver cancer prevention in patients with type 2 diabetes: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2012, 97, 2347–2353. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.E.; Serper, M.; John, B.V.; Tessiatore, K.M.; Lerer, R.; Mehta, R.; Fox, R.; Aytaman, A.; Baytarian, M.; Hunt, K.; et al. Effects of Metformin Exposure on Survival in a Large National Cohort of Patients with Diabetes and Cirrhosis. Clin. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, P.P.; Singh, A.G.; Murad, M.H.; Sanchez, W. Statins are associated with a reduced risk of hepatocellular cancer: A systematic review and meta-analysis. Gastroenterology 2013, 144, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.; Mehaffey, J.H.; Hawkins, R.B.; Hsu, A.; Schirmer, B.; Hallowell, P.T. Bariatric surgery is associated with reduction in non-alcoholic steatohepatitis and hepatocellular carcinoma: A propensity matched analysis. Am. J. Surg. 2020, 219, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Bertot, L.C.; Jeffrey, G.P.; Wallace, M.; MacQuillan, G.; Garas, G.; Ching, H.L.; Adams, L.A. Nonalcoholic fatty liver disease-related cirrhosis is commonly unrecognized and associated with hepatocellular carcinoma. Hepatol. Commun. 2017, 1, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Noninvasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.; Berhane, S.; Teng, M.; Cox, T.; Tada, T.; Toyoda, H.; Kumada, T.; Kagebayashi, C.; Satomura, S.; Johnson, P.J. Biomarker-based prognosis in hepatocellular carcinoma: Validation and extension of the BALAD model. Br. J. Cancer 2014, 110, 2090–2098. [Google Scholar] [CrossRef]
- Mottin, C.C.; Moretto, M.; Padoin, A.V.; Swarowsky, A.M.; Toneto, M.G.; Glock, L.; Repetto, G. The Role of Ultrasound in the Diagnosis of Hepatic Steatosis in Morbidly Obese Patients. Obes. Surg. 2004, 14, 635–637. [Google Scholar] [CrossRef]
- Nishida, N.; Kudo, M. Immune checkpoint blockade for the treatment of human hepatocellular carcinoma. Hepatol. Res. 2018, 48, 622–634. [Google Scholar] [CrossRef]
- Bangaru, S.; Marrero, J.A.; Singal, A.G. Review article: New therapeutic interventions for advanced hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2020, 51, 78–89. [Google Scholar]
- Shimada, S.; Mogushi, K.; Akiyama, Y.; Furuyama, T.; Watanabe, S.; Ogura, T.; Ogawa, K.; Ono, H.; Mitsunori, Y.; Ban, D.; et al. Comprehensive molecular and immunological characterization of hepatocellular carcinoma. EBioMedicine 2019, 40, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Julich-Haertel, H.; Urban, S.K.; Krawczyk, M.; Willms, A.; Jankowski, K.; Patkowski, W.; Kruk, B.; Krasnodębski, M.; Ligocka, J.; Schwab, R.; et al. Cancer-associated circulating large extracellular vesicles in cholangiocarcinoma and hepatocellular carcinoma. J. Hepatol. 2017, 67, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Li, S.; Li, G.; Zhang, S.; Tang, X.; Ni, S.; Jian, X.; Xu, C.; Zhu, J.; Lu, M. The role of extracellular vesicles in mediating progression, metastasis and potential treatment of hepatocellular carcinoma. Oncotarget 2017, 8, 3683–3695. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A.; Ishiguro, K.; Yan, I.K.; Patel, T. Extracellular Vesicle-Based Therapeutic Targeting of β-Catenin to Modulate Anticancer Immune Responses in Hepatocellular Cancer. Hepatol. Commun. 2019, 3, 525–541. [Google Scholar] [CrossRef]
- Rosenblum, D.; Gutkin, A.; Kedmi, R.; Ramishetti, S.; Veiga, N.; Jacobi, A.M.; Schubert, M.S.; Friedmann-Morvinski, D.; Cohen, Z.R.; Behlke, M.A.; et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 2020, 6, eabc9450. [Google Scholar] [CrossRef]
| Author | Publication Year | Study Population/Follow-Up Period | Incidence of HCC Caused by NAFLD-Related Liver Cirrhosis | Findings |
|---|---|---|---|---|
| Bugianesi et al. [18] | 2002 | 641 HCC patients with liver cirrhosis/1990– | 44 patients had CC. | Obesity and diabetes were significantly more common in patients with HCC. |
| Marrero et al. [19] | 2002 | 105 HCC patients/2000– | 29 patients had CC; half associated with NAFLD (13 patients). | Fifty-three cases were detected by surveillance. |
| Malik et al. [20] | 2009 | 98 NASH patients with cirrhosis underwent LT/1997–2008 | 17 (17%) developed HCC. | Survival after LT was 88% during 2.5 years of follow-up. |
| Yatsuji et al. [8] | 2009 | 68 NASH patients with cirrhosis/1990-2006 | 11.3% developed HCC over 5 years. | |
| Hashimoto et al. [21] | 2009 | 34 NASH-HCC patients/ 1990–2007 | 5-year cumulative incidence of HCC 7.6%. | Five-year survival 82.8%. |
| Ascha et al. [9] | 2010 | 510 cirrhosis patients/2003–2007 | 195 patients with NASH cirrhosis of whom 25 (12.8%) developed HCC. | 64/315 (20.3%) of HCV-cirrhotic patients developed HCC (p = 0. 03). |
| Tokushige et al. [22] | 2011 | 14,530 HCC patients/2006–2009 | NAFLD-HCC 2%. The prevalence of cirrhosis was 62% in NAFLD-HCC patients and 52% in those with “unknown” HCC. | ALC-HCC proportion 7.2%. The prevalence of cirrhosis was 78% in ALC-HCC patients. |
| Kodama et al. [23] | 2013 | 72 patients with NASH cirrhosis patients/1990–2010 | 10/72 NASH-cirrhosis patients developed HCC. 10.5% over 5 years | 6/85 ALD-cirrhosis patients developed HCC. 12.3% incidence of HCC over 5 years; ALD-cirrhosis patients. |
| Wong et al. [24] | 2014 | 61,868 LT patients (10,061 HCC)/2002–2012 | NASH-related HCC; 4-fold increase | |
| Tateishi et al. [25] | 2015 | 5326 HCC patients with non-viral etiologies/1991–2010 | NAFLD-HCC 596 patients (11.2%), of whom 368 (63.4%) had cirrhosis. | Child-Pugh classes A; 439 (76.5%), B; 120 (20.9%), C; 15 (2.6%). |
| Mittal et al. [26] | 2015 | 1500 HCC patients/2005–2010 | 8% NAFLD 8% patients, of whom only 58.3% had cirrhosis | Patients with NAFLD-related HCC did not undergo HCC surveillance in the 3 years prior to HCC diagnosis. |
| Younossi et al. [27] | 2015 | 4979 HCC patients/2004–2009 | 701 NAFLD-HCC patients (14.1%) | NAFLD-HCC exhibited a 9% annual increase. NAFLD increased 1-yr mortality: OR 1.21, 95% CI 1.01–1.45. |
| Kanwal et al. [28] | 2018 | 296,707 NAFLD patients/ 2004–2008 | 490 developed HCC (0.21/1000 person- yrs). Annual incidence of HCC in cirrhotic patients 10.6 (range 1.6–23.7)/1000 person-yrs. | |
| Vilar-Gomez et al. [29] | 2018 | 458 biopsy-confirmed NAFLD patients/1995–2013 | <F3, 17%; 95% CI, 8–31% vs. F4, 2.3%, 95% CI, 1–12% | Annual incidence of HCC: Child-Pugh A5; 1.8, A6; 4.7. |
| Author | Publication year | Study Population/Follow-UP Period | Incidence of HCC in Non-Cirrhotic Patients | Characteristics of HCC in Non-Cirrhotic Patients |
|---|---|---|---|---|
| Guzman et al. [30] | 2008 | 50 HCC patients underwent explant treatment or liver resection/2004–2007 | 3 cases/5 NAFLD+5 CC cases lacked cirrhosis. | |
| Paradis et al. [31] | 2009 | 128 surgically resected HCC patients/1995–2007 | 31 cases with MS were non-cirrhotic. | Some HCCs with MS arose via malignant transformation of a pre-existing liver cell adenoma. |
| Kawada et al. [13] | 2009 | 1168 surgically resected HCC patients/1990–2006 | 8 cases (1%) were NASH, 6 were non-cirrhotic. | |
| Yasui et al. [32] | 2011 | 87 NASH-HCC patients/1993–2010 | F1-3; 43 cases | Liver cirrhosis was less common in males. |
| Ertle et al. [33] | 2011 | 162 NAFLD/NASH-HCC patients/2007–2008 | Non-cirrhotic 41.7%, | |
| Dyson et al. [34] | 2014 | 632 HCC patients/2000–2010 | Non-cirrhotic 31/136 (22.8%). | NAFLD-HCC was associated with a lower prevalence of cirrhosis (77.2%). |
| Perumpail et al. [35] | 2015 | 44 HCC patients/2010–2012 | Non-cirrhotic: 6 cases | |
| Mittal et al. [36] | 2016 | 1500 HCC patients/2005–2010 | Non-cirrhotic: 13% | |
| Mohamad et al. [37] | 2016 | 83 NAFLD-HCC patients/2003–2012 | Non-cirrhotic: 36 cases | HCC patients that were non-cirrhotic (compared to cirrhotic) were older (67.5 ± 12.3 vs. 62.7 ± 8.1 years); less likely to be obese (52 vs. 83%) or to have type 2 diabetes (38 vs. 8%); more likely to have single nodules (80.6 vs. 52.2%) of larger size (>5 cm) (77.8 vs. 10.6%); more likely to undergo hepatic resection (66.7% vs. 17%); and less likely to receive loco-regional therapy (22.3 vs. 61.7%) or DDLT (0 vs. 72.3%) |
| Gawrieh et al. [38] | 2019 | 5144 HCC patients/2000–2014 | 11.7% were non-cirrhotic; of whom 26.3% had NAFLD | Older age, more commonly female, less frequently black. Larger tumors, less frequently fulfilled the Milan criteria, more frequently underwent resection, and experienced better overall survival than liver cirrhosis patients. |
| Bengtsson et al. [14] | 2019 | 1562 HCC patients/2004–2017 | NAFLD-HCC 225 patients 26.3% (14.4%), of whom 83 (37%) were non-cirrhotic | Older age, a lower prevalence of diabetes, and more frequent resection.Mortality was similar to that from liver cirrhosis. |
| Kodama et al. [39] | 2019 | 104 NAFLD-HCC patients/2000–2016 | F0-2; 35 cases F3-4; 69 cases | HCCs in non-cirrhotic patients were larger than in others and evidenced lower histological activity. The recurrence rate was significantly lower in NAFLD-HCC patients who were not cirrhotic (p < 0.01). Risk factors for recurrence were the male gender, lower serum albumin levels, and advanced fibrosis. |
| Tobari et al. [40] | 2020 | 857 NAFLD patients/1991–2018 | 48 patients with non-cirrhotic and 71 with cirrhotic HCCs | Risk factors for HCC in non-cirrhotic patients were the male gender, light drinking, and a high FIB4 index. |
| Kanwal et al. [41] | 2020 | 271,906 NAFLD patients/2004–2008 | 22,794 developed cirrhosis, and 253 HCC, of whom 64 were non-cirrhotic | The risk of HCC was 6.4-fold higher in patients with diabetes, obesity, dyslipidemia, and hypertension (HR: 6.42, 95% CI: 0.89–46.07). |
| Author | Publication Year | Study Population/Follow-Up Period | Diagnostic Method for NAFLD | HCC CumulativeIncidence | Risk Factors |
|---|---|---|---|---|---|
| Hashimoto et al. [21] | 2009 | 382 NASH patients (34 NASH-HCC patients)/1990–2007 | Biopsy | 7.6%—5 yrs | Fibrosis, OR: 4.232, 95% CI: 1.847–9.698. Age, OR: 1.108, 95% CI: 1.028–1.195. AST, OR: 0.956, 95% CI: 0.919–0.995. Activity, OR: 0.154, 95% CI: 0.037–0.638. |
| Kawamura et al. [55] | 2012 | 6508 NAFLD patients/12 yrs | Ultrasound; Fibrosis graded by biopsy in 104 cases | 0.02%—4 yrs 0.2%—8 yrs 0.5%—12 yrs | F3/4, HR: 25.03, 95%CI: 9.02–69.52. DM, HR: 3.21, 95%CI: 1.09–9.50. AST level ≥40 IU/L, HR: 8.20, 95% CI: 2.56–26.26. Platelet count <15 × 104/μL, HR: 7.19, 95% CI: 2.26–23.26. Age ≥60 years, HR: 4.27, 95% CI: 1.30–14.01. |
| Tokushige et al. [57] | 2013 | 34 NAFLD-related cirrhosis patients/1990–2011 | Biopsy | 11.3%—5 yrs | Child-Pugh, HR: 3.09, 95% CI: 1.374–6.934. Serum GGT, HR: 1.01, 95% CI: 1.002–1.022. Age, HR: 1.12, 95% CI 1.014–1.226. |
| Seko et al. [58] | 2017 | 312 NASH patients/1999–2014 | Biopsy | 1.9%—5 yrs 4.2%—6 yrs 8.3%—10 yrs | F3/4, HR: 24.4, 95% CI: 2.07–288.2. PNPLA3 genotype GG, HR 6.36, 95% CI 1.36–29.80. |
| Author | Publication Year | Study Population/Follow-Up Period | Treatment | Overall Survival | Disease-Free Survival | Other |
|---|---|---|---|---|---|---|
| Reddy et al. [67] | 2012 | 52 NASH-HCC patients/ 2002–2010 | DDLT, resection, RFA | 1-yr: 90%, 3-yr: 72%, 5-yr: 65% | 1-yr: 84%, 3-yr: 70%, 5-yr: 60% | |
| Wong et al. [24] | 2014 | 61,868 end-stage liver disease patients (10,061 HCC patients)/2002–2012 | DDLT | 1-yr: 87.5% 3-yr: 79.8%, 5-yr: 65.5% | NA | |
| Piscaglia et al. [15] | 2016 | 756 patients (145 NAFLD patients)/2010–2012 | DDLT, resection, RFA | 1-yr: 90–95%, 3-yr: 85–90% | NA | Survival was significantly shorter in NAFLD-HCC patients (25.5 months) (95% CI 21.9–29.1 months, p = 0.017). |
| Malik et al. [20] | 2009 | 98 NASH cirrhosis patients (17 HCC patients)/1997–2008 | DDLT | 1-yr: 85–90% | NA | |
| Hernandez-Alejandro et al. [69] | 2012 | 102 NASH patients (17 HCC patients)/2000–2011 | DDLT | NA | 1-yr: 95%, 3-yr: 95%, 5-yr: 85% | |
| Cauchy et al. [70] | 2013 | 560 HCC patients (62 MS patients)/2000–2011 | Resection | 1-yr: 83%, 3-yr: 75%, 5-yr: 59% | 1-yr: 83%, 3-yr: 70%, 5-yr: 66% | |
| Wakai et al. [71] | 2011 | 225 HCC patients (17 NAFLD patients)/1990–2007 | Resection | Patients with NAFLD exhibited better disease-free survival than did those infected with HBV or HCV. | ||
| Takuma et al. [72] | 2011 | 36 cirrhosis-associated HCC patients/1992–2009 | Resection, RFA, PEI, MCT | 1-yr: 94%, 3-yr: 85%, 5-yr: 54% | 1-yr: 89%, 3-yr: 68%, 5-yr: 54% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kogiso, T.; Tokushige, K. The Current View of Nonalcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma. Cancers 2021, 13, 516. https://doi.org/10.3390/cancers13030516
Kogiso T, Tokushige K. The Current View of Nonalcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma. Cancers. 2021; 13(3):516. https://doi.org/10.3390/cancers13030516
Chicago/Turabian StyleKogiso, Tomomi, and Katsutoshi Tokushige. 2021. "The Current View of Nonalcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma" Cancers 13, no. 3: 516. https://doi.org/10.3390/cancers13030516
APA StyleKogiso, T., & Tokushige, K. (2021). The Current View of Nonalcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma. Cancers, 13(3), 516. https://doi.org/10.3390/cancers13030516





